Abstract
Objective
Oxidative stress plays an important role in type 2 diabetes-related endothelial dysfunction. We hypothesized that resveratrol protects against oxidative stress-induced endothelial dysfunction in aortas of diabetic mice by inhibiting tumor necrosis factor-alpha (TNFα)-induced activation of NAD(P)H oxidase and preserving phosphorylation of endothelial nitric oxide synthase (eNOS).
Methods and Results
We examined endothelial-dependent vasorelaxation to acetylcholine (ACh) in diabetic mice (Leprdb) and normal controls (m Leprdb). Relaxation to ACh was blunted in Leprdb compared with m Leprdb while endothelial-independent vasorelaxation to sodium nitroprusside (SNP) was comparable. Resveratrol improved ACh-induced vasorelaxation in Leprdb without affecting dilator response to SNP. Impaired relaxation to ACh in Leprdb was partially reversed by incubating the vessels with NAD(P)H oxidase inhibitor apocynin and a membrane-permeable superoxide dismutase mimetic TEMPOL. Dihydroethidium (DHE) staining showed an elevated superoxide (O2.−) production in Leprdb while both resveratrol and apocynin significantly reduced O2.− signal. Resveratrol increased nitrite/nitrate levels and eNOS (Ser1177) phosphorylation, and attenuated H2O2 production and nitrotyrosine (N-Tyr) content in Leprdb aortas. Furthermore, resveratrol attenuated the mRNA and protein expression of TNFα. Genetic deletion of TNFα in diabetic mice (dbTNF−/dbTNF−) was associated with a reduced NAD(P)H oxidase activity and vascular O2.− production and an increased eNOS (Ser1177) phosphorylation, suggesting that TNFα plays a pivotal role in aortic dysfunction in diabetes by inducing oxidative stress and reducing NO bioavailability.
Conclusions
Resveratrol restored endothelial function in type 2 diabetes by inhibiting TNFα-induced activation of NAD(P)H oxidase and preserving eNOS phosphorylation, suggesting the potential for new treatment approaches to promote vascular health in metabolic diseases.
Keywords: Antioxidants, Nitric Oxide, Nitric Oxide Synthase, Superoxide, Vasodilation
Introduction
Epidemiological studies indicate that resveratrol, a plant polyphenol widely consumed in the Mediterranean diet, is associated with reduced risk of cardiovascular diseases,1 which account for the majority of the mortality in type 2 diabetes.2 Vascular dysfunction is the initial step in the occurrence of many disease states in the cardiovascular system concurrent with diabetes.3 Obesity is an established risk factor for type 2 diabetes. Recent studies suggest that resveratrol, by activating silent mating type information regulation 2 homolog 1 (SIRT1), safely mimics the effects of dietary restriction in laboratory animals.4 In diet-induced obese mice, resveratrol improves insulin sensitivity, lowers plasma glucose, and increases mitochondrial capacity.5 Furthermore, resveratrol attenuates aortic dysfunction in aging and high fat-induced obesity animal models.6 However, there is no study regarding the effects of resveratrol on insulin sensitivity and vascular dysfunction in type 2 diabetic mice.
Oxidative stress is known to be the key mechanism in the pathogenesis of diabetes-related vascular dysfunction. Oxidative stress is due to excessive production of reactive oxygen species (ROS). The inactivation of nitric oxide (NO, a potent vasodilator synthesized by endothelial NO synthase, eNOS) by ROS is recognized to be a crucial factor in reducing NO bioavailability and the development of endothelial dysfunction.7 NAD(P)H oxidase is a key source of superoxide (O2.−) in the vasculature.8 Previous work shows that anti-tumor necrosis factor–alpha (TNFα)-treatment inhibits NAD(P)H oxidase activation in coronary arterioles from type 2 diabetic mice.9 TNFα is reported to down-regulate eNOS expression in fat and muscle of obese rodents.10 Several studies also reported that resveratrol attenuates TNFα expression activated by lipopolysaccharides (LPS),11, 12 leading us to hypothesize that resveratrol protects against vascular dysfunction occurring during diabetes by attenuating TNFα-induced vascular oxidative stress, therefore improving NO bioavailability. To test this, we examined the effects of resveratrol on hyperglycemic status and vascular function using a type 2 diabetic mouse model.
Methods
Animal Models
The procedures followed were in accordance with approved guidelines set by the Laboratory Animal Care Committee at the University of Missouri. Heterozygote control mice (m Leprdb) (Background Strain: C57BLKS/J), homozygote type 2 diabetic mice (Leprdb) (Background Strain: C57BLKS/J) and Leprdb null for TNFα (dbTNF−/dbTNF−) (Background Strain: C57BL/6J) were purchased from Jackson Laboratory and maintained on a normal rodent chow diet. Male, 20–35g m Leprdb, 40–60 g Leprdb and dbTNF−/dbTNF− mice of either sex were used in this study. At the age of 10 weeks, m Leprdb and Leprdb mice were either treated with resveratrol (20 mg/kg/day, Cayman Chemical) or vehicle (0.5% methylcellulose) orally for 4 weeks.13
mRNA Expression of TNFα by Real-time Polymerase Chain Reaction
We have used a quantitative real time RT-PCR technique to analyze mRNA expression of TNFα in mouse aortas, using the Strategen MX3000 as reported.14 Quantification was performed using the efficiency-corrected ΔΔCT method and β-actin was used for internal normalization.14
Functional Assessment of Murine Aortas
2 mm of aortic rings were isometrically mounted in a myograph (model 610M, DMT, Denmark) and an optimal passive tension (15 mN) was applied. Aortic rings were precontracted with 1 µmol/L phenolnephrine (PE). Dose-response curve was obtained by cumulative addition of acetylcholine (ACh, 1 nmol/L to 10 µmol/L), and sodium nitroprusside (SNP, 1 nmol/L to 10 µmol/L). Relaxation at each concentration was measured and expressed as the percentage of force generated in response to PE. The contributions of TNFα, NAD(P)H oxidase and O2.− in vasorelaxation were assessed by incubating the vessels with recombinant TNFα (R&D, 10 ng/ml, 90 min),15 NAD(P)H oxidase inhibitor apocynin (100 µmol/L, 60 min), and TEMPOL (a membrane-permeable superoxide dismutase mimetic, 3 mmol/L, 60 min), respectively.16
Protein Expression of TNFα, gp91phox, eNOS, phospho-eNOS, and Nitrotyrosine (N-Tyr) by Western Blot Analyses
TNFα, gp91phox, eNOS, phospho-eNOS, and N-Tyr protein expressions were detected in aortas by Western Blot. The relative amounts of protein expression were quantified to those of the corresponding m Leprdb control.
