Abstract
A link between energy balance and reproduction is critical for the survival of all species. Energy-consuming reproductive processes need to be aborted in the face of a negative energy balance, yet knowledge of the pathways mediating this link remains limited. Fasting and food restriction that inhibit fertility also upregulate the hypothalamic melanin-concentrating hormone (MCH) system that promotes feeding and decreases energy expenditure; MCH knockout mice are lean and have a higher metabolism but remain fertile. MCH also modulates sleep, drug abuse behavior, and mood, and MCH receptor antagonists are currently being developed as antiobesity and antidepressant drugs. Despite the clinical implications of MCH, the direct postsynaptic effects of MCH have never been reported in CNS neurons. Using patch-clamp recordings in brain slices from multiple lines of transgenic GFP mice, we demonstrate a strong inhibitory effect of MCH on an exclusive population of septal vGluT2-GnRH neurons that is activated by the puberty-triggering and preovulatory luteinizing hormone surge-mediating peptide, kisspeptin. MCH has no effect on kisspeptin-insensitive GnRH, vGluT2, cholinergic, or GABAergic neurons located within the same nucleus. The inhibitory effects of MCH are reproducible and nondesensitizing and are mediated via a direct postsynaptic Ba2+-sensitive K+ channel mechanism involving the MCHR1 receptor. MCH immunoreactive fibers are in close proximity to vGluT2-GFP and GnRH-GFP neurons. Importantly, MCH blocks the excitatory effect of kisspeptin on vGluT2-GnRH neurons. Considering the role of MCH in regulating energy balance and of GnRH and kisspeptin in triggering puberty and maintaining fertility, MCH may provide a critical link between energy balance and reproduction directly at the level of the kisspeptin-activated vGluT2-GnRH neuron.
Keywords: fertility, gonadotropins, HPG axis, obesity, starvation
Nutritional status and availability of energy stores exert a profound impact on reproductive function (1–3). Reproduction is an expensive energy-consuming process, and thus it is important that puberty, pregnancy, and lactation occur only when metabolic fuel is available (4). Availability of metabolic fuel is conveyed to the brain by peripherally generated signals, such as leptin, insulin, and ghrelin, as well as by centrally released peptides such as neuropeptide Y, melanocortins, and melanin-concentrating hormone (MCH). One or more of these signals can directly or indirectly link energy balance with reproduction at one or more levels of the hypothalamic-pituitary-gonadal (HPG) axis. Thus, insulin and leptin may indirectly influence GnRH neurons (2, 5–7); leptin could regulate the HPG axis via kisspeptin-containing hypothalamic neurons (8, 9). Kisspeptin and its receptor are critical for reproduction (10–12); both kisspeptin and kisspeptin receptor knockout mice fail to enter puberty, and humans with loss of function mutations in the kisspeptin receptor exhibit hypogonadotropic hypogonadism and are infertile (13–15). Mechanistically, kisspeptin is a potent activator of GnRH neurons (16–19); it enhances pituitary gonadotropin release, triggering a cascade that is essential for entering puberty (13, 20) and for maintaining ovulation and fertility (21).
MCH neurons, which are mostly located in the lateral hypothalamus and in the zona incerta, may also target GnRH neurons directly, as MCH fibers are in close apposition with GnRH neurons (22). MCH acts via the G-protein-coupled receptors MCHR1 (23–27) and MCHR2 (28–30); only MCHR1 is present in the rodent brain, and 50–55% of rat GnRH neurons express MCHR1 (22). Intracerebral infusions of MCH can suppress (31) or enhance (32, 33) pituitary gonadotropin release, depending on the estrogenic milieu. Fasting and food restriction, which has an inhibitory effect on fertility as evidenced by decreased circulating gonadotropins and anovulation (34), upregulates the MCH system (35, 36). An activated MCH system decreases energy expenditure and increases food intake. In contrast, MCH deficiency in mice leads to leanness and increased metabolism. However, despite their leanness, MCH knockout (37, 38) and MCHR1 knockouts (39) remain fertile. The MCH system may also be involved in the regulation of sleep (40–42), drug addiction (43, 44), and mood (45). MCHR1 antagonists are currently being developed as antiobesity and antidepressant drugs (46, 47).
Despite the various functions attributed to MCH, the dramatic phenotype of the MCH knockout mice and the clinical importance of MCH, there is little information on the electrophysiological effects of MCH on CNS neurons. Available studies are restricted to hypothalamic neurons that respond to MCH with presynaptic inhibition of transmitter release (48, 49). Direct effects of MCH on membrane potential of CNS neurons have never been reported.
MCH neurons densely innervate the rodent medial septum/diagonal band of Broca (MSDB) (50, 51) that contains cholinergic, GABAergic, and vGluT2-glutamatergic neurons as well as two subpopulations of GnRH neurons, only one of which is activated by the puberty-triggering peptide kisspeptin (19).
In an effort to identify the cellular targets of MCH in the brain and to understand the mechanisms linking energy balance to reproduction, the goal of the present study was to test the hypothesis that MCH would modulate the activity of kisspeptin-activated and kisspeptin-insensitive MSDB GnRH neurons. Using electrophysiological recordings and anatomical labeling methods in multiple lines of transgenic GFP mice, we report strong, inhibitory effects of MCH on kisspeptin-activated vGluT2-GnRH neurons. Kisspeptin-insensitive GnRH, cholinergic, GABAergic, and glutamatergic MSDB neurons did not respond to MCH. The observed inhibitory effects of MCH are mediated via a direct postsynaptic mechanism and are of greater magnitude than has been reported for any CNS neuron. These actions of MCH on kisspeptin-activated GnRH neurons may provide a critical link between energy balance and reproductive physiology.
