Abstract
The cytoplasmic signaling protein TNF receptor-associated factor 5 (TRAF5) has been implicated in several biological roles in Tlymphocyte responses. However, a clear connection between in vivo TRAF5 immune cell functions and specific signaling pathways has not been made. This study shows that TRAF5 associated strongly with the viral oncogenic CD40 mimic latent membrane protein 1 (LMP1), in contrast to weaker association with CD40, for which it has been shown to play a modest role. LMP1 uses specific TRAFs differently than CD40, resulting in amplified and dysregulated CD40-like activation of B lymphocytes. When the cytoplasmic domain of LMP1 is expressed as a transgenic replacement for CD40 in mouse B cells, the resulting mouse exhibits measures of B-cell hyperactivity such as splenomegaly, lymphadenopathy, elevated serum IL-6, elevated serum autoantibodies, and abnormal splenic architecture. Thus, in contrast to CD40, TRAF5 may have an important nonredundant role as a positive mediator of LMP1 signaling and functions in B cells. To test this hypothesis, mice were created that express mCD40LMP1 in place of CD40, and are either sufficient or deficient in TRAF5. Results revealed that TRAF5 plays a critical role in LMP1-mediated c-Jun kinase signaling and is required for much of the abnormal phenotype observed in mCD40LMP1 transgenic mice. This is the first report showing a major requirement for TRAF5 in signaling by a specific receptor both in vitro and in vivo, as well as playing an important role in biological function in B lymphocytes.
Keywords: B lymphocyte, latent membrane protein 1, TNF receptor-associated factor, lymphocyte activation, TNF receptor superfamily
The Epstein–Barr virus-encoded latent membrane protein 1 (LMP1) is a functional mimic of CD40 expressed as a six transmembrane receptor on the cell surface (1). LMP1 self-oligomerizes, resulting in constitutive signaling (1, 2). Although LMP1 can initiate signaling without a ligand, the unique nature of its cytoplasmic domain results in sustained and amplified activation independent of its transmembrane regions. Thus, a hybrid molecule in which the transmembrane domains of LMP1 are replaced with the transmembrane and extracellular portions of CD40 induces B-cell activation that is higher and more prolonged than levels achieved by endogenous CD40 (3–5). Expression of LMP1 by B cells also results in increased survival (5, 6) and class switching (7). The heightened activation state caused by LMP1 expression in mice leads to B-cell hyperactivity, resulting in splenomegaly, lymphadenopathy, and production of elevated serum IL-6 and autoantibodies (4). The amplified and constitutive CD40-like signal provided by LMP1 can also contribute to generation of Reed–Sternberg cells (8) and B-cell lymphoma (9).
Like CD40, LMP1 binds and uses cytoplasmic adaptor molecules known as TNF receptor-associated factors (TRAFs). Interestingly, LMP1 uses TRAFs 1, 2, and 3 differently than CD40. TRAFs 1 and 2 cooperate to promote certain CD40-mediated signals to B cells, but TRAF3 inhibits TRAF2-dependent CD40 signaling (10, 11). In contrast, LMP1 can activate B cells independently of TRAFs 1 and 2, but its signaling is inhibited in the absence of TRAF3 (10, 11). LMP1 associates with TRAF5 in cell lines (12, 13), but the role of TRAF5 in LMP1 function has remained a mystery. Early characterization of a TRAF5−/− mouse found only a modest contribution of TRAF5 to CD40 signaling, so it may have been concluded that TRAF5 also plays little role in LMP1 signaling (14). However, because it is now appreciated that distinct receptors can use TRAFs in different, sometimes opposing ways, we believed that whether and how LMP1 uses TRAF5 should be directly addressed.
TRAF5 is a putative positive regulator of a number of TNF receptor superfamily receptors (15–17), and LMP1 associates with TRAF5, suggesting that TRAF5 may also play a positive role in LMP1 signaling (13). To test this, mice expressing a hybrid mouse (m) CD40-LMP1 transgene on a CD40−/− background were bred to TRAF5-deficient mice to generate mCD40LMP1 transgenic (LMP-tg) mice that were also TRAF5 deficient (LMP1-tg TRAF5−/−). These mice allowed the flexibility to study in vivo effects of TRAF5 deficiency on LMP1 biological effects as well as the biochemical effects of TRAF5 deficiency on LMP1 signaling in primary B lymphocytes.
Results
Effect of TRAF5 on Spleen and Lymph Node Size in LMP1-tg Mice.