NADP(H) Oxidase Activity
NAD(P)H oxidase activity was assayed in homogenized aorta samples using lucigenin-derived chemiluminescence assay as previously reported.9, 17 O2.− production was measured in the presence of 5 µmol/L lucigenin. The reaction was started by adding NAD(P)H (100 µmol/L). The relative light units (RLU) of chemiluminescence were read in a luminometer (Fluoroscan Ascent FL).
Detection of Vascular O2.− Production by Ethidium Bromide (EB) Fluorescence Assay
Dihydroethidium (DHE), an oxidative fluorescent dye, was used to localize O2.− production in situ as previously reported.18 To determine the role of TNFα and NAD(P)H oxidase in O2.− production in aortas in type 2 diabetes, the vessels were treated with apocynin (100 µmol/L, 60 min) or recombinant TNFα (R&D, 10 ng/ml, 90 min). The specificity of O2.− production was examined in the presence of TEMPOL (1 mmol/L, 60 min). Quantification of fluorescence intensity was determined by using Image-J (NIH, Bethesda, MD) and normalized to m Leprdb.
Measurement of Serum Hydrogen Peroxide (H2O2)
Serum production of H2O2 was determined by using QuantiChrom™ peroxide assay kit (BioAssay Systems).
Measurement of Nitrite/Nitrate
Aortas were homogenized and supernatants were collected for the quantification of nitrite/nitrate level using amperometric sensors (WPI, World Precision Instruments) according to previous publication.19 Briefly, nitrate was converted to nitrite using Nitralyzer Nitrate to Nitrite Reduction Kit (WPI). 2 mm sensor (ISO-NOP) was calibrated by chemical generation of NO. The currents (pA) detected by the sensor represent the concentration of nitrite in vessel samples and were normalized to the protein concentration.
Data Analysis
All data were presented as mean±SD except as specifically stated. Statistical comparisons under various treatments were performed with one-way ANOVA, and intergroup differences were tested with Tukey inequality. Significance was accepted at P < 0.05.
Results
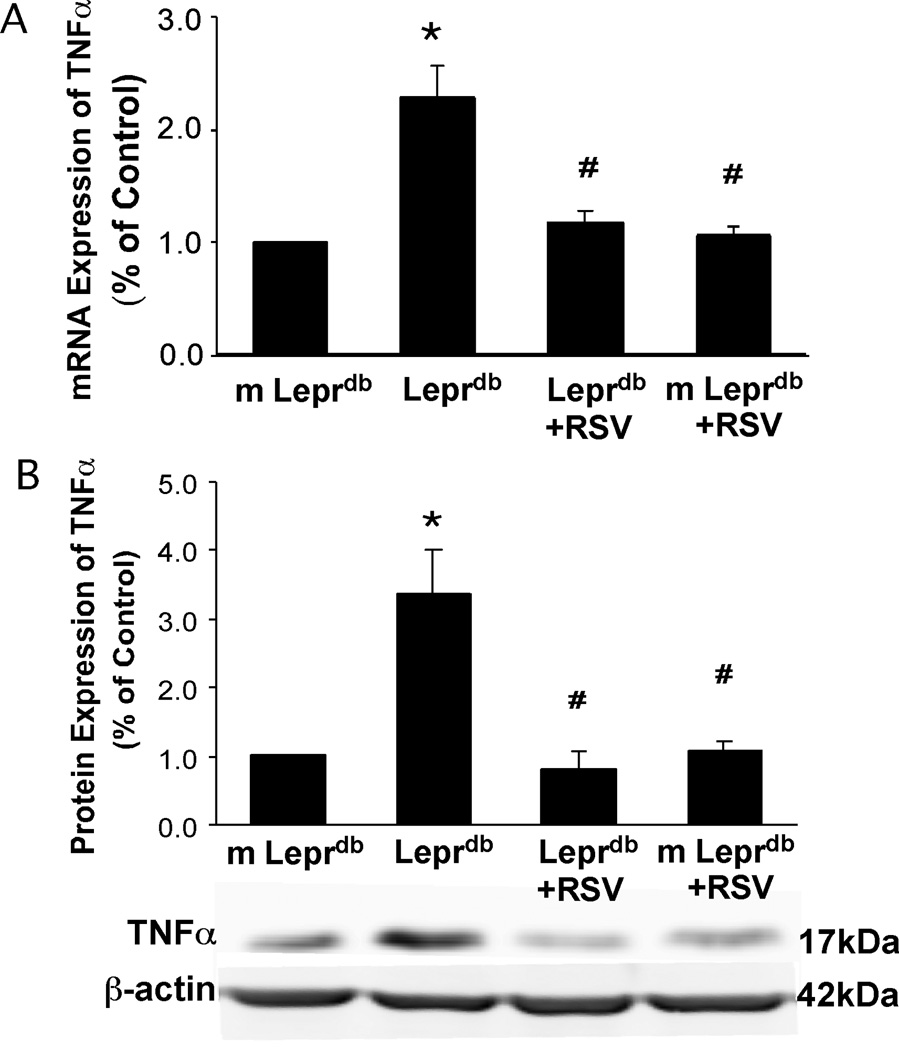
Resveratrol Attenuated mRNA and Protein Expression of TNFα in Type 2 Diabetes
mRNA and protein expression of TNFα were examined in isolated aortas of m Leprdb and Leprdb mice either treated with resveratrol (m Leprdb+RSV and Leprdb+RSV) or vehicle (m Leprdb and Leprdb). mRNA and protein expression of TNFα were significantly elevated in Leprdb compared with m Leprdb. Resveratrol treatment reduced TNFα mRNA and protein expression in Leprdb without affecting that in m Leprdb (Figure 1).
Figure 1.
mRNA (A) and protein expression (B) of TNFα (fold change) were significantly higher in Leprdb than in m Leprdb. Resveratrol (RSV) reduced TNFα expression (both mRNA and protein) in Leprdb. n=5 separate experiments. *p<0.05 vs. m Leprdb; #p<0.05 vs. Leprdb.