Results
MCH Inhibits vGluT2-GnRH Neurons via a MCHR1-Transduced Direct Postsynaptic Mechanism.
MCH inhibited a unique subpopulation of vGluT2-GnRH neurons in the MSDB that can be identified in slices prepared from either vGluT2-GFP or GnRH-GFP mice (19, 52). MCH (1 μM) produced a 3- to 20-mV hyperpolarization (mean: 8.1 ± 1.5 mV; n = 15) in 59% of vGluT2-GnRH neurons recorded in brain slices prepared from 17 postpubertal mice (n = 22 cells; 35–160 days of age). In brain slices prepared from 300 prepubertal mice (12–30 days of age), MCH inhibited 60% of neurons tested (n = 327) and produced a statistically similar hyperpolarization of 6.7 ± 0.3 mV (range 2–20 mV; n = 149). Because neurons from both prepubertal and postpubertal mice responded similarly to MCH, the remaining studies were conducted in slices prepared from prepubertal mice.
The inhibitory effect of MCH was similar in amplitude in both prepubertal males and females and showed little desensitization to a second application of agonist administered 5–15 min after the first (females, first MCH application: 8.2 ± 0.7 mV, second MCH application: 8.1 ± 0.7 mV; n = 20 cells recorded from 18 mice; males, first MCH application: 7.8 ± 0.8 mV, second MCH application: 7.9 ± 0.9 mV; n = 15 cells recorded from 14 mice; Student's paired t test, not significant; Fig. 1 A and B). Neuropeptide-glutamic acid-isoleucine (n = 9), cocaine- and amphetamine-regulated transcript (n = 11) and nesfatin (n = 10) had no effect on MCH responsive or nonresponsive vGluT2-GnRH neurons; all three peptides can be produced by MCH neurons (51, 53, 54). Similarly, leptin (n = 5) and NPY (n = 19), which also signal availability of energy stores, had no effect on the kisspeptin-activated vGluT2-GnRH neurons.
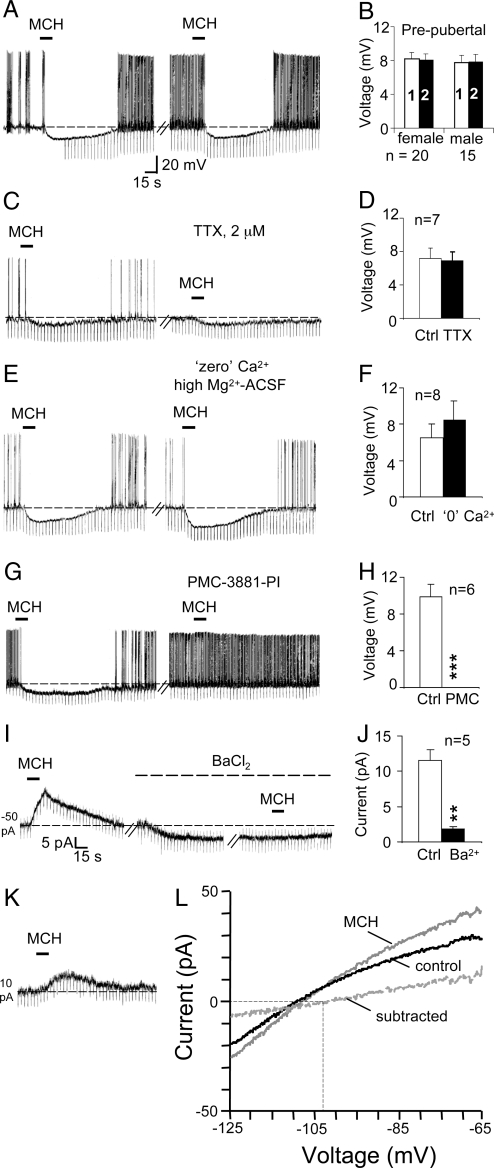
Fig. 1.
MCH inhibits vGluT2-GnRH neurons via a direct postsynaptic mechanism involving MCH1R and an opening of Ba2+-sensitive K+ channels. (A) Chart record from an example current-clamp recording shows that two consecutive applications of 1 μM MCH at an interval of 8.5 min produced a comparable hyperpolarization of 13 mV. (B) Bar charts summarize the data for two consecutive applications of MCH applied at intervals of 5–15 min. Note the similar magnitude of the response in the two sexes, the reproducibility of the inhibitory effects, and a lack of desensitization. (C–F) Chart records and bar charts show that MCH inhibition does not change in the presence of TTX or in “zero” Ca2+/high Mg2+ ACSF. (G, H) Chart record and bar chart shows the effect of MCH before and after bath application of a MCH1R antagonist. Broken lines indicate base line, and number in mV denotes resting membrane potential in quiescent neurons. Downward vertical deflections are in response to −0.01 nA current pulses delivered every 4 s to monitor the input resistance of the recorded neuron. (I, J) In an example voltage-clamp recording (holding potential: −65 mV) MCH produced a 15-pA outward current. Bath-applied BaCl2, a K+ channel blocker, produced a 6-pA inward current and blocked MCH inhibition. (K) Cell in which MCH induced a 7-pA outward current that is closer to the mean current observed under our experimental conditions. (L) I-V curves, obtained using slow steady-state ramps in a voltage-clamped neuron, in the absence and in the presence of MCH. Subtracted current is also shown. The MCH current reversed at −103 mV, close to the calculated EK of −101 mV.