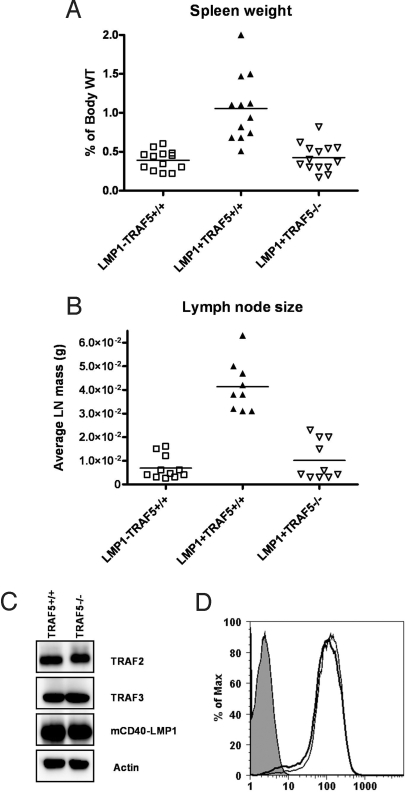
Mice expressing the mCD40LMP1 transgene (LMP1-tg) have a phenotype consistent with B-cell hyperactivity, including grossly enlarged spleen and lymph nodes (4). As expected, LMP1-tg (LMP1+TRAF5+/+) mice had enlarged spleens and lymph nodes compared with littermate controls negative for the mCD40-LMP1 transgene (Fig. 1A). However, LMP1+TRAF5−/− mice had spleen sizes that were on average 62% smaller than spleens from LMP1-tg mice (Fig. 1A). Similarly, the average lymph node size from LMP1-tg TRAF5-deficient mice was 76% smaller than lymph nodes from LMP1-tg mice (Fig. 1B). Both spleen and lymph nodes from LMP1+TRAF5−/− mice were similar in size to nontransgenic littermate controls (LMP1−TRAF5+/+) (Fig. 1 A and B). These results cannot be explained by decrease in surface expression of the mCD40-LMP1 transgene (Fig. 1C) or reduced expression of TRAFs 2 and 3 in LMP1+TRAF5−/− mice (Fig. 1D). Thus, the LMP1-mediated increase in secondary lymphoid organ size requires TRAF5.
Fig. 1.
Spleen and lymph node weights in LMP1+TRAF5+/+ and LMP1+TRAF5−/− mice. Spleens and lymph nodes were harvested from 3- to 4-month-old LMP1−TRAF5+/+ (open squares), LMP1+TRAF5+/+ (closed triangles), and LMP1+TRAF5−/− (open triangles) mice and weighed. To account for variability in mouse size, spleen weights are represented as a percentage of overall body weight (A). Cervical, axillary, and brachial lymph nodes were harvested and weighed, and are represented as average lymph node weight (B). Purified splenic B cells from LMP1+TRAF5−/− mice and littermate controls were lysed in 2× SDS lysis buffer and separated by SDS/PAGE. TRAFs, mCD40-LMP1, and actin were detected by Western blot (C). Surface expression of mCD40-LMP1 on purified splenic B cells was detected by using an anti-mCD40-FITC mAb or isotype control mAb for staining by immunofluorescence flow cytometry (D).
Serum IL-6, Autoantibodies, and Spontaneous Germinal Centers (GCs) in LMP1+TRAF5−/− Mice.
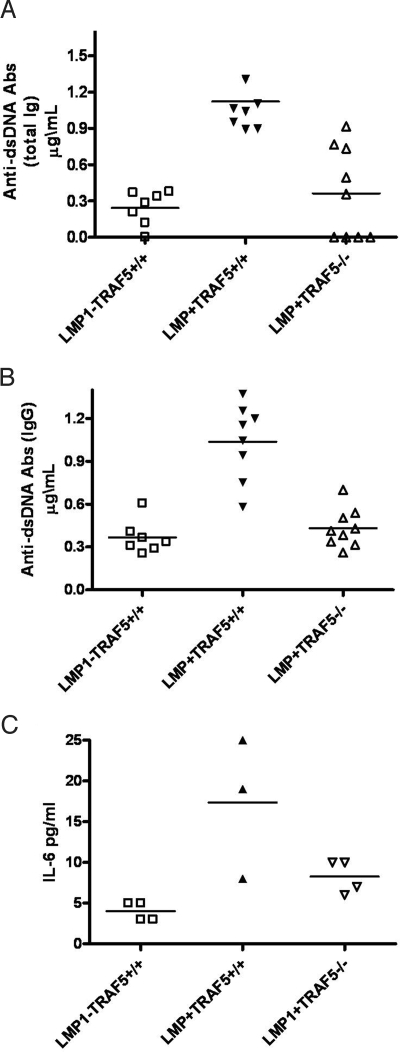
Replacement of CD40 with LMP1 in vivo leads to elevated serum IL-6 and anti-dsDNA Abs (4). Evaluation of serum from LMP1−TRAF5+/+, LMP1+TRAF5+/+, and LMP1+TRAF5−/− mice showed that total anti-dsDNA Abs (Fig. 2A), IgG anti-dsDNA Abs (Fig. 2B), and IL-6 (Fig. 2C) returned to normal levels when LMP1 was expressed in the absence of TRAF5. LMP1+TRAF5−/− mice had substantially less serum anti-dsDNA Abs (60% fewer IgG and 40% fewer total Ig) compared with LMP1+TRAF5+/+ littermates (Fig. 2 A and B). LMP1+TRAF5−/− mice also had on average 70% less serum IL-6 than LMP1+TRAF5+/+ mice, with serum IL-6 values similar to nontransgenic mice (Fig. 2C).