Resveratrol Improved Aortic Endothelial Dysfunction in Type 2 Diabetes
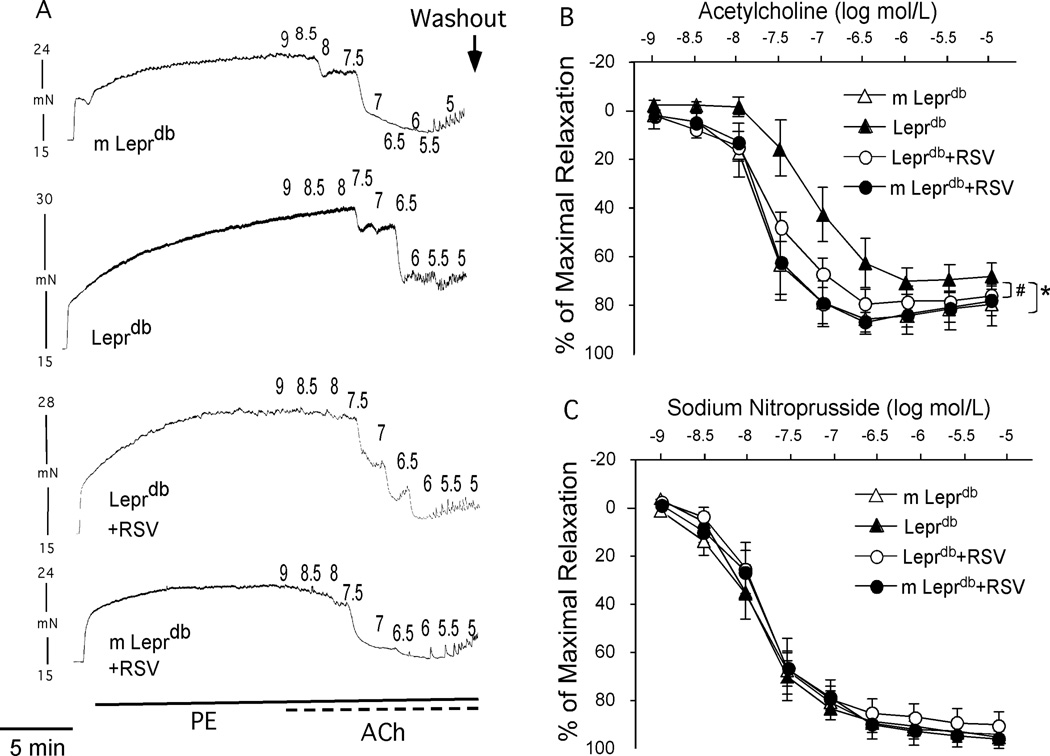
Isolated aortic rings from m Leprdb and Leprdb either treated with resveratrol or vehicle dilated dose-dependently to endothelium-dependent and endothelium–independent agonists (ACh and SNP respectively). Relaxation to ACh was blunted in Leprdb compared with m Leprdb while endothelial-independent vasorelaxation to SNP was comparable. Resveratrol improved ACh-induced vasorelaxation in Leprdb without affecting dilator response to SNP (Figure 2).
Figure 2.
Representative traces (A) and concentration-response curve (B) showed that endothelial-dependent vasorelaxation to ACh was significantly impaired in aortas of Leprdb. Resveratrol treatment restored impaired vasorelaxation. (C) Endothelial-independent vasorelaxation to SNP was not statistically different among groups. n=7–12 mice. *p<0.05 vs. m Leprdb; #p<0.05 vs. Leprdb.
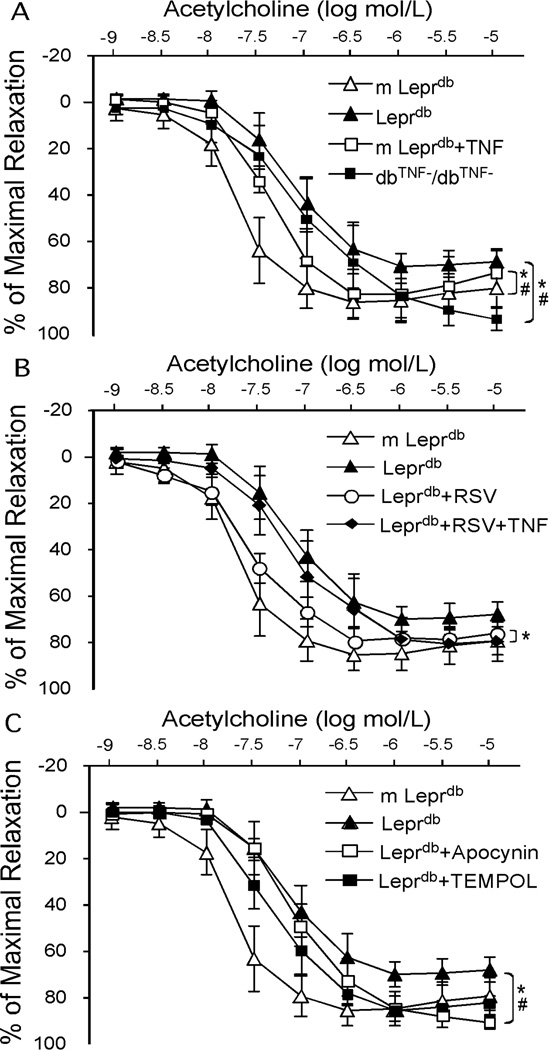
Role of TNFα and NAD(P)H Oxidase in Diabetes-induced Aortic Vascular Dysfunction
In dbTNF−/dbTNF−, ACh-induced vasorelaxation was greater than that in Leprdb while recombinant TNFα incubation impaired endothelial-dependent vasorelaxation in m Leprdb (Figure 3A). Although resveratrol restored endothelial function in diabetic mice, the improvement was abolished by incubating the vessel with recombinant TNFα (Figure 3B). Furthermore, administration of apocynin and TEMPOL rescued impaired vasorelaxation to ACh in Leprdb (Figure 3C).
Figure 3.
Genetic deletion of TNFα (dbTNF−/dbTNF−) (A, n=8 mice), apocynin and TEMPOL (C, n=4 mice) partially restored impaired vasorelaxation in Leprdb. TNFα incubation impaired endothelial function in m Leprdb (A, n=6 mice) and abolished the improvement in endothelial function by resveratrol treatment in Leprdb (B, n=6 mice). *p<0.05 vs. m Leprdb; #p<0.05 vs. Leprdb.