The MCH-induced hyperpolarization in vGluT2-GnRH neurons persisted in TTX (control: 7.1 ± 1.3 mV; TTX: 7 ± 1 mV; n = 7; not significant, Student's paired t test) and in “zero” Ca2+/high Mg2+ ACSF (control: 6.5 ± 1.5 mV; “zero” Ca2+/high Mg2+: 8.5 ± 2 mV; n = 8 cells; not significant), suggesting involvement of a direct postsynaptic mechanism (Fig. 1 C–F). As expected, the MCH-induced inhibitory effect was blocked by the MCHR1-antagonist PMC-3881-PI (control: 9.8 ± 1.4 mV; antagonist: 0 ± 0 mV; n = 6; P < 0.001, Student's paired t test; Fig. 1 G and H).
To prevent the confounding effects of small changes in membrane resistance, the conductance change associated with the MCH inhibition was measured under voltage-clamp conditions. At a holding potential of −65 mV, MCH produced a 6.5 ± 1 pA outward current (Fig. 1 I and K) that was associated with a significant increase in membrane conductance (control: 0.8 ± 0.2 nS; MCH: 1 ± 0.2 nS; P < 0.01; n = 22), suggesting a net opening of channels. The MCH-induced outward current was significantly reduced in the presence of BaCl2, a blocker of K+ channels (control: 11.6 ± 1.4 pA; Ba2+: 1.8 ± 0.4 pA; n = 5; Student's paired t test; P < 0.01; Fig. 1 I and J). Ba2+ alone produced a 10.6 ± 3.3 pA inward current in voltage-clamp recordings (n = 5). Consistent with the involvement of a K+ current, MCH-induced outward current reversed at mean reversal potential of −101 ± 1.7 mV (n = 5), which is near the calculated Ek of −101 mV (Fig. 1L). Thus, MCH-induced inhibition of vGluT2-GnRH neurons involves opening of Ba2+-sensitive K+ channels.
MCH Selectively Inhibits a Subpopulation of Kisspeptin-Activated vGluT2-GnRH Neurons in the MSDB.
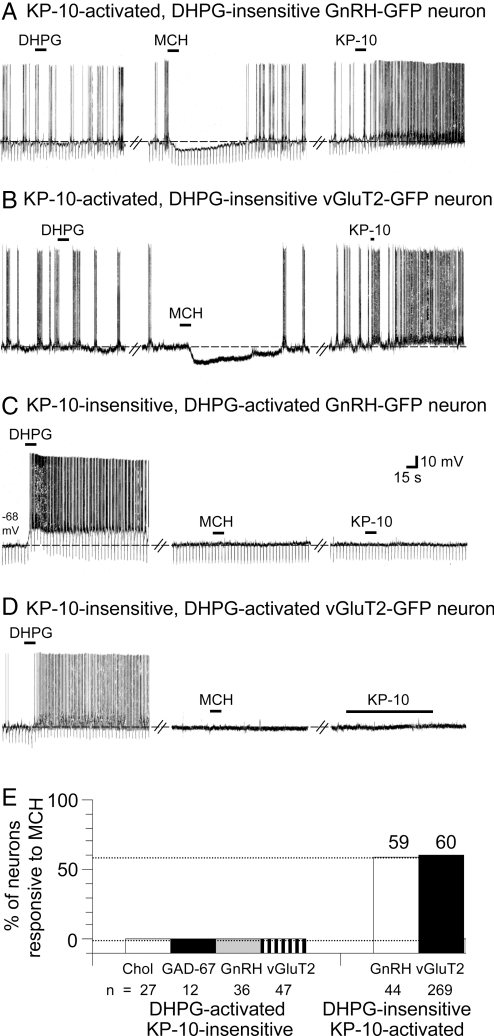
The above-described effects of MCH occurred in a very unique population of neurons within the MSDB. The MCH-inhibited vGluT2-GnRH neurons could be distinguished from other major neuronal populations within the MSDB by their lack of excitatory response to the Group I glutamate metabotropic-receptor agonist DHPG and by their strong and persistent activation by the neuropeptide, kisspeptin, the natural ligand of GPR54 (19, 52). Although DHPG-sensitivity was examined in every neuron tested, because of the strong and prolonged nature of the kisspeptin response, kisspeptin agonist was applied only at the end of the experiment. Sensitivity to kisspeptin was assessed using the bioactive fragment kissspeptin-10 (KP-10). A total of 75 MCH-responsive vGluT2-GnRH neurons were confirmed to be KP-10-sensitive at the end of the experiment (Fig. 2A, B, and E).
Fig. 2.