Fig. 2.
Serum anti-dsDNA antibodies and IL-6 in LMP1+TRAF5+/+ and LMP1+TRAF5−/− mice. Serum was collected from LMP1−TRAF5+/+ (open squares), LMP1+TRAF5+/+ (closed triangles), and LMP1+TRAF5−/− (open triangles) mice of 3–4 months of age. The collected serum was used in ELISAs specific for total anti-dsDNA antibodies (A) or IgG-specific anti-dsDNA antibodies (B). The serum was also used in IL-6-specific ELISAs (C). The IL-6 data are representative of two independent experiments.
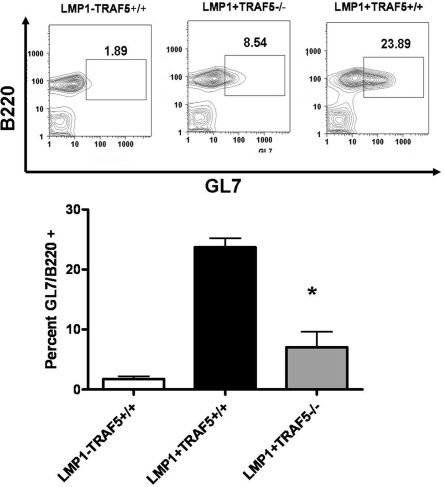
In addition to high levels of IL-6 and autoantibodies, LMP1-tg mice also have spontaneous splenic GCs (4). Similar to results for IL-6 and anti-dsDNA antibodies, LMP1+TRAF5−/− mice had fewer GC B cells (18) than did LMP1+TRAF5+/+ mice (Fig. 3). LMP1+TRAF5−/− mice also had fewer CD138+ cells in the spleen than LMP1+TRAF5+/+ mice (Fig. S1).
Fig. 3.
Spontaneous germinal centers in LMP1+TRAF5+/+ and LMP1+TRAF5−/− mice. Spleens were collected from LMP1−TRAF5+/+ (white bar), LMP1+TRAF5+/+ (black bar), and LMP1+TRAF5−/− (gray bar) mice of 3–4 months of age. The spleens were homogenized and stained with anti-B220 PerCp mAb and GL7 mAb. *, P < 0.005.
Effect of TRAF5 on Unique LMP1-Associated Cell Subset Distribution.
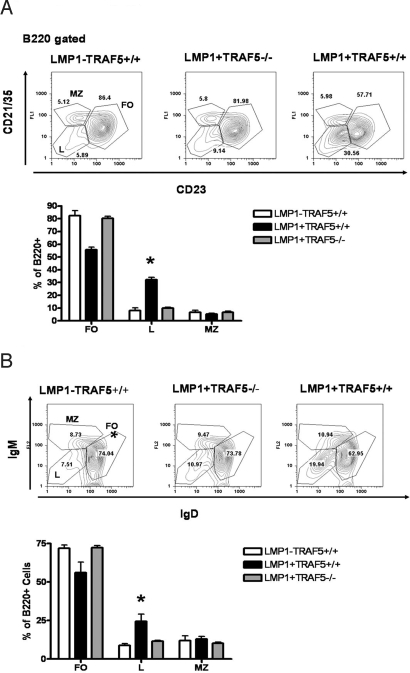
An interesting feature of the LMP1-tg mouse is an expansion of cells that are B220+ but have low expression of CD23, CD21/35, IgM, and IgD (4). We refer to this as “population L,” because its expansion is seen when LMP1 is expressed in vivo. Spleens from LMP1+TRAF5−/− mice have a marked reduction in population L, with occurrence of this similar in frequency to that in spleens from mice that lack mCD40-LMP1 (Fig. 4 A and B). Population L is heterogeneous, composed of plasmacytoid dendritic cells (DCs) and likely other DC subsets (Fig. S2A), immature B cells (Fig. S2B), and some B1 B cells (Fig. S2B). LMP1-tg mice lacking TRAF5 showed a reduction in CD11c+PDCA-1+ cells in the spleen (Fig. S2A). However, TRAF5 deficiency had no effect on CD5+ or AA4.1+ B cells (Fig. S2B).
Fig. 4.
Population L in LMP1+TRAF5+/+ and LMP1+TRAF5−/− mice. Spleens were collected from LMP1−TRAF5+/+ (white bars), LMP1+TRAF5+/+ (black bars), and LMP1+TRAF5−/− (gray bars) mice of 3–4 months of age. The spleens were homogenized and stained with anti-B220 PerCp mAb, anti-CD21/35 FITC mAb, and anti-CD23 PE mAb (A) or anti-B220 PerCp mAb, anti-IgD FITC mAb, and anti-IgM PE mAb (B). *, P < 0.0001.
Direct Role of TRAF5 in LMP1-Mediated JNK Activation and IL-6 Secretion by B-Cells.