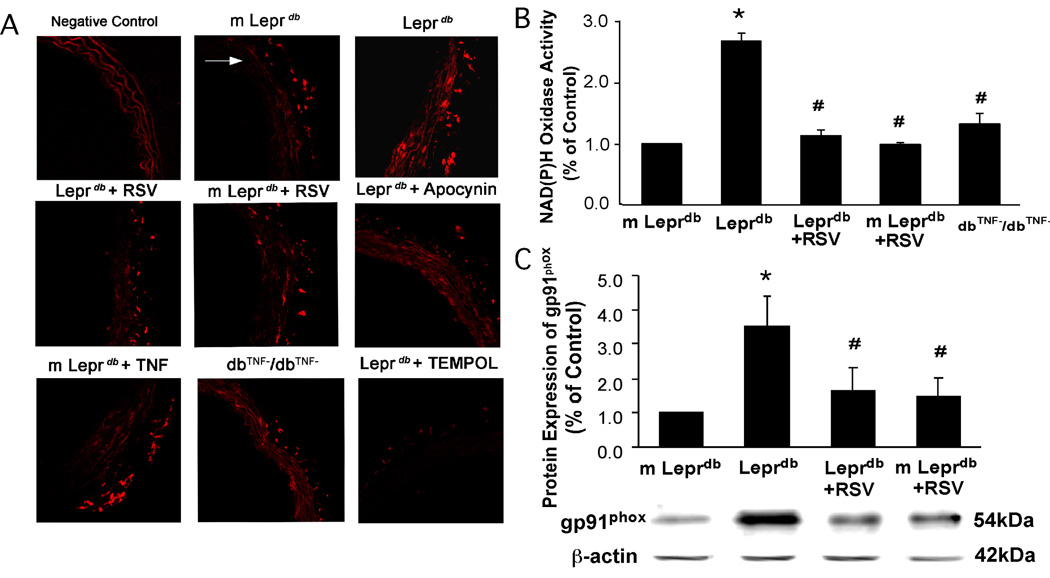
Resveratrol Inhibited NAD(P)H Activation and Subsequent O2.− Production
O2.− production was significantly elevated in diabetic mice compared with control mice and resveratrol decreased vascular O2.− production (Figure 4A). Although dbTNF−/dbTNF− showed decreased O2.− production compared with Leprdb, the O2.− production in m Leprdb was enhanced after incubating the vessels with recombinant TNFα. In Leprdb, vascular O2.− production was reduced to the level comparable to m Leprdb by incubating the vessel with apocynin (Figure 4A). Quantification of fluorescence intensity is available in online supplemental materials (Figure II. Please see www.ahajournals.org). Figure 4B and 4C show that NAD(P)H oxidase activity and its subunit, gp91phox protein expression were significantly elevated in diabetic mice. Resveratrol inhibited NAD(P)H oxidase activation and gp91phox expression. dbTNF−/dbTNF− mice also showed reduced NAD(P)H oxidase activity compared with that of Leprdb (Figure 4B).
Figure 4.
(A) Resveratrol, apocynin and genetic deletion of TNFα significantly reduced aortic O2.− production in Leprdb. Incubating aortas of m Leprdb with TNFα increased O2.− production. White arrow indicates the lumen of aorta. (B) and (C), Resveratrol significantly decreased NAD(P)H oxidase activity and gp91phox protein expression in Leprdb. NAD(P)H oxidase activity was lower in dbTNF−/dbTNF− than in Leprdb (B). n=4 separate experiments. *P < 0.05 vs. m Leprdb; #P<0.05 vs. Leprdb.
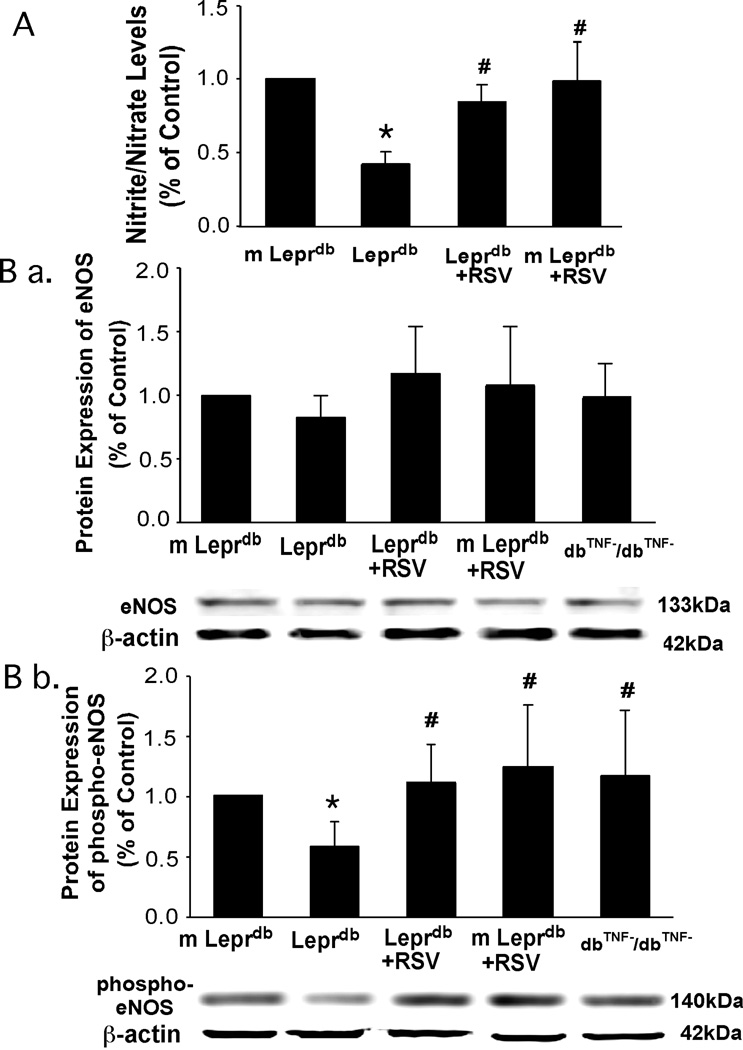
Resveratrol Increased NO Bioavailability and eNOS Phosphorylation
Aortic nitrite/nitrate levels were reduced in diabetic mice and resveratrol enhanced aortic NO release (Figure 5A). Although total eNOS protein expression was comparable among groups, eNOS phosphorylation was decreased in diabetic mice. Resveratrol increased eNOS phosphorylation at Ser1177 in the aorta of diabetic mice (Figure 5B). Protein expression of phospho-eNOS was greater in dbTNF−/dbTNF− compared with that in Leprdb (Figure 5B).