MCH-inhibited vGluT2-GnRH neurons are uniquely activated by kisspeptin but not by a Group I metabotropic glutamate receptor agonist, DHPG. (A) Chart record from a current-clamped DHPG-insensitive GnRH-GFP neuron shows that the neuron responded to MCH (1 μM, 15 s) with a 7-mV hyperpolarization that lasted for 110 s. The same neuron responded to the bioactive kisspeptin fragment, KP-10 (100 nM, 15 s) with a prolonged excitation (washout not shown). (B) vGluT2-GFP neuron with similar properties. The neuron was insensitive to DHPG but responded to MCH with a 13-mV hyperpolarization that lasted for 187 s; the neuron was also activated by KP-10. (C) Shows that a DHPG-activated GnRH-GFP neuron that is insensitive to KP-10 is also insensitive to MCH. (D) Similarly, no effect of MCH was seen in a DHPG-activated, KP-10-insensitive vGluT2-GFP neuron. (E) Bar chart summarizes the data and also shows that cholinergic and GAD67-GFP neurons located within the same nucleus do not respond to MCH; both cholinergic and GABAergic neurons are activated by DHPG but not by KP-10. Note that a similar percentage of DHPG-insensitive vGluT2-GFP and GnRH-GFP neurons was inhibited by MCH; these neurons most likely represent the same neuronal population, as GnRH neurons co-localize vGluT2 (19).
MCH had no effect on the 36 KP-10-insensitive GnRH-GFP cells that were recorded in brain slices prepared from 30 mice (Fig. 2 C and E) or on the 47 KP-10-insensitive vGluT2-GFP neurons that were recorded from 42 mice (Fig. 2 D and E); these neurons were strongly activated by DHPG. MCH also had no effect on the cholinergic (n = 27) or on the GABAergic neurons (n = 12; Fig. 2E); both neuronal populations are similarly activated by DHPG but not by KP-10 (19, 55, 56). Thus, MCH selectively inhibits the KP-10-activated, DHPG-insensitive neurons within the MSDB.
MCH Interrupts Kisspeptin-Induced Activation of vGluT2-GnRH Neurons.
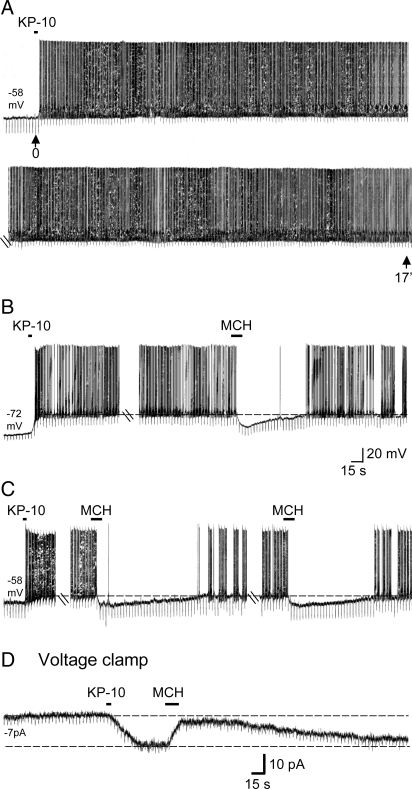
Because of a strong link between energy balance and reproduction, we next determined whether MCH, by virtue of its inhibitory activity, could oppose the long-lasting excitatory effect of kisspeptin. Under control conditions, KP-10 activation lasts an average of 16 ± 1.5 min (Fig. 3A) (19). In 12 cells, 100 nM KP-10 was applied for 5 s and, after establishment of the excitatory response to KP-10, MCH was applied for 15 s. MCH interrupted the excitatory effect of KP-10 and produced a 10.9 ± 1.1 mV hyperpolarization (Fig. 3B). This interruption lasted an average of 1.8 ± 0.3 min (n = 12). In addition, because of the nondesensitizing nature of the MCH response, repeated applications of MCH continued to block KP-10 activation (Fig. 3C). A similar interruption was observed in voltage-clamp recordings (n = 3) (Fig. 3D).
Fig. 3.
MCH opposes kisspeptin-induced activation in vGluT2-GnRH neurons. (A) KP-10 (100 nM, 5 s) induced a prolonged excitation that lasted >17 min. (B) Cell that depolarized and started firing in response to KP-10, only to be interrupted by a subsequent MCH application. MCH inhibition lasted for 100 s. (C) Second cell in which MCH interrupted KP-10-induced firing for 140 s and for 120 s on reapplication. (D) Voltage-clamp recording showing that KP-10 induced a 15-pA inward current. MCH induced a 12-pA current, almost completely reversing the KP-10-induced inward current, which returned to its original magnitude 240 s later.
MCH Immunoreactive Fibers Are Present in the Vicinity of Septal vGluT2-GnRH Neurons.