Our in vivo observations led us to ask whether TRAF5 directly impacts LMP1 signaling in B cells or whether in vivo effects are due to an indirect requirement for TRAF5 by other receptors downstream of LMP1 signaling. Purified primary B lymphocytes from LMP1+TRAF5+/+ and LMP1+TRAF5−/− mice were stimulated ex vivo with anti-mCD40 mAbs to determine the direct effect of TRAF5 deficiency on LMP1-mediated IL-6 secretion, JNK activation, and NF-κB activation.
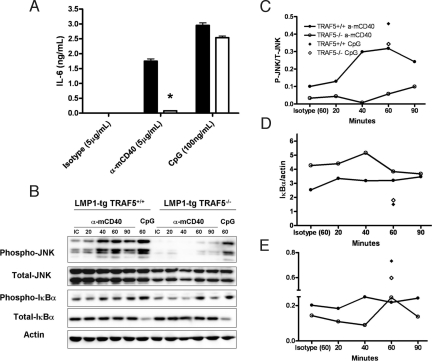
B cells from LMP1+TRAF5+/+ mice secreted IL-6 in response to both anti-mCD40 and CpG oligonucleotide (a TLR9 ligand), whereas B cells from LMP1+TRAF5−/− mice were defective in their capacity to secrete IL-6 when stimulated through mCD40-LMP1 (Fig. 5A). However, B cells from LMP1+TRAF5−/− mice produced levels of IL-6 similar to levels produced by B cells from LMP1+TRAF5+/+ mice when given a control TLR9 stimulus (Fig. 5A). We also conducted a multiplex cytokine assay to ask whether TRAF5 was involved in secretion of other cytokines induced by LMP1 stimulation. Similar to IL-6, TRAF5 was important in secretion of TNF-α and IL-17 by LMP1-stimulated B cells (Fig. S3A). IL-10 and IL-12 were not significantly affected by TRAF5 deficiency (Fig. S3B). This indicates that TRAF5 is important in many, but not all LMP1-induced, signaling effects.
Fig. 5.
The effect of TRAF5 deficiency on LMP1-mediated JNK activation and IL-6 secretion in vitro. Splenic B cells were purified from LMP1+TRAF5+/+ and LMP1+TRAF5−/− mice of 8–12 weeks of age by negative selection (>95% purity). Purified B cells were stimulated with 5 μg/mL anti-mCD40 (HM40.3), 5 μg/mL isotype control, or 100 ng/mL CpG-B (2084) in 48-well plates, and at 48 h their supernatants were collected for use in an IL-6-specific ELISA (A). Purified B cells were stimulated in vitro with 10 μg/mL anti-mCD40 (HM40.3), 10 μg/mL isotype control, or 100 ng/mL CpG-B (2084) for 20–90 min and used in a phospho-JNK Western blot (B). P-JNK was normalized to total JNK (C), P-IκBα was normalized to total IκBα (D), and total IκBα was normalized to actin (E). *, P < 0.001.
Like CD40, LMP1 activates JNK and NF-κB, important regulators of IL-6 production (19). We thus tested whether activation of JNK and/or NF-κB was affected in primary TRAF5-deficient B cells expressing LMP1. Stimulation of B cells from LMP1+TRAF5+/+ mice through mCD40LMP1 led to an accumulation of phosphorylated JNK that was maximal at 60 min. Interestingly, B cells from LMP1+TRAF5−/− mice were unable to activate JNK when stimulated through mCD40-LMP1 (Fig. 5 B and C). As a positive control for JNK activation, B cells from both groups were stimulated with CpG DNA for 60 min. TRAF5−/− B cells activated JNK in response to this TLR9 stimulus to levels similar to those from TRAF5 sufficient B cells (Fig. 5 B and C). The impact of TRAF5 deficiency on NF-κB activation was more subtle. Degradation of IκBα was not affected by the absence of TRAF5 (Fig. 5 A and D). However, phosphorylation of IκBα was less prominent in B cells that were TRAF5 deficient (Fig. 5 A and E). The alternative NF-κB pathway was not affected by TRAF deficiency because nuclear RelB and p52 levels were not altered in mCD40-LMP1-stimulated primary B cells (Fig. S4).
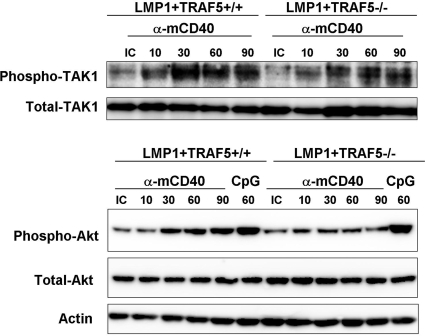
Previous reports suggest that transforming growth factor β-activated kinase 1 (TAK1) is important for LMP1-mediated JNK activation (20, 21). In primary B cells, we did not observe a substantial effect of TRAF5 deficiency on TAK1 activation after stimulation of mCD40-LMP1 (Fig. 6Upper). There are also reports of LMP1 activating the protein kinase B (PKB/Akt) pathway (22). Interestingly, Akt phosphorylation after LMP1 stimulation was inhibited in B cells lacking TRAF5 (Fig. 6 Lower). Like JNK, Akt activation by CpG was not affected by TRAF5 deficiency (Fig. 6 Lower).