Figure 5.
Aortic nitrite/nitrate level (A) and phosphorylation of eNOS at Ser1177 (B b.) were lower in Leprdb, while total eNOS expression (B a.) was similar among groups. Resveratrol enhanced nitrite/nitrate production and eNOS phosphorylation. eNOS phosphorylation was higher in dbTNF−/dbTNF− than in Leprdb (B b.). n=5 separate experiments. *P<0.05 vs. m Leprdb; #P<0.05 vs. Leprdb.
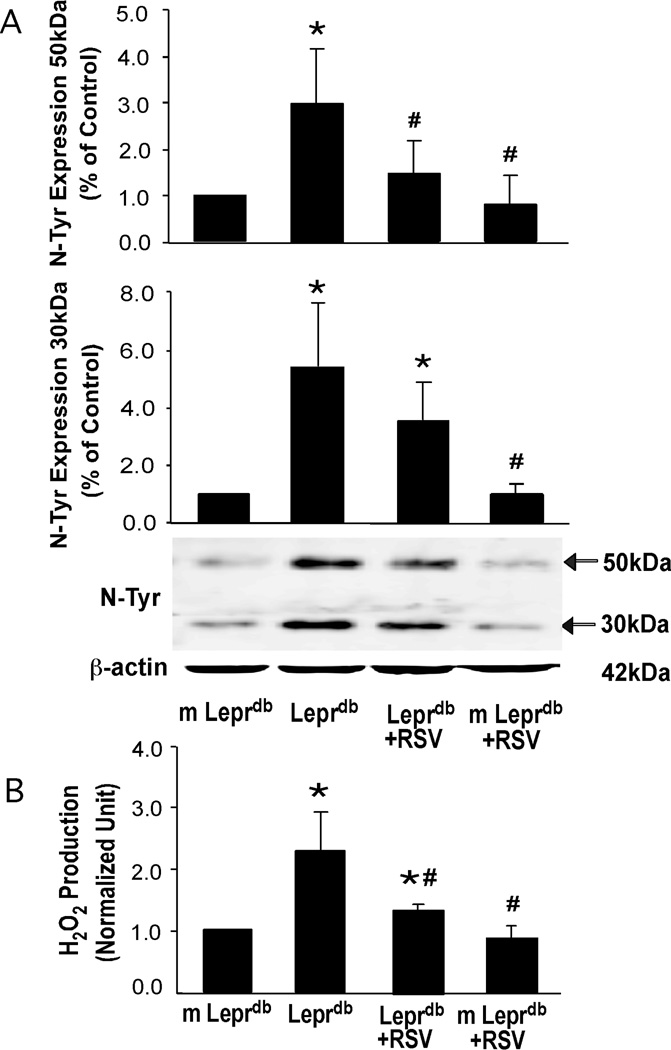
Resveratrol Decreased N-Tyr Expression and Serum H2O2 Production
Western blot analysis (Figure 6A) of N-Tyr protein expression in aortas from m Leprdb and Leprdb revealed a significantly higher level of N-Tyr in Leprdb. Figure 6B shows elevated serum H2O2 production in Leprdb compared with control mice. Resveratrol significantly diminished the aortic N-Tyr protein expression and serum H2O2 level in diabetic mice without affecting those in control mice (Figure 6A and 6B).
Figure 6.
Aortic N-Tyr expression (A) and serum H2O2 production (B) were higher in Leprdb than in m Leprdb. Resveratrol decreased N-Tyr content and H2O2 levels in Leprdb. n=4 separate experiments. *P<0.05 vs. m Leprdb; #P<0.05 vs. Leprdb.
Discussion
The pathogenic relationships among obesity, type 2 diabetes, and its vascular complications, remain poorly understood. Intensification of oxidative stress and inflammation has been implicated to accelerate vascular dysfunction in type 2 diabetes despite anti-hyperglycemic treatment. In past decades, numerous interventions have been put forward to counteract the vulnerability of the vasculature to oxidative challenges and inflammation in diabetes. Our major findings are: 1) Chronic resveratrol administration rescues aortic dysfunction in type 2 diabetes. 2) Resveratrol has potent effects that inhibit TNFα expression in aortas of diabetic mice. 3) Resveratrol attenuates macrovascular oxidative stress partially by inhibiting TNFα-induced NAD(P)H oxidase activation. 4) Resveratrol greatly improves aortic NO bioavailability through enhancing eNOS phosphorylation in type 2 diabetes. 5) Resveratrol does not induce weight loss, nor improve hyperglycemic status and insulin sensitivity in type 2 diabetic Leprdb mice.
Role of Resveratrol in Weight Loss and Hyperglycemic Status
The Leprdb mouse is a genetic model of non-insulin-dependent diabetes (type 2 diabetes), which has defects in receptors for the obese gene product, leptin.20 The defects of leptin receptors, especially its long form Ob-Rb in Leprdb lead to impairments of leptin regulation on food intake and body weight mainly via activation of the hypothalamic leptin receptors.21 and to a lesser extent via its peripheral and varied targets,22 and result in the expression of diabetes, preceded by hyperinsulinemia, hyperglycemia, and extreme obesity.23
Resveratrol reverses hyperglycemic status and improves insulin sensitivity in high fat-induced obese rodents and in the type 1 diabetic animal model.5, 24, 25 Oral administration of resveratrol at a dose of 22.4 mg/kg/day was shown to improve insulin sensitivity and to slightly reduce the body weight of 1-year-old mice fed a high fat diet.24 At a dose of 400 mg/kg/day, resveratrol prevented diet-induced obesity and alleviated obesity-related insulin resistance.5 In streptozotocin-induced type 1 diabetic rats, resveratrol dose-dependently decreased plasma glucose and lipid levels.25 In contrast, our results show that resveratrol does not alter body weight nor reduce hyperinsulinemic hyperglycemia in Leprdb mice (Table I and Figure I. Please see www.ahajournals.org). Body weight is considered important in the control of type 2 diabetes26 and the calorie restriction mimetic effects of resveratrol have been proposed to largely explain its anti-aging benefits.4 Our work suggests there are therapeutic effects of resveratrol, especially on vascular complications, that can also occur independently of weight loss and hyperglycemic status.
