Abstract
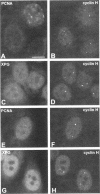
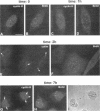
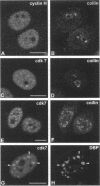
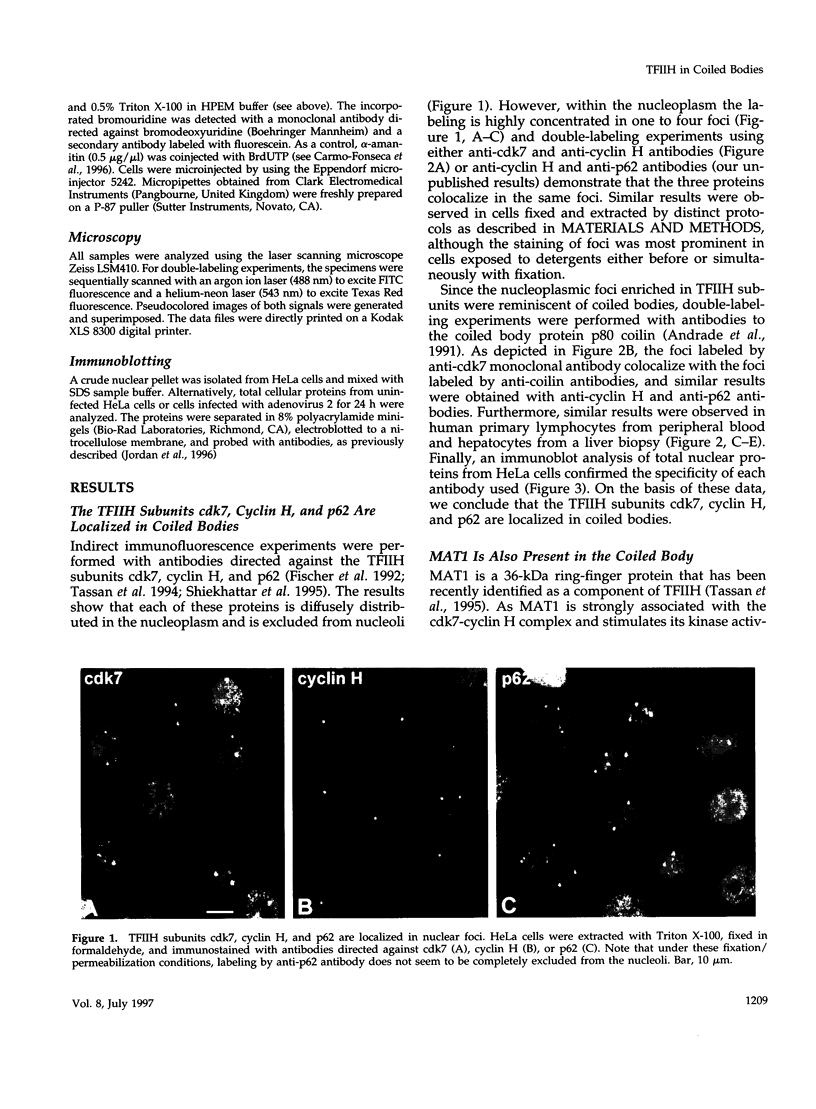
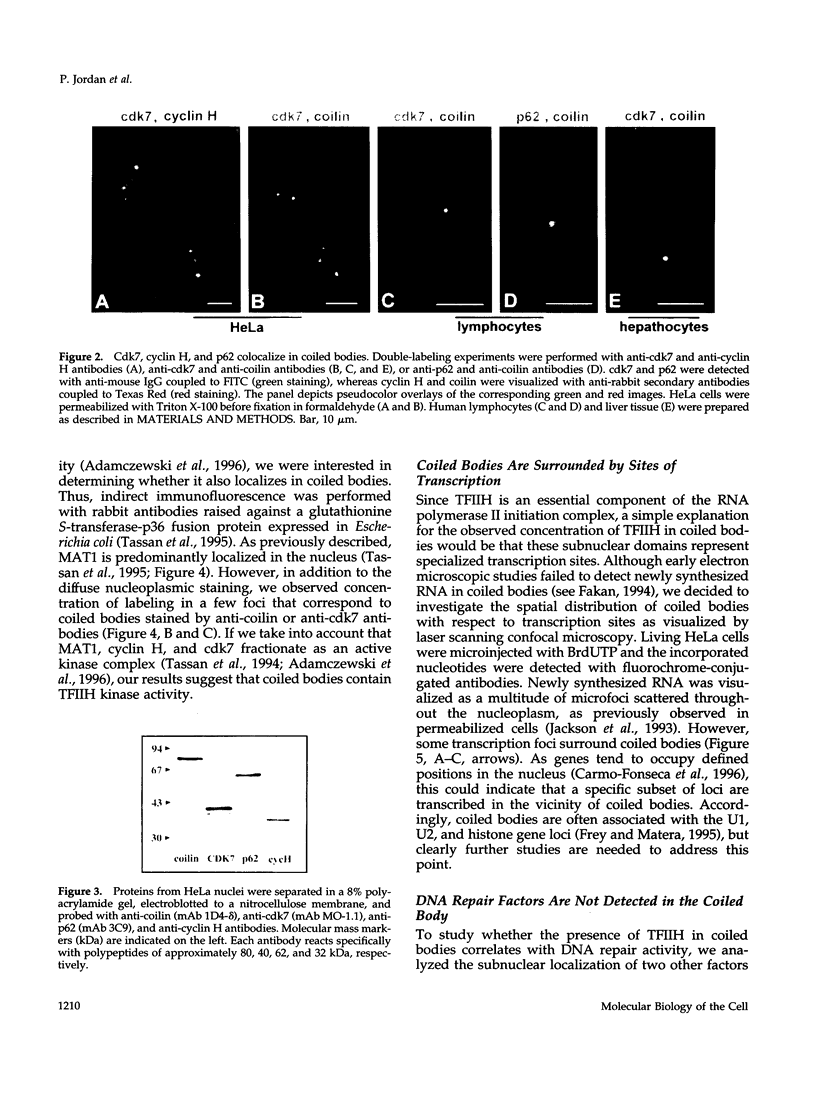
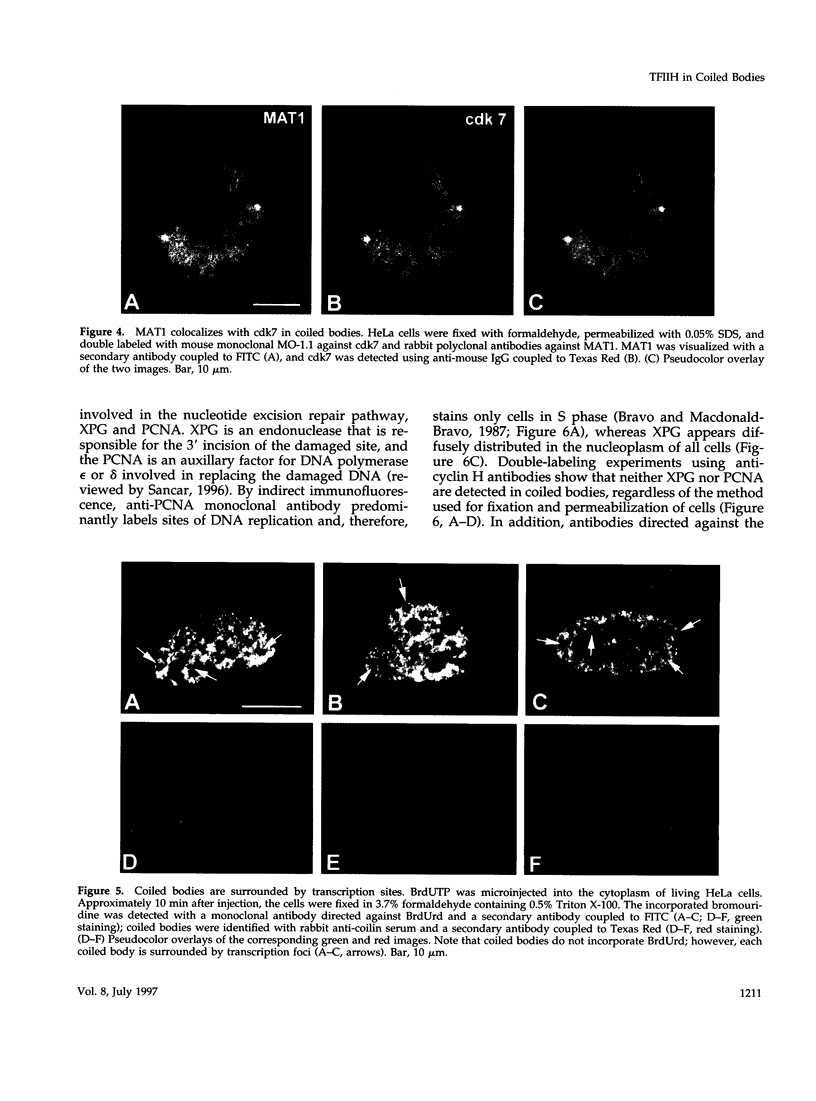
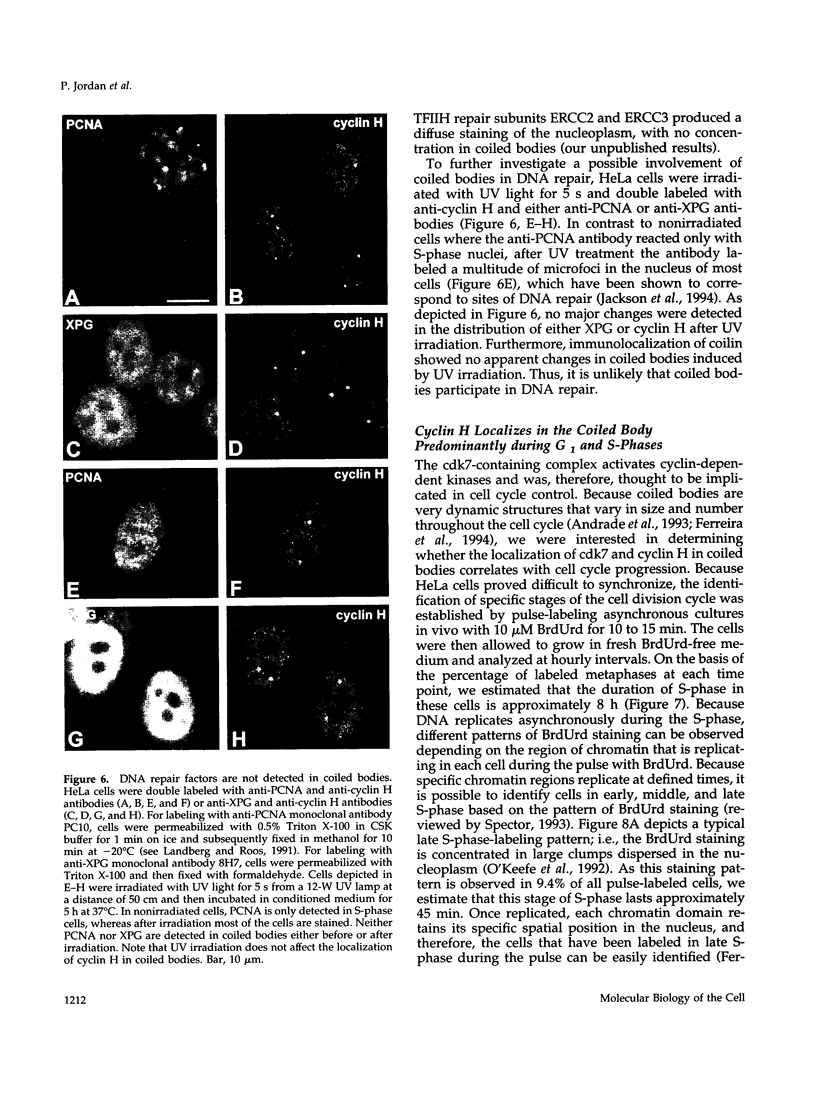
TFIIH is a general transcription factor for RNA polymerase II that in addition is involved in DNA excision repair. TFIIH is composed of eight or nine subunits and we show that at least four of them, namely cdk7, cyclin H, MAT1, and p62 are localized in the coiled body, a distinct subnuclear structure that is transcription dependent and highly enriched in small nuclear ribonucleoproteins. Although coiled bodies do not correspond to sites of transcription, in vivo incorporation of bromo-UTP shows that they are surrounded by transcription foci. Immunofluorescence analysis using antibodies directed against the essential repair factors proliferating cell nuclear antigen and XPG did not reveal labeling of the coiled body in either untreated cells or cells irradiated with UV light, arguing that coiled bodies are