Fig. 6.
TAK1 and Akt activation in B lymphocytes from LMP1+TRAF5+/+ and LMP1+TRAF5−/− mice. Purified B cells were stimulated in vitro with 10 μg/mL anti-mCD40 (HM40.3) or 10 μg/mL isotype control for 10–90 min and used in a phospho-TAK1 Western blot (Upper), or stimulated in vitro with 10 μg/mL anti-mCD40 (HM40.3), 10 μg/mL isotype control, or 100 ng/mL CpG-B (2084) for 10–90 min and used in a phospho-Akt Western blot (Lower).
Discussion
The association between TRAF5 and the cytoplasmic domain of LMP1 was reported a decade ago (12). However, the functional role of TRAF5 in LMP1 signaling received little attention, because the majority of the focus in LMP1 signaling studies has been more well studied TRAF molecules. One possible reason for this is that early investigation of the TRAF5−/− mouse suggested that TRAF5 has a modest role in CD40 signaling (14). Based on our previous work showing that LMP1 uses TRAF molecules in different ways compared with CD40 (10, 11), we believed it was important to address this knowledge gap, both in vivo and in vitro.
Our results show clearly that TRAF5 is an important positive regulator of LMP1 biological effects in vivo. Strikingly, all of the pronounced in vivo features characteristic of transgenic expression of the LMP1 cytoplasmic domain, including enlarged secondary lymphoid organs, expansion of an interesting cell subpopulation (population L), and elevated serum IL-6 and autoantibodies were all returned to normal levels if TRAF5 was not available.
It is unknown whether population L influences the phenotype of LMP1-tg mice or whether expansion of this subset results from B-cell hyperreactivity caused by LMP1 expression. However, B-cell stimulations in vitro, and the observation of a similar population of cells in the spleens of an unrelated strain of autoimmune prone mice (BXSB-Yaa) (23) suggest that B-cell hyperreactivity is the cause rather than the result of this influx of CD11c+ cells (23). Similar to the LMP1-tg mice, BXSB-Yaa mice have high serum IL-6, which accelerates development of CD11c+ cells (24). Taken together, these results demonstrate that TRAF5 makes essential contributions to LMP1 in vivo function. This information is relevant to potential design of therapies that seek to block LMP1 signaling, particularly because TRAF5 does not appear to be highly important for signaling by the normal counterpart of LMP1, CD40.
The in vivo findings, however, could not establish whether TRAF5 was required for direct signals provided by LMP1. A likely candidate that could contribute to phenotypic characteristics of the LMP1-tg mouse is IL-6. We previously showed that, whereas CD40 can induce B-cell IL-6 secretion when stimulated by its membrane-bound ligand, CD154, anti-CD40 mAbs are an insufficient stimulus (25). In contrast, hybrid molecules with a CD40 external and LMP1 cytoplasmic domain respond equally well to agonistic mAb and CD154 (3), and mice expressing this molecule have elevated serum IL-6 (4), which was not found in the absence of TRAF5 (Fig. 2C).
IL-6 overexpression induces a phenotype similar to that observed in the LMP1-tg mouse, including splenomegaly, lymphadenopathy, and plasmacytosis (26). Increased IL-6 secretion also leads to increased levels of anti-dsDNA IgG in mice (27). Additionally, studies of the BXSB-Yaa mouse have shown that elevated levels of IL-6 can enhance the development of CD11c+ cells (24), providing a clue that IL-6 may contribute to the emergence of population L that is characteristic of LMP1-tg mice. Thus, the defect in LMP1-mediated IL-6 secretion may play an important role in the reduction in LMP1-associated phenotypic features observed in LMP1+TRAF5−/− mice. Because CD40-mediated IL-6 production depends on the formation of AP-1 complexes composed of phosphorylated c-Jun (28), we tested whether the activation of JNK by LMP1 was disrupted in LMP1-tg B cells that lack TRAF5. JNK phosphorylation after stimulation of LMP1 was severely defective in B cells that were TRAF5 deficient (Fig. 5B), suggesting that the defect in IL-6 secretion may be due to an inability to activate JNK in TRAF5-deficient B cells. Although TRAF5 had a profound role in LMP1-mediated JNK activation and IL-6 production, TRAF5 deficiency had no adverse effect on TLR9-induced IL-6 secretion (Fig. 5A). Similarly to JNK, LMP1, but not TLR9-mediated Akt phosphorylation was also decreased in B cells lacking TRAF5 (Fig. 6 Lower). We and others have also observed that TRAF5 deficiency has no adverse effect on CD40-mediated IL-6 secretion nor JNK activation (14) (Fig. S5), suggesting that the absence of TRAF5 specifically affects LMP1 signaling and does not result in global disruption of JNK activation or IL-6 production. This use of TRAF5 by LMP1 further demonstrates that LMP1 employs TRAF molecules differently than CD40. This also suggests further promising avenues for therapeutic inhibition of LMP1 signaling, without disrupting normal CD40 function.