Role of Resveratrol in TNFα Expression and anti-TNFα Treatment in Vascular Dysfunction
Our previous study demonstrated that TNFα contributes to microvascular dysfunction in type 2 diabetes evidenced by increased TNFα mRNA and protein expression in coronary arterioles of diabetic mice and improved coronary arteriolar endothelial function in dbTNF−/dbTNF−.9 This study further supports the central role of TNFα in aortic dysfunction in type 2 diabetes and the protective effects of resveratrol in TNFα-induced vascular dysfunction. Resveratrol potently decreases TNFα mRNA and protein expression in diabetic mice aorta, suggesting profound anti-inflammatory effects of resveratrol in the macrovasculature in type 2 diabetes (Figure 1). As a potent inflammatory trigger, the central role of TNFα in vascular dysfunction has been demonstrated by the ability of agents that block the action of TNFα to treat a range of cardiovascular disorders and inflammatory conditions. Although anti-TNFα treatment has been extensively studied in vascular dysfunction accompanied by various inflammatory diseases such as rheumatoid arthritis and Crohn’s disease, the beneficial effects of TNFα inhibition have not been fully clarified in cardiovascular diseases and in vascular complications associated with type 2 diabetes.
Our results demonstrated for the first time that resveratrol administration improves aortic endothelial function in type 2 diabetic mice without affecting the dilator response in control mice. Although the Emax is only slightly decreased in diabetic mice, the EC50 is significantly higher in diabetic mice compared with control mice (Table II. Please see www.ahajournals.org), suggesting the aortas of Leprdb exhibit attenuated endothelium-dependent relaxation in response to ACh. ACh interacts with endothelial muscarinic M3 receptors and causes NO release and vasodilation.27 To rule out the possibility that the vascular dysfunction in Leprdb may be due to impaired muscarinic receptor activity, we examine adenosine-induced vasorelaxation, which is fully endothelial-dependent in murine aorta (data not shown). Our results reveal that diabetic mice show impaired adenosine-induced vasorelaxation and resveratrol restores vascular function (Figure IV. Please see www.ahajournals.org), which is consistent with the results of ACh-induced vasorelaxation. Furthermore, in dbTNF−/dbTNF−, ACh-induced vasorelaxation is greater than that of Leprdb and TNFα impairs endothelial function in m Leprdb control (Figure 3A). Although resveratrol restores endothelial function in diabetic mice, incubating the vessel with recombinant TNFα abolishes the improvement (Figure 3B). Therefore, TNFα appears to play a pivotal role in macrovascular dysfunction in type 2 diabetes and the vascular protective effects of resveratrol may be related to its TNFα inhibitory properties. Recently, long-term resveratrol treatment was also shown to improve aortic endothelial function in aged and high fat diet-induced obese mice.4 Furthermore, chronic resveratrol consumption improved endothelium-dependent vasorelaxation in hypertensive (but not normotensive) rats.28 These results suggest that resveratrol treatment has the potential to preserve vascular health when multiple cardiovascular risk factors are present.
Interestingly, in vitro administration of supra-physiological concentrations of resveratrol causes both endothelium-dependent and endothelium-independent relaxation of rat aortic ring preparations.29, 30 In endothelium-intact rings, resveratrol can dose-dependently inhibit the contractile response of the vasculature to noradrenaline. This inhibitory effect can be blocked by NO synthase inhibitor NG-nitro-L-arginine (L-NNA), suggesting an acute activation of NO formation/release induced by resveratrol. In addition, at higher concentrations resveratrol may also act directly on the vascular smooth muscle cells,29 likely by modulating the function of voltage-gated K+ (Kv) channels.30 Although direct vasoactive effects of resveratrol are likely not responsible for the improvement of endothelial function in the present study, they may contribute to the in vivo effects of chronic resveratrol treatment.
Role of Resveratrol in TNFα-induced NAD(P)H Oxidase Activation and Subsequent Oxidative/Nitrative Stress
There are multiple cellular sources of superoxide anion, including NAD(P)H oxidase, xanthine oxidase, the mitochondrial respiratory chain, and the arachidonic acid cascade (including lipoxygenase and cycloxygenase).31 Strong evidence supports the idea that activation of NAD(P)H oxidase contributes to vascular oxidative stress in virtually every forms of experimental diabetes.32, 33 Furthermore, inhibition of ROS generation by genetic depletion of gp91phox, by administration of dominant-negative Rac1, or by structurally different pharmacological inhibitors of NAD(P)H oxidase also significantly attenuate vascular O2.− production in diabetes.32, 33 Activation of NAD(P)H oxidase by TNFα is a main contributor to the generation of oxidative stress in coronary microcirculation in type 2 diabetes.9 In aortas from Leprdb, NAD(P)H oxidase activity is greatly elevated (Figure 4B). dbTNF−/dbTNF− shows decreased NAD(P)H oxidase activity (Figure 4B), and inhibiting NAD(P)H oxidase activation by apocynin (100 µmol/L) potently reduces vascular O2.− production (Figure 4A) as well as improving endothelial function in diabetic mice (Figure 3C), while a xanthine oxidase inhibitor allopurinol (10 µmol/L), or the mitochondrial respiratory chain inhibitor rotenone (1 µmol/L), did not improve aortic function (data not shown). Although a recent study posit that apocynin at high concentrations may exert direct antioxidant effects, at concentrations less than 1,000 µmol/L, apocynin did not scavenge O2.− produced by cell-free systems, as detected by either dihydroethidium (DHE) or lucigenin assays.34 In the present study we used apocynin at a concentration of 100 µM, which in the study by Heumüller et al did not scavenge O2.− in DHE assay. Thus, we attribute the finding that low concentrations of apocynin decreased O2.− production in the aorta of Leprdb mice to the inhibition of NADPH oxidases by apocynin.
In situ detection of O2.− in aortas by DHE staining shows that the increased O2.− production mainly accumulates in the adventitia layers (Figure 4A), which is consistent with previous work using different staining methods.35 Furthermore, immunohistochemical staining revealed specific labeling of the adventitia with NAD(P)H oxidase subunits gp91phox, p22phox, p47phox and p67phox, whereas no substantial staining was observed in other areas of the aorta.35 Our results reveal that gp91phox protein expression is elevated in Leprdb mice, suggesting the up-regulation of gp91phox in diabetic mice is associated with apocynin-inhibitable, NAD(P)H oxidase-derived O2.− production. Furthermore, resveratrol reduces gp91phox expression in Leprdb mice (Figure 4C). In light of previous results from studies using genetically altered mice and structurally different inhibitors of NADPH oxidase, our data support the conclusion that resveratrol-induced down-regulation of NAD(P)H oxidase expression contributes to the attenuation of NADPH oxidase-derived O2.− production and consequent improvement of endothelial function in the aorta of Leprdb mice.
