Abstract
NifEN is a key player in the biosynthesis of nitrogenase MoFe protein. It not only shares a considerable degree of sequence homology with the MoFe protein, but also contains clusters that are homologous to those found in the MoFe protein. Here we present an investigation of the catalytic activities of NifEN. Our data show that NifEN is catalytically competent in acetylene (C2H2) and azide (N3−) reduction, yet unable to reduce dinitrogen (N2) or evolve hydrogen (H2). Upon turnover, C2H2 gives rise to an additional S = 1/2 signal, whereas N3− perturbs the signal originating from the NifEN-associated FeMoco homolog. Combined biochemical and spectroscopic studies reveal that N3− can act as either an inhibitor or an activator for the binding and/or reduction of C2H2, while carbon monoxide (CO) is a potent inhibitor for the binding and/or reduction of both N3− and C2H2. Taken together, our results suggest that NifEN is a catalytic homolog of MoFe protein; however, it is only a “skeleton” version of the MoFe protein, as its associated clusters are simpler in structure and less versatile in function, which, in turn, may account for its narrower range of substrates and lower activities of substrate reduction. The resemblance of NifEN to MoFe protein in catalysis points to a plausible, sequential appearance of the two proteins in nitrogenase evolution. More importantly, the discrepancy between the two systems may provide useful insights into nitrogenase mechanism and allow reconstruction of a fully functional nitrogenase from the “skeleton” enzyme, NifEN.
Keywords: catalysis, MoFe protein
Nitrogenase catalyzes the key step in global nitrogen cycle: the reduction of dinitrogen (N2) to ammonia (NH3). This process not only represents the major entry point of reduced nitrogen into food chain, but embodies the formidable chemistry of breaking the triple bond of N2 under ambient conditions (1). The best characterized Mo-nitrogenase is a binary enzyme system comprising two redox-active metalloproteins. One, designated Fe protein, is a α2-homodimer with one [Fe4S4] cluster bridged between subunits and one ATP binding site located in each subunit; the other, termed MoFe protein, is a α2β2-heterotetramer containing two unique metal centers: the P-cluster, a [Fe8S7] cluster ligated between each αβ subunit dimer; and the FeMo-cofactor (FeMoco), a [MoFe7S9X-homocitrate] cluster (X = C, N or O) buried within each α subunit. Nitrogenase catalysis involves repeated association/dissociation between Fe protein and MoFe protein, and ATP-dependent electron transfer from the [Fe4S4] cluster of Fe protein, through the P-cluster, to the FeMoco of MoFe protein, where substrate reduction occurs.
The ability of FeMoco to adopt various oxidation states allows nitrogenase to catalyze reductions of a wide range of substrates. Apart from its physiological substrate (i.e., N2), nitrogenase is capable of reducing a variety of alternative substrates, such as acetylene (C2H2), azide (N3−), cyanide (CN−), and hydrazine (N2H4), each requiring accumulation of a different number of electrons at the FeMoco site for substrate binding and reduction (1). On the other hand, the redox-versatility of FeMoco presents a serious challenge to ongoing efforts to decipher the catalytic mechanism of nitrogenase. The ability of FeMoco to shuttle between different oxidation states in a rapid and uncontrolled manner allows substrates to interact only transiently with certain oxidation state(s) of FeMoco. Consequently, it is extremely difficult to capture any substrate-bound form of nitrogenase for direct examination of substrate-enzyme interactions during catalysis. Recently, a combined genetic and spectroscopic strategy was used to overcome this problem. By altering substrate accessibility and limiting electron flux, a number of substrates/intermediates were successfully trapped on MoFe protein (2). These studies, together with the identification of a central atom of FeMoco that may be involved in nitrogenase turnover (3), could prove instrumental in elucidating the mechanistic details of nitrogenase. Meanwhile, the search for alternative approaches continues, with the ultimate goal to solve the riddle of biological nitrogen fixation.