The mechanism by which TRAF5 regulates JNK activation and IL-6 production by LMP1 is currently under investigation. Because TAK1 is important for JNK activation by LMP1 via interactions with TRAF6 (20, 21), we investigated TAK1 activation in TRAF5−/− B cells. LMP1-mediated TAK activation was not substantially altered in B cells from LMP1+TRAF5−/− mice (Fig. 6 Upper). JNK activation by TLR8 is TAK1 independent (29). It is possible that TRAF5 influences JNK activation by a TAK1-independent mechanism as well. One possibility is that TRAF5 directly promotes JNK activation as TRAF2 does for CD40 (30). A second, not mutually exclusive, possibility may be that TRAF5 indirectly influences JNK activation through the stabilization of other TRAFs. The significance of TRAF–TRAF interactions and LMP1 signaling is part of our ongoing investigations.
Here, we have shown a role for TRAF5 as a necessary positive regulator of JNK activation by LMP1. The consequence of TRAF5 deficiency for primary B cells expressing LMP1 is an inability to secrete IL-6, providing a link between TRAF5 and IL-6. The inability of B cells from the LMP1+TRAF5−/− mice to secrete IL-6 in response to LMP1 may partly explain dramatic reduction of the phenotype displayed by LMP1-tg mice. The observed positive role of TRAF5 in production of other cytokines, particularly TNF-α, may also play a role in the phenotype of LMP1-tg mice. Although more studies are required to pinpoint the mechanism by which TRAF5 influences the exaggerated phenotype observed in the LMP1-tg mouse, work here provides clear evidence that TRAF5 is a critical positive regular of LMP1 signaling and has profound influence over B-cell activity. This work provides us with a new understanding of the consequences of TRAF5 deficiency for LMP1 signaling both in vitro and in vivo, which is important information to consider in LMP1's pathogenic effects in humans (31–33).
Materials and Methods
Mice.
TRAF5−/− mice (14, 34) were bred to CD40−/− mice to create TRAF5−/−/CD40−/− mice. These mice were bred to CD40−/−/mCD40-LMP1-tg mice (4) to create TRAF5−/−/CD40−/−/mCD40-LMP1-tg mice. Both TRAF5−/− mice and mCD40-LMP1-tg mice were extensively backcrossed to C57BL/6 mice, and all strains used in the experiments contained in this report were littermates. PCR was used to screen for the targeted germ-line allele(s) for TRAF5 and CD40 and for the mCD40-LMP1 transgene. Mice were maintained under pathogen-free conditions at the University of Iowa. Use of mice in this study was in accordance with a protocol approved by the University of Iowa Animal Care and Use Committee.
Antibodies and Reagents.
α-B220-peridinin chlorophyll protein (PerCp), α-IgM-phycoerythrin (PE), α-IgD-FITC, α-CD21/35-FITC, α-CD23-PE, α-MHC class II-FITC, α-CD11c-allophycocyanin (APC), α-CD40-FITC, PDCA-1-PE, AA4.1-APC, CD5-APC, and GL7-Alexa Fluor 647 Abs were purchased from eBiosciences. Abs to IκBα, phospho-JNK, phospho-TAK1, TAK1, phospho-Akt, Akt, and p52 were purchased from Cell Signaling Technologies. The anti-JNK and RelB Abs were purchased from Santa Cruz Biotechnology. Anti-CD40 mAb (HM40.3) was purchased from eBiosciences, and the isotype control Ab (G235-1) was purchased from BD Pharmingen.
Flow Cytometry.
Whole splenocytes were depleted of erythrocytes by hypotonic lysis and stained with α-B220, α-IgM, α-IgD, or α-B220, α-CD21/35, and α-CD23, α-B220, α-MHC class II, and α-CD11c Abs. For the detection of immature and B1 B cells, ΑΑ4.1 and α-CD5 mAbs were used. DC subsets were stained with α-B220 α-CD11c, α-MHC class II, and α-PDCA1 mAbs. For all staining, the standard protocol described in ref. 4 was used.
Detection of Serum IL-6 and Serum Autoantibodies.
Serum was collected from unimmunized mice (12–16 weeks of age) and used in ELISAs to detect IL-6 (R&D Systems) or anti-dsDNA Abs (Alpha Diagnostic International).
Detection of Cytokine Production In Vitro.
Primary B cells were isolated by using an anti-CD43 negative selection kit (Miltenyi Biotec) and stimulated with anti-mCD40 mAbs (HM40.3) or isotype control Abs for 24 and 48 h (IL-6) or 4, 6, and 24 h (TNF-α). After stimulation, supernatants were collected and used in cytokine-specific ELISAs.
Proximal Signaling Assays.