The increase in vascular NAD(P)H oxidase-induced O2.− production may initiate profound oxidative/nitrative stress. On the one hand, inactivation of NO by O2.− anion is recognized to be a key mechanism underlying reduced NO bioavailability and the development of endothelial dysfunction.7 The interaction of O2.− and NO produces peroxynitrite (ONOO−), which leads to protein tyrosine nitration to generate nitrotyrosine.36 Nitrotyrosine was initially considered as a specific marker of peroxynitrite generation, but now is generally regarded as an index of reactive nitrogen species, rather than a specific indicator of peroxynitrite formation. Accumulating evidence suggests that diabetes is associated with increased nitrosative stress and peroxynitrite formation.37 Toxic actions of peroxynitrite and/or nitrotyrosine in the cardiovascular system are also supported by evidence showing that the degree of cell death and/or dysfunction correlates with levels of nitrotyrosine in endothelial cells, myocytes and fibroblasts from heart biopsies of diabetic patients.38,39 Nitrotyrosine content is higher in endothelial cells of microvasculature in diabetic patients, which correlates with endothelial dysfunction,40 suggesting that nitrotyrosine level is associated with diabetes-related endothelial dysfunction. On the other hand, O2.− can be dismutased spontaneously or by the catalytic effects of superoxide dismutase (SOD) to produce H2O2.41 We show that aortic N-Tyr protein content is elevated in diabetic mice and is significantly reduced by resveratrol treatment (Figure 6A). Furthermore, resveratrol also attenuated H2O2 levels measured in the serum of diabetic mice (Figure 6B), suggesting that resveratrol has potent anti-oxidative activities. Resveratrol also up-regulates natural antioxidant enzymes. Superoxide dismutase (SOD)-1 and glutathione peroxidase (GPx) protein expression are decreased in Leprdb and resveratrol up-regulated aortic SOD-1 and GPx protein expression, whereas SOD-3 and catalase protein expression were similar among groups (Figure III. Please see www.ahajournals.org). More interestingly, in dbTNF−/dbTNF−, SOD-1 and GPx protein expression were similar to Leprdb, indicating that the genetic deletion of TNFα in Leprdb did not affect the expression of antioxidant enzyme while resveratrol elevated antioxidant enzyme expression in Leprdb. These results may explain why aortic function in dbTNF−/dbTNF− fails to return to the levels of the control and resveratrol treated Leprdb.
Whether these antioxidant effects result primarily from a direct scavenging effect or are the result of the activation of pathways that up-regulate natural antioxidant defenses in the cells, or via the inhibition of oxidase enzymes, etc. is not yet clear. Because the resveratrol concentration in the plasma of treated mice was reported to be much lower42 than the resveratrol concentration needed in vitro to effectively scavenge free radicals (1 µmol/L to 100 µmol/L),6, 14, 43 we posit that direct anti-oxidant effects of resveratrol are likely play only a secondary role in vasoprotection.
Therefore, our results provide direct evidence that chronic resveratrol administration inhibits TNFα-induced NAD(P)H oxidase activation and subsequent vascular oxidative stress in vivo, as well as up-regulating antioxidant enzymes, which is postulated to be the beneficial effects of dietary supplement of resveratrol on vascular dysfunction in type 2 diabetes.
Role of Resveratrol in the Prevention of TNFα-induced Imapirment of NO Bioavailability
In rat aortic endothelial cells, resveratrol treatment (100 µmol/L, 16 hours) increased nitrite/nitrate level 2-fold. This effect was completely abolished by knockdown of SIRT1 with siRNA.6 Resveratrol inhibits the acetylation of endogenous eNOS on lysine residues in vitro, suggesting that SIRT1 activation may play a fundamental role in regulating endothelial NO and endothelium-dependent vascular tone by deacetylating eNOS.6 Our study examined the effects of resveratrol on the (Ser1177) phosphorylation of eNOS. Although total eNOS protein expression is not statistically different among groups, phospho-eNOS protein content is reduced in the aorta of diabetic mice (Figure 5B). Resveratrol treated Leprdb mice exhibit increased (Ser1177) phosphorylation of eNOS (Figure 5B), which is associated with an increased NO bioavailability, as evidenced by increased aortic nitrite/nitrate level (Figure 5A). TNFα plays an important role in regulating eNOS expression and activity, as evidenced by down-regulation of mRNA and protein expression of eNOS following TNFα stimulation in endothelial cells.44 TNFα also inhibits endothelial NO synthase gene promoter activity in bovine aortic endothelial cells.45 Therefore, TNFα exerts transcriptional, as well as post-transcriptional, effects on eNOS gene expression. Since dbTNF−/dbTNF− also shows elevated phospho-eNOS expression in aortas (Figure 5B), we postulate that resveratrol’s effects on enhancing eNOS phosphorylation and improving NO bioavailability may be related to its role in inhibiting TNFα expression.
Collectively, the vasoprotective activity of resveratrol against vascular oxidative stress and decreased NO bioavailability has been explained by the capacity of this polyphenol to inhibit the activation of vascular NAD(P)H oxidase and down-regulation of eNOS phosphorylation induced by TNFα. The detailed characterization of resveratrol’s therapeutic effects in vascular tissue may identify novel therapeutic approaches that modulate this system to ameliorate the heightened cardiovascular risk associated with obesity and type 2 diabetes. We believe that a better understanding of the mechanisms by which resveratrol attenuates vascular oxidative stress as well as improves NO bioavailability in diabetes will lead to novel pharmacological interventions using resveratrol to prevent or delay the development of vascular complications in type 2 diabetic patients.
Supplementary Material
Acknowledgments
None.
Funding Sources
This study was supported by grants from the American Diabetes Association (to ZU) and the American Heart Association (110350047A, to CZ) and NIH (RO1-HL077566 and RO1-HL085119, to CZ).
Footnotes
Subject Codes:
(91) Oxidant stress
(95) Endothelium/vascular type/nitric oxide
(190) Type 2 diabetes
Conflicts of Interest:
None.