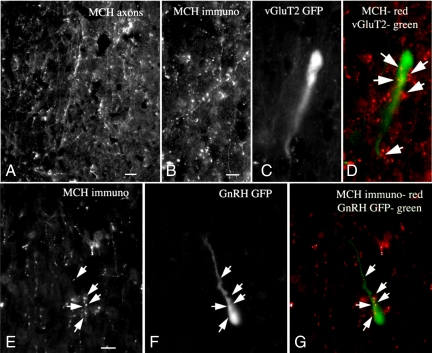
Because MCH inhibited KP-10-activated vGluT2-GnRH neurons, we specifically determined the anatomical relationship between MCH-immunoreactive fibers and vGluT2-GFP and GnRH-GFP neurons using vGluT2-GFP (n = 5) and GnRH-GFP (n = 5) mice and a well-characterized antisera against MCH (51). In sections prepared from vGluT2-GFP mice, MCH immunoreactive axons stained red were found throughout the MSDB and appeared in close juxtaposition to vGluT2 cell bodies and dendrites. In some cells, many MCH boutons appeared to contact single GFP cells (Fig. 4 A–D). Similarly, in the GnRH-GFP mice, MCH axons appeared in close juxtaposition to GFP cell bodies and dendrites (Fig. 4 E–G). Elimination of either the primary or secondary antibody gave no staining, and substitution with antisera against hypocretin/orexin resulted in a different pattern of staining. Thus MCH axons contact GnRH as well as the vGluT2 neurons. Ultrastructural analysis would help to address the question of whether these contacts are synaptic in nature.
Fig. 4.
MCH-immunoreactive fibers contact vGluT2 and GnRH-GFP neurons in the MSDB. (A) MCH axons in the MSDB. Scale bar, 15 μm. (B-D) Same microscope field showing MCH axons (B), a vGluT2-GFP neuron (C), and MCH axons (in red) contacting the vGluT2-GFP neurons (in green) (D). Scale bar, 9 μm. (E–G) Microscope field showing MCH axons (E), GnRH-GFP neuron (F), and red MCH axons in apparent contact with green GnRH-GFP neurons. Scale bar, 10 μm. Note the similar shape of the vGluT2-GFP and GnRH-GFP neurons.
Discussion
In the present study, we report a direct postsynaptic inhibitory action of the orexigenic peptide MCH on CNS neurons for the first time. These effects suggest an additional function of MCH in the brain. The inhibitory effects of MCH occur in an exclusive subset of basal forebrain neurons, namely, the vGluT2-GnRH neurons. The MCH-induced inhibition in vGluT2-GnRH neurons is mediated via a MCH1-receptor-transduced postsynaptic mechanism that involves an opening of Ba2+-sensitive K+ channels. MCH-immunoreactive fibers are located in close apposition to vGluT2-GFP and GnRH-GFP neurons. Interestingly, the inhibitory effects of MCH can interrupt or block the persistent excitatory effect of the hypothalamic peptide kisspeptin, which is essential for reproduction. Given the role of MCH in energy balance and of kisspeptin in triggering puberty and in sustaining ovulation and fertility, MCH inhibition of kisspeptin-sensitive vGluT2-GnRH neurons suggests a MCH-mediated link between energy balance and reproduction directly at the level of the GnRH neuron.
In the present study, brief 15-s applications of MCH produced a strong and reproducible inhibition in a highly selective population of vGluT2-GnRH neurons that is persistently activated by the Gq-coupled receptor GPR54 ligand kisspeptin but not by the Gq-coupled Group I glutamate metabotropic receptor agonist DHPG. In contrast, MCH had no effect on DHPG-activated, kisspeptin-insensitive GnRH, vGluT2, cholinergic or GABAergic neurons that are located within the same basal forebrain area. Thus the observed inhibitory effects of MCH in the MSDB are highly selective for a neuronal population that is involved in triggering puberty and sustaining key reproductive functions that are essential to fertility. Therefore, these findings suggest a novel function of MCH. In addition, the absence of MCH inhibition in kisspeptin-insensitive, DHPG-activated GnRH neurons provides an additional line of evidence in favor of two populations of GnRH neurons that serve different functions by virtue of their neuromodulator responses (19).
MCH has been shown to alter the firing rate of ventromedial hypothalamic and arcuate neurons; whether these effects are mediated via a direct mechanism has not been determined (57). In cultured lateral hypothalamic neurons, MCH inhibits voltage-dependent calcium channels and decreases both glutamatergic and GABAergic synaptic transmission without involvement of K+ currents and without having a direct effect on membrane potential and conductance (48, 49). In the present study, conducted in murine brain slices, MCH produced a 2- to 20-mV hyperpolarization and a marked increase in membrane conductance that persisted after blockade of synaptic transmission, suggesting a direct effect of MCH on membrane properties. These effects suggest an additional function of MCH in the brain. The MCH-induced changes in membrane properties reversed near Ek and did not occur in the presence of barium, suggesting involvement of K+ channels. Considering that MCHR1 is coupled to the G proteins, Gi and Go, it is not surprising that MCH is inhibitory. Nevertheless, earlier studies in non-neuronal kidney cell lines found that MCH raised intracellular calcium (25, 26). In neurons, an increase in calcium is generally associated with excitation, underlining the complications of studying neuronal receptors in non-neurons.
Unlike MCH, NEI, CART, or nesfatin had no apparent effect on the membrane properties of kisspeptin-activated vGluT2-GnRH neurons. The absence of leptin and NPY effects on these neurons is consistent with the absence of leptin receptors in GnRH neurons (6); NPY effects have been reported only in lactating mice (58).
The physiological significance of the observed inhibitory effects of MCH is underscored by our MCH immunoreactivity studies. The high density of MCH fibers that we observed in MSDB in mice is consistent with earlier reports in rats (50, 51, 59). The close appositions observed between GnRH-GFP neurons and MCH-ir fibers in mice are consistent with the study in rats (22). In addition, in our study, close appositions were noted between vGluT2-GFP neurons and MCH fibers that are consistent with the observed inhibitory effects of MCH on vGluT2-GnRH neurons.