Primary B cells were isolated as for the in vitro cytokine production experiments above. Cells were stimulated with 100 nM CpG-B (2084), 10 μg/mL anti-mCD40 mAb (HM40.3), or 10 μg/mL isotype control Ab (G235-1) for 0, 10, 20, 40, 60, and 90 min. After stimulation, cells were lysed in SDS lysis buffer (1% SDS, 2% β-mercaptoethanol, 62.5 mM Tris, pH 6.8), and lysates were sonicated and boiled before separation by SDS/PAGE (30). The proteins in the gels were transferred to PVDF membranes and blocked in 2% milk for 2–5 h. Degradation of IκBα, characteristic of canonical NF-κB activation, was detected by the use of anti-IκBα Abs (Cell Signaling Technologies). JNK, TAK1, Akt, p38, and Erk phosphorylation was detected by using Abs specific to phosphorylated forms of these proteins (Cell Signaling Technologies).
NF-κB2 Activation.
Primary B cells were isolated as described above. A total of 1 × 106 cells was stimulated with 5 μg/mL anti-mCD40 (HM40.3) or isotype control Ab (G235-1) for 6 or 24 h in 24-well plates. After stimulations, cells were harvested and treated as above. Nuclear RelB and p52 were detected via Western blot.
Statistical Analysis.
P values were generated by using Student's t test (unpaired, two-tailed, at 95% confidence interval).
Supplementary Material
Acknowledgments.
We thank Drs. Laura Stunz and Thomas Waldschmidt (University of Iowa, Iowa City, IA) for suggestions and technical advice. This work was supported by National Institutes of Health Grants AI49993 and CA099997 (to G.A.B.) and is the result of work supported in part with resources and the use of facilities at the Iowa City Veterans Affairs Medical Center (Iowa City, IA). Z.J.K. received support from American Heart Association Predoctoral Fellowship 0715658Z.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903786106/DCSupplemental.
References
- 1.Fennewald S, van Santen V, Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984;51:411–419. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liebowitz D, Wang D, Kieff E. Orientation and patching of the latent infection membrane protein encoded by Epstein-Barr virus. J Virol. 1986;58:233–237. doi: 10.1128/jvi.58.1.233-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown KD, Hostager BS, Bishop GA. Differential signaling and tumor necrosis factor receptor-associated factor (TRAF) degradation mediated by CD40 and the Epstein-Barr virus oncoprotein latent membrane protein 1 (LMP1) J Exp Med. 2001;193:943–954. doi: 10.1084/jem.193.8.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stunz LL, et al. Expression of the cytoplasmic tail of LMP1 in mice induces hyperactivation of B lymphocytes and disordered lymphoid architecture. Immunity. 2004;21:255–266. doi: 10.1016/j.immuni.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Busch LK, Bishop GA. The EBV transforming protein, latent membrane protein 1, mimics and cooperates with CD40 signaling in B lymphocytes. J Immunol. 1999;162:2555–2561. [PubMed] [Google Scholar]
- 6.Dirmeier U, et al. Latent membrane protein 1 of Epstein-Barr virus coordinately regulates proliferation with control of apoptosis. 2005;24:1711–1717. doi: 10.1038/sj.onc.1208367. [DOI] [PubMed] [Google Scholar]
- 7.Rastelli J, et al. LMP1 signaling can replace CD40 signaling in B cells in vivo and has unique features of inducing class-switch recombination to IgG1. Blood. 2008;111:1448–1455. doi: 10.1182/blood-2007-10-117655. [DOI] [PubMed] [Google Scholar]
- 8.Vockerodt M, et al. The Epstein-Barr virus oncoprotein, latent membrane protein-1, reprograms germinal centre B cells towards a Hodgkin's Reed-Sternberg-like phenotype. J Pathol. 2008;216:83–92. doi: 10.1002/path.2384. [DOI] [PubMed] [Google Scholar]
- 9.Homig-Holzel C, et al. Constitutive CD40 signaling in B cells selectively activates the noncanonical NF-κB pathway and promotes lymphomagenesis. J Exp Med. 2008;205:1317–1329. doi: 10.1084/jem.20080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie P, Hostager BS, Munroe ME, Moore CR, Bishop GA. Cooperation between TNF receptor-associated factors 1 and 2 in CD40 signaling. J Immunol. 2006;176:5388–5400. doi: 10.4049/jimmunol.176.9.5388. [DOI] [PubMed] [Google Scholar]
- 11.Xie P, Hostager BS, Bishop GA. Requirement for TRAF3 in signaling by LMP1 but not CD40 in B lymphocytes. J Exp Med. 2004;199:661–671. doi: 10.1084/jem.20031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devergne O, et al. Role of the TRAF binding site and NF-kappa B activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J Virol. 1998;72:7900–7908. doi: 10.1128/jvi.72.10.7900-7908.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie P, Bishop GA. Roles of TNF receptor-associated factor 3 in signaling to B lymphocytes by carboxyl-terminal activating regions 1 and 2 of the EBV-encoded oncoprotein latent membrane protein 1. J Immunol. 2004;173:5546–5555. doi: 10.4049/jimmunol.173.9.5546. [DOI] [PubMed] [Google Scholar]