One aspect yet to be explored in this regard is the identification of enzymatic systems homologous to nitrogenase. NifEN serves as an ideal candidate for this purpose. Better known as a scaffold protein in FeMoco assembly, NifEN is a α2β2 heterotetramer that shares considerable sequence homology with MoFe protein (4). Additionally, it contains cluster-binding sites that are homologous to those in MoFe protein: the “P-cluster site” at the αβ subunit interface, which houses a P-cluster homolog; and the “FeMoco site” within the α subunit, which hosts the conversion of a FeMoco precursor to a mature cluster before its transfer to MoFe protein (4). While the P-cluster homolog was identified earlier as a [Fe4S4]-type cluster (5), the FeMoco precursor was captured on NifEN only recently, which was subsequently identified as a Fe-only homolog closely resembling the core structure of mature FeMoco (6). Thus, NifEN seems to have equivalents for both clusters that are involved in electron transfer within MoFe protein (Fig. 1). One question naturally follows: Can NifEN substitute for MoFe protein as the redox partner of Fe protein and catalyze the reduction of at least some substrates of MoFe protein? Here we present a combined biochemical and spectroscopic investigation of the catalytic activities of NifEN. Our data suggest that NifEN is a catalytically competent homolog of MoFe protein; however, it has a narrower substrate profile and a lower efficiency than MoFe protein. This finding suggests that NifEN represents a “skeleton” version of MoFe protein, which, in turn, may provide significant insights into the evolution and mechanism of nitrogenase.
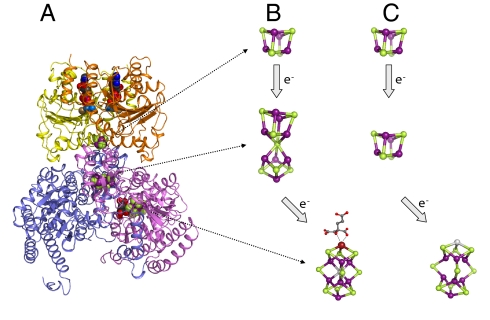
Fig. 1.
Electron transfer during nitrogenase catalysis. (A) Crystal structure of MgADP·AlF4−-stabilized complex between Fe protein and one αβ-dimer of MoFe protein. (B) Electron transfer pathway between Fe protein and MoFe protein. (C) Hypothetical electron transfer pathway between Fe protein and NifEN. It has been proposed that, during nitrogenase catalysis, electrons flow from the [Fe4S4] cluster of the Fe protein to the P-cluster ([Fe8S7]) and then the FeMoco ([MoFe7S9X-homocitrate], where X = C, N, or O) of MoFe protein. Likewise, electrons could flow from the [Fe4S4] cluster of the Fe protein to the [Fe4S4] cluster and then the FeMoco homolog of NifEN. Figures are generated in PYMOL using 1N2C and 1M1N PDB coordinates (3, 21). The two subunits of Fe protein are colored yellow and orange, and the α- and β-subunits of MoFe protein are colored blue and violate. Atoms of clusters are colored as follows: Fe, purple; S, green; Mo, burgundy; C, dark gray; and O, red. Note that homocitrate is missing from the NifEN-associated FeMoco homolog, and Mo is either absent or replaced by Fe (colored light gray) in the FeMoco homolog structure.
Results