Functionally, MCH has been linked to the sleep-wake cycle (40–42), to drug abuse (43, 44), and to mood disorders; MCH receptor antagonists have antidepressive and anxiolytic effects (43, 47). However, most work on MCH has emphasized its role as a critical hypothalamic regulator of energy homeostasis with effects both on feeding behavior and energy expenditure (38). MCH1R antagonists are currently strong candidates for the treatment of obesity (47, 60). Within the MSDB, the observed inhibitory actions of MCH on vGluT2-GnRH neurons presumably link energy balance to reproduction. It is well known that the physiological controls of reproduction and energy balance are closely intertwined, perhaps to maximize chances of individual survival and propagation of species. The GnRH neurons are a key element of the HPG axis; they integrate various internal homeostatic and external environmental signals and serve as the final pathway via which the brain controls fertility in all mammalian species; a lack of GnRH leads to infertility (61). GnRH neurons project to the median eminence and the released GnRH enhances pituitary gonadotropin secretion, which in turn release the sex steroids from the gonads. The results of the present study suggest that MCH, via its inhibitory actions on GnRH neurons, should reduce pituitary gonadotropin release, which is consistent with a study in behaving rats (31). Stimulatory effects of MCH on gonadotropin release have also been reported, albeit in anesthetized, ovariectomized, adrenalectomized rats (32). MCH may also influence gonadotropin release via the median eminence (62), although the posterior rather than the anterior lobe of the pituitary receives a denser MCH innervation (51).
Another interesting finding in this study is that MCH, via its opposing inhibitory and nondesensitizing effect, could interrupt the persistent and profound excitatory effect of kisspeptin on GnRH neurons. Thus, MCH could potentially act as a brake for the puberty-triggering and other effects of the kisspeptin-GPR54 system, such as by inducing the luteinizing hormone (LH) surge that precedes ovulation. In this study, the inhibitory effects of MCH were observed equally well in both prepubertal and postpubertal male and female mice, suggesting a general role of MCH in linking energy balance to reproduction in both sexes. These inhibitory actions of MCH on GnRH neurons could help to curtail reproductive activity under conditions that upregulate the MCH system, such as during food deprivation. Normally, a greater than 15% reduction in body weight, as can occur after a 48-h fast, reduces gonadotropin release and delays cyclicity and ovulation in mice (63). Similarly, in mice with various genetic mutations associated with a lean phenotype, fertility is often compromised. Thus, the adipose-specific knockouts of raptor that weigh 18% less than controls (64) and mice with transketolase insufficiency that have reduced adipose tissue (65) show reduced fertility. However, MCH and MCHR1 knockout mice (37, 39) remain fertile despite weighing 24–28% less than controls. The presence of fertility in MCH knockout mice is consistent with the results of the present study, which suggest that MCH inhibition of GnRH neurons may be required for reducing reproductive function in certain physiological states. Similar to the MCH knockout, muscarinic M3 receptor knockout are lean, hypophagic, and fertile despite a 22–25% reduction in body weight; interestingly these mice have significantly reduced MCH levels (66). Furthermore, Fto knockouts, which show no alterations in MCH expression, remain fertile despite weighing 30–40% less than controls (67). This is consistent with the view that an upregulated MCH system may be required for reducing fertility in conditions of starvation. Thus, in the absence of an upregulated MCH system, lean mice may continue to be reproductively active, as MCH may be required to inhibit GnRH neurons and perhaps other components of the HPG axis to suppress fertility.
In the absence of starvation, under normal physiological conditions, the MCH neurons are quiescent during wakefulness but fire action potentials during rapid eye movement (REM) sleep (42). In addition, c-fos studies show that ≈60% of MCH neurons are activated during sleep after a period of REM sleep deprivation (40). The results of our study would predict a decrease in gonadotropin release during REM sleep and after REM sleep deprivation. Indeed, in rats, REM sleep deprivation has been shown to reduce LH and FSH levels (68); and in adult men, REM sleep was found to be uniformly associated with decreasing LH concentrations (69).
In conclusion, the observed direct inhibitory effects of MCH on a subpopulation of GnRH neurons, which is essential for triggering puberty and producing the preovulatory LH surge, provides evidence for a critical link between energy balance and reproduction at the level of the GnRH neuron itself. Because MCH antagonists are currently being developed as potential antidepressant and antiobesity drugs, it may be prudent to consider their actions on the reproductive system, especially if used to treat depression in anorexic individuals.
Materials and Methods
Whole-cell patch clamp recordings were performed on GnRH, vGluT2, and GABAergic neurons in brain slices prepared from established lines of transgenic GFP mice. MCH and other agonists were applied using a Y-tube. Immunocytochemistry was performed to determine MCH innervation of vGluT2-GnRH neurons using a well-established antibody [for detailed methods, see SI].
Supplementary Material
Acknowledgments.
This work was supported by National Institution of Health grants MH61465, NS41454, and NS48476, and by the State of Connecticut, Department of Mental Health and Addiction Services.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908200106/DCSupplemental.