- 14.Nakano H, et al. Targeted disruption of Traf5 gene causes defects in CD40- and CD27-mediated lymphocyte activation. Proc Natl Acad Sci USA. 1999;96:9803–9808. doi: 10.1073/pnas.96.17.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida TK, et al. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci USA. 1996;93:9437–9442. doi: 10.1073/pnas.93.18.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aizawa S, et al. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NFkappa B activation. J Biol Chem. 1997;272:2042–2045. doi: 10.1074/jbc.272.4.2042. [DOI] [PubMed] [Google Scholar]
- 17.Akiba H, et al. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. J Biol Chem. 1998;273:13353–13358. doi: 10.1074/jbc.273.21.13353. [DOI] [PubMed] [Google Scholar]
- 18.Naito Y, et al. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol Cell Biol. 2007;27:3008–3022. doi: 10.1128/MCB.02047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baccam M, Woo S-Y, Vinson C, Bishop GA. CD40-mediated transcriptional regulation of the IL-6 gene in B lymphocytes: Involvement of NF-κB, AP-1, and C/EBP. J Immunol. 2003;170:3099–3108. doi: 10.4049/jimmunol.170.6.3099. [DOI] [PubMed] [Google Scholar]
- 20.Uemura N, et al. TAK1 is a component of the Epstein-Barr virus LMP1 complex and is essential for activation of JNK but not of NF-κB. J Biol Chem. 2006;281:7863–7872. doi: 10.1074/jbc.M509834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan J, et al. Elucidation of the c-Jun N-terminal kinase pathway mediated by Epstein-Barr virus-encoded latent membrane protein 1. Mol Cell Biol. 2004;24:192–199. doi: 10.1128/MCB.24.1.192-199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornburg NJ, et al. LMP1 signaling and activation of NF-kappaB in LMP1 transgenic mice. Oncogene. 2006;25:288–297. doi: 10.1038/sj.onc.1209023. [DOI] [PubMed] [Google Scholar]
- 23.Hirofumi Amano, et al. Selective expansion of a monocyte subset expressing the CD11c dendritic cell marker in the Yaa model of systemic lupus erythematosus. Arthritis Rheum. 2005;52:2790–2798. doi: 10.1002/art.21365. [DOI] [PubMed] [Google Scholar]
- 24.Maeda K, et al. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6. Sle1.Yaa animals. Blood. 2009;113:4534–4540. doi: 10.1182/blood-2008-12-192559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baccam M, Bishop GA. Membrane-bound CD154, but not CD40-specific antibody, mediates NF-kappaB-independent IL-6 production in B cells. Eur J Immunol. 1999;29:3855–3866. doi: 10.1002/(SICI)1521-4141(199912)29:12<3855::AID-IMMU3855>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 26.Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–368. doi: 10.1016/s1359-6101(02)00027-8. 2002. [DOI] [PubMed] [Google Scholar]
- 27.Richards HB, et al. Interleukin 6 dependence of anti-DNA antibody production: Evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188:985–990. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanden Bush TJ, Bishop GA. TLR7 and CD40 cooperate in IL-6 production via enhanced JNK and AP-1 activation. Eur J Immunol. 2008;38:400–409. doi: 10.1002/eji.200737602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin J, et al. TLR8-mediated NF-κB and JNK activation are TAK1-independent and MEKK3-dependent. J Biol Chem. 2006;281:21013–21021. doi: 10.1074/jbc.M512908200. [DOI] [PubMed] [Google Scholar]
- 30.Hostager BS, Haxhinasto SA, Rowland SL, Bishop GA. Tumor necrosis factor receptor-associated factor 2 (TRAF2)-deficient B lymphocytes reveal novel roles for TRAF2 in CD40 signaling. J Biol Chem. 2003;278:45382–45390. doi: 10.1074/jbc.M306708200. [DOI] [PubMed] [Google Scholar]
- 31.Herbst H, Samol J, Foss HD, Raff T, Niedobitek G. Modulation of interleukin-6 expression in Hodgkin and Reed-Sternberg cells by Epstein-Barr virus. J Pathol. 1997;182:299–306. doi: 10.1002/(SICI)1096-9896(199707)182:3<299::AID-PATH856>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Neri A, et al. Epstein-Barr virus infection precedes clonal expansion in Burkitt's and acquired immunodeficiency syndrome-associated lymphoma. Blood. 1991;77:1092–1095. [PubMed] [Google Scholar]
- 33.Brady G, MacArthur GJ, Farrell PJ. Epstein Barr virus and Burkitt lymphoma. J Clin Pathol. 2007;60:1397–1402. doi: 10.1136/jcp.2007.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraus ZJ, Haring JS, Bishop GA. TNF receptor-associated factor 5 is required for optimal T cell expansion and survival in response to infection. J Immunol. 2008;181:7800–7809. doi: 10.4049/jimmunol.181.11.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.