When coupled with Fe protein, NifEN is capable of reducing C2H2 and N3−, but not CN−, N2, H+ and N2H4 (Table 1). NifEN is less active than MoFe protein in C2H2 and N3− reductions; nevertheless, it generates the same products as MoFe protein in both cases. Reduction of C2H2 by NifEN, like that by MoFe protein, is a two-electron process, as C2H4 is the only detected product. Reduction of N3− by NifEN, however, differs from that by MoFe protein in the number of electrons. While MoFe protein can reduce N3−, stepwise, by two, six, and eight electrons to N2+NH3, N2H4+NH3, and NH3, NifEN-catalyzed reaction is “stuck” at the first step, as (i) NifEN cannot reduce N2; (ii) no N2H4 production is detected; and (iii) N2H4 is not a substrate of NifEN (Table 1). Apparently, NifEN is only capable of reducing substrates with a limited amount of electrons, as reductions of both C2H2 and N3− involve no more than two electrons. Such a hypothesis is further substantiated by the inability of NifEN to reduce CN− and N2: the former involves four or six electrons; the latter, six. In addition, two reactions that are intimately associated with N2 turnover are also missing from the catalytic repertoire of NifEN: (i) the reduction of H+ to H2, an obligate event that occurs upon N2 binding/reduction; and (ii) the reduction of N2H4, a possible intermediate of N2 reduction, to NH3. It is likely, therefore, that the [Fe4S4] center in NifEN does not mediate electron transfer as flexibly and effectively as the [Fe8S7] P-cluster in MoFe protein. Consequently, the FeMoco homolog in NifEN is “fixed” at a more oxidized state that is prohibitive for substrates requiring more reduced states of cofactor for binding/reduction, resulting in a narrower substrate profile and a lower enzymatic efficiency of NifEN. Besides the missing P-cluster, other factors could also account for the differences between the catalytic properties of NifEN and MoFe protein, especially when N2 reduction is considered. It has been proposed that homocitrate switches from bidentate to monodentate ligation to the Mo atom of FeMoco during catalysis, which frees up a binding site for N2 on Mo (7). The absence of homocitrate and Mo from the FeMoco homolog may explain the inability of NifEN to employ such a mechanism for N2 reduction. Alternatively, protein residues surrounding the cofactor could also participate in N2 reduction. For example, both αQ191K and αH195N variants of MoFe protein do not reduce N2 (8). Interestingly, the exact mutations of MoFe protein are duplicated in the native sequence of NifEN (Fig. S1), suggesting that the immediate protein environment of the active site in NifEN may render it inactive in N2 reduction.
Table 1.
Substrate reducing activities of NifEN and MoFe protein of A. vinelandii
| Enzyme | Substrate | Product, nmol/mg protein/min |
|||||
|---|---|---|---|---|---|---|---|
| CH4 | C2H4 | C2H6 | H2 | NH3 | N2H4 | ||
| NifEN* | C2H2 (under Ar) | — | 52 ± 3 | 0 | — | — | — |
| C2H2 (under N2) | — | 56 ± 1 | 0 | — | — | — | |
| N3− (under Ar) | — | — | — | — | 71 ± 10 | 0 | |
| CN− (under Ar) | 0 | — | — | — | 5 ± 1 | — | |
| N2 | — | — | — | 0 | 0 | 0 | |
| H+ (under Ar) | — | — | — | 0 | — | — | |
| N2H4 (under Ar) | — | — | — | — | 0 | — | |
| MoFe protein | C2H2 (under Ar) | — | 2,144 ± 72 | 0 | — | — | — |
| C2H2 (under N2) | — | 2,261 ± 30 | 0 | — | — | — | |
| N3− (under Ar) | — | — | — | — | 587 ± 66 | 36 ± 4 | |
| CN− (under Ar) | 72 ± 3 | — | — | — | 53 ± 11 | — | |
| N2 | — | — | — | 604 ± 63 | 1,112 ± 41 | — | |
| H+ (under Ar) | — | — | — | 2,490 ± 65 | — | — | |
| N2H4 (under Ar) | — | — | — | — | 1,145 ± 135 | — | |
*No activities were observed of ΔnifB NifEN, a FeMoco homolog-free form of NifEN, which was generated by deletion of nifB, the gene encoding an essential product for cofactor biosynthesis.