References
- 1.Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Crown A, Clifton DK, Steiner RA. Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology. 2007;86:175–182. doi: 10.1159/000109095. [DOI] [PubMed] [Google Scholar]
- 3.Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–E832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mircea CN, Lujan ME, Pierson RA. Metabolic fuel and clinical implications for female reproduction. J Obstet Gynaecol Can. 2007;29:887–902. doi: 10.1016/S1701-2163(16)32661-5. [DOI] [PubMed] [Google Scholar]
- 5.Gottsch ML, Clifton DK, Steiner RA. Galanin-like peptide as a link in the integration of metabolism and reproduction. Trends Endocrinol Metab. 2004;15:215–221. doi: 10.1016/j.tem.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Quennell JH, et al. Leptin indirectly regulates GnRH neuronal function. Endocrinology. 2009 doi: 10.1210/en.2008-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leshan RL, et al. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci. 2009;29:3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 9.Castellano JM, et al. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- 10.Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Chan YM, Broder-Fingert S, Seminara SB. Reproductive functions of kisspeptin and Gpr54 across the life cycle of mice and men. Peptides. 2009;30:42–48. doi: 10.1016/j.peptides.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottsch ML, Clifton DK, Steiner RA. From KISS1 to kisspeptins: An historical perspective and suggested nomenclature. Peptides. 2009;30:4–9. doi: 10.1016/j.peptides.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seminara SB, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 14.Lapatto R, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 15.d'Anglemont de Tassigny X, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–4434. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumalska I, et al. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci. 2008;28:8003–8013. doi: 10.1523/JNEUROSCI.1225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Roux N, et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson-Hughes PS, Grove KL, Smith MS. Melanin concentrating hormone (MCH): A novel neural pathway for regulation of GnRH neurons. Brain Res. 2005;1041:117–124. doi: 10.1016/j.brainres.2004.11.066. [DOI] [PubMed] [Google Scholar]
- 23.Shimomura Y, et al. Isolation and identification of melanin-concentrating hormone as the endogenous ligand of the SLC-1 receptor. Biochem Biophys Res Commun. 1999;261:622–626. doi: 10.1006/bbrc.1999.1104. [DOI] [PubMed] [Google Scholar]
- 24.Bächner D, Kreienkamp H, Weise C, Buck F, Richter D. Identification of melanin concentrating hormone (MCH) as the natural ligand for the orphan somatostatin-like receptor 1 (SLC-1) FEBS Lett. 1999;457:522–524. doi: 10.1016/s0014-5793(99)01092-3. [DOI] [PubMed] [Google Scholar]
- 25.Lembo PM, et al. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nat Cell Biol. 1999;1:267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- 26.Chambers J, et al. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400:261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- 27.Saito Y, et al. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400:265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, et al. Identification and pharmacological characterization of a novel human melanin-concentrating hormone receptor, mch-r2. J Biol Chem. 2001;276:34664–34670. doi: 10.1074/jbc.M102601200. [DOI] [PubMed] [Google Scholar]
- 29.Sailer AW, et al. Identification and characterization of a second melanin-concentrating hormone receptor, MCH-2R. Proc Natl Acad Sci USA. 2001;98:7564–7569. doi: 10.1073/pnas.121170598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An S, et al. Identification and characterization of a melanin-concentrating hormone receptor. Proc Natl Acad Sci USA. 2001;98:7576–7581. doi: 10.1073/pnas.131200698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsukamura H, et al. Intracerebroventricular administration of melanin-concentrating hormone suppresses pulsatile luteinizing hormone release in the female rat. J Neuroendocrinol. 2000;12:529–534. doi: 10.1046/j.1365-2826.2000.00482.x. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez MI, Baker BI, Wilson CA. Stimulatory effect of melanin-concentrating hormone on luteinising hormone release. Neuroendocrinology. 1997;66:254–262. doi: 10.1159/000127246. [DOI] [PubMed] [Google Scholar]
- 33.Murray JF, et al. Evidence for a stimulatory action of melanin-concentrating hormone on luteinising hormone release involving MCH1 and melanocortin-5 receptors. J Neuroendocrinol. 2006;18:157–167. doi: 10.1111/j.1365-2826.2005.01397.x. [DOI] [PubMed] [Google Scholar]
- 34.Wade GN, Jones JE. Neuroendocrinology of nutritional infertility. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1277–R1296. doi: 10.1152/ajpregu.00475.2004. [DOI] [PubMed] [Google Scholar]
- 35.Qu D, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 36.Presse F, Sorokovsky I, Max JP, Nicolaidis S, Nahon JL. Melanin-concentrating hormone is a potent anorectic peptide regulated by food-deprivation and glucopenia in the rat. Neuroscience. 1996;71:735–745. doi: 10.1016/0306-4522(95)00481-5. [DOI] [PubMed] [Google Scholar]
- 37.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 38.Kokkotou E, et al. Mice with MCH ablation resist diet-induced obesity through strain-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;289:R117–R124. doi: 10.1152/ajpregu.00861.2004. [DOI] [PubMed] [Google Scholar]