Reference
- 1.Simopoulos AP. The Mediterranean diets: What is so special about the diet of Greece? The scientific evidence. J Nutr. 2001;131:3065S–3073S. doi: 10.1093/jn/131.11.3065S. [DOI] [PubMed] [Google Scholar]
- 2.Sander GE, Wilklow FE, Giles TD. Heart failure in diabetes mellitus: causal and treatment considerations. Minerva Cardioangiol. 2004;52:491–503. [PubMed] [Google Scholar]
- 3.Blann AD, Lip GY. The endothelium in atherothrombotic disease: assessment of function, mechanisms and clinical implications. Blood Coagul Fibrinolysis. 1998;9:297–306. [PubMed] [Google Scholar]
- 4.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008 doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah AM, Channon KM. Free radicals and redox signalling in cardiovascular disease. Heart. 2004;90:486–487. doi: 10.1136/hrt.2003.029389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griendling KK. NADPH oxidases: new regulators of old functions. Antioxid Redox Signal. 2006;8:1443–1445. doi: 10.1089/ars.2006.8.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 10.Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest. 2006;116:2791–2798. doi: 10.1172/JCI28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marier JF, Chen K, Prince P, Scott G, del Castillo JR, Vachon P. Production of ex vivo lipopolysaccharide-induced tumor necrosis factor-alpha, interleukin-1beta, and interleukin-6 is suppressed by trans-resveratrol in a concentration-dependent manner. Can J Vet Res. 2005;69:151–154. [PMC free article] [PubMed] [Google Scholar]
- 12.Ma ZH, Ma QY, Sha HC, Wang LC. [Effect of resveratrol on lipopolysaccharide-induced activation of rat peritoneal macrophages] Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:1363–1365. [PubMed] [Google Scholar]
- 13.Sharma S, Kulkarni SK, Chopra K. Effect of resveratrol, a polyphenolic phytoalexin, on thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Fundam Clin Pharmacol. 2007;21:89–94. doi: 10.1111/j.1472-8206.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 14.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi T, Taguchi K, Yasuhiro T, Matsumoto T, Kamata K. Impairment of PI3-K/Akt pathway underlies attenuated endothelial function in aorta of type 2 diabetic mouse model. Hypertension. 2004;44:956–962. doi: 10.1161/01.HYP.0000147559.10261.a7. [DOI] [PubMed] [Google Scholar]
- 16.Xie H, Ray PE, Short BL. NF-kappaB activation plays a role in superoxide-mediated cerebral endothelial dysfunction after hypoxia/reoxygenation. Stroke. 2005;36:1047–1052. doi: 10.1161/01.STR.0000157664.34308.cc. [DOI] [PubMed] [Google Scholar]
- 17.Chow SE, Hshu YC, Wang JS, Chen JK. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol. 2007;102:1520–1527. doi: 10.1152/japplphysiol.00881.2006. [DOI] [PubMed] [Google Scholar]
- 18.Bagi Z, Koller A, Kaley G. Superoxide-NO interaction decreases flow- and agonist-induced dilations of coronary arterioles in Type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003;285:H1404–H1410. doi: 10.1152/ajpheart.00235.2003. [DOI] [PubMed] [Google Scholar]
- 19.Berkels R, Purol-Schnabel S, Roesen R. A new method to measure nitrate/nitrite with a NO-sensitive electrode. J Appl Physiol. 2001;90:317–320. doi: 10.1152/jappl.2001.90.1.317. [DOI] [PubMed] [Google Scholar]
- 20.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 21.Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 22.Ninomiya Y, Shigemura N, Yasumatsu K, Ohta R, Sugimoto K, Nakashima K, Lindemann B. Leptin and sweet taste. Vitam Horm. 2002;64:221–248. doi: 10.1016/s0083-6729(02)64007-5. [DOI] [PubMed] [Google Scholar]
- 23.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 24.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E1339–E1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- 26.Hermansen K, Mortensen LS. Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf. 2007;30:1127–1142. doi: 10.2165/00002018-200730120-00005. [DOI] [PubMed] [Google Scholar]
- 27.Boulanger CM, Morrison KJ, Vanhoutte PM. Mediation by M3-muscarinic receptors of both endothelium-dependent contraction and relaxation to acetylcholine in the aorta of the spontaneously hypertensive rat. Br J Pharmacol. 1994;112:519–524. doi: 10.1111/j.1476-5381.1994.tb13104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rush JW, Quadrilatero J, Levy AS, Ford RJ. Chronic resveratrol enhances endothelium-dependent relaxation but does not alter eNOS levels in aorta of spontaneously hypertensive rats. Exp Biol Med (Maywood) 2007;232:814–822. [PubMed] [Google Scholar]
- 29.Chen CK, Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 1996;27:363–366. doi: 10.1016/0306-3623(95)02001-2. [DOI] [PubMed] [Google Scholar]
- 30.Novakovic A, Bukarica LG, Kanjuh V, Heinle H. Potassium channels-mediated vasorelaxation of rat aorta induced by resveratrol. Basic Clin Pharmacol Toxicol. 2006;99:360–364. doi: 10.1111/j.1742-7843.2006.pto_531.x. [DOI] [PubMed] [Google Scholar]
- 31.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 32.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 33.Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L, Duriez M, Vilar J, Brandes RP, Levy BI, Shah AM, Silvestre JS. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol. 2006;169:719–728. doi: 10.2353/ajpath.2006.060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 35.Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, Cohen RA. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998;82:810–818. doi: 10.1161/01.res.82.7.810. [DOI] [PubMed] [Google Scholar]
- 36.Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- 37.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 39.Pacher P, Szabo C. Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr Opin Pharmacol. 2006;6:136–141. doi: 10.1016/j.coph.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, Da Ros R, Motz E. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation. 2002;106:1211–1218. doi: 10.1161/01.cir.0000027569.76671.a8. [DOI] [PubMed] [Google Scholar]
- 41.Papa S, Skulachev VP. Reactive oxygen species, mitochondria, apoptosis and aging. Mol Cell Biochem. 1997;174:305–319. [PubMed] [Google Scholar]
- 42.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia Z, Liu M, Wu Y, Sharma V, Luo T, Ouyang J, McNeill H. N-acetylcysteine attenuates TNF-alpha-induced human vascular endothelial cell apoptosis and restores eNOS expression. Eur J Pharmacol. 2006;550:134–142. doi: 10.1016/j.ejphar.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 45.Anderson HD, Rahmutula D, Gardner DG. Tumor necrosis factor-alpha inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem. 2004;279:963–969. doi: 10.1074/jbc.M309552200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.