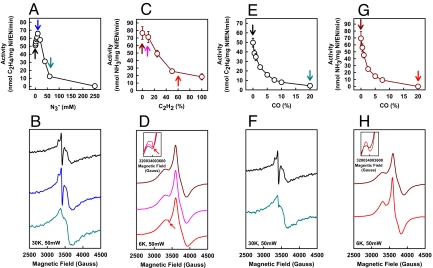
The relatively low efficiency of NifEN is further demonstrated by its increased demand for reducing power, i.e., a lower solution potential and a higher excess of reductase, to achieve maximum activities. Optimal reductions of both C2H2 and N3− by NifEN occur at 0.4 mM dithionite (ca −490 mV) (9, *) and a Fe protein/NifEN ratio of approximately 70 (Fig. 2 A–D). In comparison, MoFe protein requires 20 mM dithionite (ca −440 mV) (9) and a Fe protein/MoFe protein ratio of 30 for maximum activities. Despite these discrepancies, both NifEN and MoFe protein require Fe protein to function as an ATPase during catalysis. Neither C2H2 nor N3− is reduced by NifEN if ATP is absent, if ATP is replaced by ADP or nonhydrolysable ATP analogs, or if Fe protein is replaced by its A157S variant that is specifically defective in ATP hydrolysis (Fig. 2 E and F). Moreover, in the presence of ATP, an extra EPR feature appears upon C2H2 turnover at 6 K, which overlays with the S = 1/2 signal that originates from the NifEN-associated clusters (Fig. 3A, arrow). This EPR feature becomes predominant with increasing temperatures and, at 30 K, reveals its identity as a S = 1/2 signal with a sharp inflection at g = 2.02 (Fig. 3A, box). Such a feature is not observed at 6 K in the absence of ATP, and the NifEN-originated signal becomes somewhat featureless at 30 K (Fig. 3B, box). No additional EPR feature can be observed upon N3− turnover. Nevertheless, the g = 2.07 feature of the NifEN-associated signal decreases in magnitude, which is most pronounced at 6 K (Fig. 3 C and D, boxes). The appearance of a EPR signal (upon C2H2 turnover) or the perturbation of a particular EPR feature (upon N3− turnover) likely reflect the interactions between substrates and cofactor homolog on NifEN, which affect the redox properties of the latter. It is important to note that a similar S = 1/2 signal has been observed during MoFe protein-catalyzed C2H2 turnover (10), suggesting that the active centers in NifEN (i.e., FeMoco homolog) and MoFe protein (i.e., FeMoco) undergo similar redox changes upon C2H2 turnover.
Fig. 2.
Substrate-reducing activities of NifEN. (A and B) Dependence of C2H2 (A) and N3− (B) reducing activities on dithionite concentration. (C and D) Dependence of C2H2 (C) and N3− (D) reducing activities on Fe protein/NifEN ratio. (E and F) Dependence of C2H2 (E) and N3− (F) reducing activities on ATP hydrolysis. AMPPNP, ATPγS, non-hydrolysable ATP analogs. A157S Fe protein, a Fe protein variant that is specifically defective in ATP hydrolysis (13).
Fig. 3.
EPR properties of NifEN in the presence of C2H2 and N3−. (A and B) Temperature-dependency of the EPR spectrum of NifEN under turnover (A) and nonturnover (B) conditions of C2H2. (C and D) Temperature-dependency of the EPR spectrum of NifEN under turnover (C) and nonturnover (D) conditions of N3−. Turnover samples (A and C) contain ATP, which is absent from nonturnover samples (B and D).
The distinct turnover-associated signals offer a unique opportunity for combined biochemical/spectroscopic investigations of the interplay between the two substrates of NifEN and the effect of carbon monoxide (CO) on both substrates. The impact of N3− on C2H2 reduction is bimodular. Below 10 mM, N3− acts as an allosteric activator for C2H2 reduction; above 10 mM, however, N3− is an effective inhibitor of the same reaction (Fig. 4A). Consistent with the change in activity, the C2H2 turnover signal (Fig. 4B, black trace) increases in size at 10 mM N3− (Fig. 4B, blue trace), yet it is replaced by the broad, nonturnover state signal at 60 mM N3− (Fig. 4B, green trace). Conversely, the effect of C2H2 on N3− reduction is more straightforward. An increase in C2H2 concentration results in a consistent decrease in N3− reduction, although 100% C2H2 does not inhibit the reaction completely (Fig. 4C). Consistent with the inhibition of N3− turnover, the g = 2.07 feature of the NifEN-associated signal becomes more pronounced with increasing C2H2 concentrations and, at 60% C2H2, the C2H2 turnover feature appears (Fig. 4D Inset), suggesting that C2H2 out-competes N3− for binding/reduction under these conditions. Reductions of both C2H2 and N3− are completely inhibited by 20% CO (Fig. 4 E and G), as demonstrated further by a return of the respective turnover signal to the resting state (Fig. 4 F and H). A similar pattern of CO inhibition on C2H2 and N3− reductions has been observed in the case of MoFe protein, so has the mutual inhibition between C2H2 and N3− (1, 11). However, the allosteric activation of C2H2 reduction by N3− and the inability of C2H2 to fully inhibit N3− reduction are unveiled in the case of NifEN. A plausible model can be proposed that involves a common site where C2H2 and N3− compete for binding, and an additional site for N3− binding that (i) enhances the C2H2 reduction at low concentrations of N3− and (ii) allows N3− reduction to occur even when the other site is completely blocked by C2H2 (Fig. 5).