- 39.Marsh DJ, et al. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci USA. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verret L, et al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willie JT, Sinton CM, Maratos-Flier E, Yanagisawa M. Abnormal response of melanin-concentrating hormone deficient mice to fasting: Hyperactivity and rapid eye movement sleep suppression. Neuroscience. 2008;156:819–829. doi: 10.1016/j.neuroscience.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci USA. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgescu D, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pissios P, et al. Dysregulation of the mesolimbic dopamine system and reward in MCH−/− mice. Biol Psychiatry. 2008;64:184–191. doi: 10.1016/j.biopsych.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Adamantidis A, de Lecea L. Sleep and metabolism: Shared circuits, new connections. Trends Endocrinol Metab. 2008;19:362–370. doi: 10.1016/j.tem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Nahon JL. The melanocortins and melanin-concentrating hormone in the central regulation of feeding behavior and energy homeostasis. C R Biol. 2006;329:623–638. doi: 10.1016/j.crvi.2006.03.021. discussion 653–655. [DOI] [PubMed] [Google Scholar]
- 47.Shimazaki T, Yoshimizu T, Chaki S. Melanin-concentrating hormone MCH1 receptor antagonists: A potential new approach to the treatment of depression and anxiety disorders. CNS Drugs. 2006;20:801–811. doi: 10.2165/00023210-200620100-00002. [DOI] [PubMed] [Google Scholar]
- 48.Gao XB, van den Pol AN. Melanin-concentrating hormone depresses L-, N-, and P/Q-type voltage-dependent calcium channels in rat lateral hypothalamic neurons. J Physiol. 2002;542:273–286. doi: 10.1113/jphysiol.2002.019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol. 2001;533:237–252. doi: 10.1111/j.1469-7793.2001.0237b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bittencourt JC, Elias CF. Melanin-concentrating hormone and neuropeptide EI projections from the lateral hypothalamic area and zona incerta to the medial septal nucleus and spinal cord: A study using multiple neuronal tracers. Brain Res. 1998;805:1–19. doi: 10.1016/s0006-8993(98)00598-8. [DOI] [PubMed] [Google Scholar]
- 51.Bittencourt JC, et al. The melanin-concentrating hormone system of the rat brain: An immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 52.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587:1401–1411. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cvetkovic V, et al. Characterization of subpopulations of neurons producing melanin-concentrating hormone in the rat ventral diencephalon. J Neurochem. 2004;91:911–919. doi: 10.1111/j.1471-4159.2004.02776.x. [DOI] [PubMed] [Google Scholar]
- 54.Fort P, et al. The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience. 2008;155:174–181. doi: 10.1016/j.neuroscience.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 55.Wu M, Hajszan T, Leranth C, Alreja M. Nicotine recruits a local glutamatergic circuit to excite septohippocampal GABAergic neurons. Eur J Neurosci. 2003;18:1155–1168. doi: 10.1046/j.1460-9568.2003.02847.x. [DOI] [PubMed] [Google Scholar]
- 56.Wu M, Hajszan T, Xu C, Leranth C, Alreja M. Group I metabotropic glutamate receptor activation produces a direct excitation of identified septohippocampal cholinergic neurons. J Neurophysiol. 2004;92:1216–1225. doi: 10.1152/jn.00180.2004. [DOI] [PubMed] [Google Scholar]
- 57.Davidowa H, Li Y, Plagemann A. Hypothalamic ventromedial and arcuate neurons of normal and postnatally overnourished rats differ in their responses to melanin-concentrating hormone. Regul Pept. 2002;108:103–111. doi: 10.1016/s0167-0115(02)00153-2. [DOI] [PubMed] [Google Scholar]
- 58.Xu J, Kirigiti MA, Cowley MA, Grove KL, Smith MS. Suppression of basal spontaneous gonadotropin-releasing hormone neuronal activity during lactation: Role of inhibitory effects of neuropeptide Y. Endocrinology. 2009;150:333–340. doi: 10.1210/en.2008-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- 60.McBriar MD. Recent advances in the discovery of melanin-concentrating hormone receptor antagonists. Curr Opin Drug Discov Devel. 2006;9:496–508. [PubMed] [Google Scholar]
- 61.Gibson MJ, Wu TJ, Miller GM, Silverman AJ. What nature's knockout teaches us about GnRH activity: Hypogonadal mice and neuronal grafts. Horm Behav. 1997;31:212–220. doi: 10.1006/hbeh.1997.1387. [DOI] [PubMed] [Google Scholar]
- 62.Gallardo MG, Chiocchio SR, Tramezzani JH. Changes of melanin-concentrating hormone related to LHRH release in the median eminence of rats. Brain Res. 2004;1030:152–158. doi: 10.1016/j.brainres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 64.Polak P, et al. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Xu ZP, Wawrousek EF, Piatigorsky J. Transketolase haploinsufficiency reduces adipose tissue and female fertility in mice. Mol Cell Biol. 2002;22:6142–6147. doi: 10.1128/MCB.22.17.6142-6147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamada M, et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- 67.Fischer J, et al. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 68.Peder M, Porkka-Heiskanen T, Laakso ML, Johansson G. Rapid eye movement sleep deprivation depresses plasma FSH and LH in castrated rats. Physiol Behav. 1989;45:1167–1170. doi: 10.1016/0031-9384(89)90104-2. [DOI] [PubMed] [Google Scholar]
- 69.Fehm HL, Clausing J, Kern W, Pietrowsky R, Born J. Sleep-associated augmentation and synchronization of luteinizing hormone pulses in adult men. Neuroendocrinology. 1991;54:192–195. doi: 10.1159/000125875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.