Fig. 4.
Interactions between C2H2, N3− and CO in NifEN-catalyzed reactions. (A and B) C2H2-reducing activities (A) and EPR properties (B) of NifEN with increasing N3− concentrations. Black, no N3−; blue, 20 mM N3−; green, 60 mM N3−. (C and D) N3−-reducing activities (C) and EPR properties (D) of NifEN with increasing C2H2 concentrations. Burgundy, no C2H2; pink, 10% C2H2; red, 60% C2H2. (E and F) C2H2-reducing activities (E) and EPR properties (F) of NifEN with increasing CO concentrations. Black, no CO; green, 20% CO. (G and H) N3−-reducing activities (G) and EPR properties (H) of NifEN with increasing CO concentrations. Burgundy, no CO; red, 20% CO.
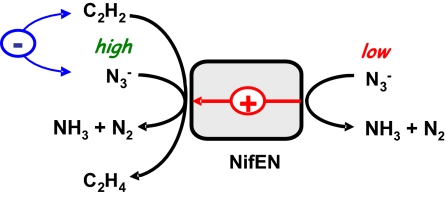
Fig. 5.
Plausible model of C2H2-and N3−-binding to NifEN. N3− may have two binding sites at the active cofactor site of NifEN: one site only allows the binding of N3−; whereas the other also allows the binding of C2H2. It is possible that, at high concentrations, N3− competes with C2H2 for binding at the shared site; and at low concentrations, N3− allosterically enhances the C2H2 reduction upon binding to the unshared site. Based on this model, C2H2 cannot inhibit N3− reduction completely, as the unshared site allows a certain level of N3− reduction to occur even when the same reaction is completely blocked by C2H2 at the shared site. Conversely, N3− can inhibit C2H2 reduction completely, as both sites can be occupied fully by N3−.
The “moonlighting” function of NifEN as a C2H2 and N3− reductase adds an interesting twist to the current theory of nitrogenase evolution. It has been proposed that duplication and divergence of a common ancestral gene gave rise to both NifEN and MoFe protein (12). This hypothesis implies a parallel evolvement of NifEN and MoFe protein upon branching at the genetic level. The ability of NifEN to reduce some MoFe protein substrates, however, suggests a sequential order in which NifEN and MoFe protein may have appeared during evolution. NifEN could be the predecessor to MoFe protein, evolving into an effective enzyme with a wide range of substrates while orienting itself solely toward FeMoco biosynthesis. In this scenario, NifEN might have acted as a detoxifying enzyme in the primitive earth environment, where toxic substances such as C2H2 and N3− were abundant. Regardless of what events transpired during evolution, NifEN is only the second enzyme identified to date that is capable of reducing C2H2 and N3−.
Apart from its value from the evolutionary perspective, identification of NifEN as a catalytic homolog of MoFe protein bears significant implications for nitrogenase mechanism. The biggest obstacle to detailed investigations of interactions between nitrogenase and its various substrates is the empirical difficulty in achieving a particular oxidation state of the active center (i.e., FeMoco) that allows the binding and reduction of certain substrate(s). The low electron flux through the Fe protein/NifEN system restricts the supply of electrons to the active center (i.e., FeMoco homolog), rendering it in a more oxidized state to which only select substrates, such as C2H2 and N3−, can bind. Thus, the reactions of C2H2 and N3− are effectively “uncoupled” from those of the other substrates, permitting the observation of an interesting allosteric effect of N3− on C2H2 reduction under low electron flux. More importantly, the slow turnover of NifEN may prove advantageous in naturally enriching a C2H2-bound form of NifEN and eventually allow successful crystallization of the first intermediate-bound nitrogenase homolog, a feat yet to be accomplished for this complex enzyme system.
Despite the inability of NifEN to catalyze the reduction of the full spectrum of MoFe protein substrates, what NifEN cannot do is perhaps even more interesting than what it can do, as comparison between NifEN and MoFe protein may reveal features missing in the former that are responsible for the catalytic capacity of the latter. The most notable deficiency of NifEN is its inability to reduce N2 and evolve H2. Could it be the [Fe4S4] cluster that does not match up with the redox capacity of the [Fe8S7] P-cluster? Could it be the missing heterometal or homocitrate that does the trick? Or, could it be the cluster environment that plays a pivotal role in substrate turnover? In any case, NifEN represents a “skeleton” enzyme on which a fully functional one could be (re)built. For example, a normal P-cluster could be reconstructed by restoring the missing ligands (Fig. S1). Further, a mature FeMoco could be generated by incorporating heterometal and homocitrate into the FeMoco homolog (13). Finally, residues surrounding the FeMoco homolog could be systematically altered for improved substrate accessibility (Fig. S1). Such an “add-on” approach (i.e., approach to restore the catalytic features in NifEN) can be combined with a “subtraction” approach (i.e., approach to remove the catalytic features from MoFe protein) and, together, they could provide a comprehensive description of factors essential for nitrogenase catalysis. With a lot of careful design and a bit of luck, a nitrogen-fixing system may be (re)created that permits a controlled electron flow and the capture of physiologically-relevant intermediate(s) during turnover. While it is too early to predict the outcome of these studies, one thing is certain: NifEN has been a key player in the functionality of nitrogenase and will continue to bring us surprises in the future.
Materials and Methods
Unless noted otherwise, all chemicals and reagents were obtained from Fisher Scientific or Sigma-Aldrich.
Protein Purification.
A. vinelandii strains DJ1041 (expressing His-tagged NifEN) and DJ1141 (expressing His-tagged MoFe protein and non-tagged Fe protein) were grown in 180–L batches in a 200–L New Brunswick fermentor (New Brunswick Scientific) as described previously (14). Nontagged Fe protein, His-tagged MoFe protein and His-tagged NifEN were purified as described elsewhere (14, 15).
Activity Analysis.
All nitrogenase activity assays were carried out as described earlier (14, 16). H2 and C2H4 were analyzed as published elsewhere (17). Ammonium was determined by a high-performance liquid chromatography fluorescence method (18).
EPR Spectroscopy.
All electron paramagnetic resonance spectroscopy (EPR) samples were prepared anaerobically. Turnover samples were prepared as described elsewhere (19, 20), which contained 15 mg NifEN protein, 1 mg Fe protein, 6 mM Na2ATP, 8 mM MgCl2, 50 mM phosphocreatine, 0.20 mg/mL creatine phosphokinase, 10% glycerol, 0.4 mM Na2S2O4, and 25 mM Tris-HCl (pH 8.0) in the presence of 60% C2H2 or 20 mM N3−. Nonturnover samples were prepared the same way except that ATP was omitted. Spectra were collected as described previously (16) in perpendicular mode using a Bruker ESP 300 Ez spectrophotometer (Bruker) interfaced with an Oxford Instruments ESR-9002 liquid helium continuous-flow cryostat (Oxford Instruments).
Supplementary Material
Acknowledgments.
We thank Professor Andrew Borovik at UCI for his assistance on EPR measurement. This work was supported by National Institutes of Health grant GM-67626 (to M.W.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907872106/DCSupplemental.
The theoretical solution potential of dithionite was calculated based on previously-published equations (9).
References
- 1.Burgess BK, Lowe DJ. Mechanism of molybdenum nitrogenase. Chem Rev. 1996;96:2983–3012. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- 2.Barney BM, et al. Breaking the N2 triple bond: Insights into the nitrogenase mechanism. Dalton Trans. 2006;19:2277–2284. doi: 10.1039/b517633f. [DOI] [PubMed] [Google Scholar]
- 3.Einsle O, et al. Nitrogenase MoFe-protein at 1.16 Å resolution: A central ligand in the FeMo-cofactor. Science. 2002;297:1696–1700. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, Fay AW, Lee CC, Yoshizawa J, Ribbe MW. Assembly of nitrogenase MoFe protein. Biochemistry. 2008;47:3973–3981. doi: 10.1021/bi7025003. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin PJ, et al. The Azotobacter vinelandii NifEN complex contains two identical [4Fe-4S] clusters. Biochemistry. 1998;37:10420–10428. doi: 10.1021/bi980435n. [DOI] [PubMed] [Google Scholar]
- 6.Corbett MC, et al. Structural insights into a protein-bound iron-molybdenum cofactor precursor. Proc Natl Acad Sci USA. 2006;103:1238–1243. doi: 10.1073/pnas.0507853103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durrant MC, Francis A, Lowe DJ, Newton WE, Fisher K. Evidence for a dynamic role for homocitrate during nitrogen fixation: The effect of substitution at the α-Lys426 position in MoFe-protein of Azotobacter vinelandii. Biochem J. 2006;397:261–270. doi: 10.1042/BJ20060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher K, Dilworth MJ, Newton WE. Differential effects on N2 binding and reduction, HD formation, and azide reduction with α-195His- and α-191Gln-substituted MoFe proteins of Azotobacter vinelandii nitrogenase. Biochemistry. 2000;39:15570–15577. doi: 10.1021/bi0017834. [DOI] [PubMed] [Google Scholar]
- 9.Mayhew SG. The redox potential of dithionite and SO2− from equilibrium reactions with flavodoxins, methyl viologen, and hydrogen plus hydrogenase. Eur J Biochem. 1978;85:535–547. doi: 10.1111/j.1432-1033.1978.tb12269.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee HI, et al. Electron inventory, kinetic assignment (En), structure, and bonding of nitrogenase turnover intermediates with C2H2 and CO. J Am Chem Soc. 2005;127:15880–15890. doi: 10.1021/ja054078x. [DOI] [PubMed] [Google Scholar]
- 11.Rivera-Ortiz JM, Burris RH. Interactions among substrates and inhibitors of nitrogenase. J Bacteriol. 1975;123:537–545. doi: 10.1128/jb.123.2.537-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fani R, Gallo R, Liò P. Molecular evolution of nitrogen fixation: The evolutionary history of the nifD, nifK, nifE, and nifN genes. J Mol Evol. 2000;51:1–11. doi: 10.1007/s002390010061. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, et al. FeMo cofactor maturation on NifEN. Proc Natl Acad Sci USA. 2006;103:17119–17124. doi: 10.1073/pnas.0602647103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgess BK, Jacobs DB, Stiefel EI. Large-scale purification of high activity Azotobacter vinelandii nitrogenase. Biochim Biophys Acta. 1980;614:196–209. doi: 10.1016/0005-2744(80)90180-1. [DOI] [PubMed] [Google Scholar]
- 15.Ribbe MW, Hu Y, Guo M, Schmid B, Burgess BK. The FeMoco-deficient MoFe protein produced by a nifH deletion strain of Azotobacter vinelandii shows unusual P-cluster features. J Biol Chem. 2002;277:23469–23476. doi: 10.1074/jbc.M202061200. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Fay AW, Dos Santos PC, Naderi F, Ribbe MW. Characterization of Azotobacter vinelandii nifZ deletion strains: Indication of stepwise MoFe protein assembly. J Biol Chem. 2004;279:54963–54971. doi: 10.1074/jbc.M408983200. [DOI] [PubMed] [Google Scholar]
- 17.Gavini N, Burgess BK. FeMo cofactor synthesis by a nifH mutant with altered MgATP reactivity. J Biol Chem. 1992;267:21179–21186. [PubMed] [Google Scholar]
- 18.Corbin JL. FeMo cofactor synthesis by a nifH mutant with altered MgATP reactivity. Appl Environ Microbiol. 1984;47:1027–1030. [Google Scholar]
- 19.Benton PM, et al. Localization of a substrate binding site on the FeMo-cofactor in nitrogenase: Trapping propargyl alcohol with an α-70-substituted MoFe protein. Biochemistry. 2003;42:9102–9109. doi: 10.1021/bi034595x. [DOI] [PubMed] [Google Scholar]
- 20.Maskos Z, Fisher K, Sørlie M, Newton WE, Hales BJ. Variant MoFe proteins of Azotobacter vinelandii: Effects of carbon monoxide on electron paramagnetic resonance spectra generated during enzyme turnover. J Biol Inorg Chem. 2005;10:394–406. doi: 10.1007/s00775-005-0648-2. [DOI] [PubMed] [Google Scholar]
- 21.Schindelin H, Kisker C, Schlessman JL, Howard JB, Rees DC. Structure of ADP·AIF4−-stabilized nitrogenase complex and its implications for signal transduction. Nature. 1997;387:370–376. doi: 10.1038/387370a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.