probably not involved in DNA repair mechanisms. The localization of cyclin H in the coiled body was predominantly detected during the G1 and S-phases of the cell cycle, whereas in G2 coiled bodies were very small or not detected. Finally, both cyclin H and cdk7 did not colocalize with P80 coilin after disruption of the coiled body, indicating that these proteins are specifically targeted to the small nuclear ribonucleoprotein-containing domain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamczewski J. P., Rossignol M., Tassan J. P., Nigg E. A., Moncollin V., Egly J. M. MAT1, cdk7 and cyclin H form a kinase complex which is UV light-sensitive upon association with TFIIH. EMBO J. 1996 Apr 15;15(8):1877–1884. [PMC free article] [PubMed] [Google Scholar]
- Andrade L. E., Chan E. K., Raska I., Peebles C. L., Roos G., Tan E. M. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J Exp Med. 1991 Jun 1;173(6):1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade L. E., Tan E. M., Chan E. K. Immunocytochemical analysis of the coiled body in the cell cycle and during cell proliferation. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1947–1951. doi: 10.1073/pnas.90.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D. W., Murphy C., Wu Z., Wu C. H., Gall J. G. In vitro assembly of coiled bodies in Xenopus egg extract. Mol Biol Cell. 1994 Jun;5(6):633–644. doi: 10.1091/mbc.5.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D. L. Regulation of transcriptional elongation by RNA polymerase II. Curr Opin Genet Dev. 1995 Apr;5(2):210–216. doi: 10.1016/0959-437x(95)80010-7. [DOI] [PubMed] [Google Scholar]
- Blangy A., Lane H. A., d'Hérin P., Harper M., Kress M., Nigg E. A. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995 Dec 29;83(7):1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Bohmann K., Ferreira J. A., Lamond A. I. Mutational analysis of p80 coilin indicates a functional interaction between coiled bodies and the nucleolus. J Cell Biol. 1995 Nov;131(4):817–831. doi: 10.1083/jcb.131.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmann K., Ferreira J., Santama N., Weis K., Lamond A. I. Molecular analysis of the coiled body. J Cell Sci Suppl. 1995;19:107–113. doi: 10.1242/jcs.1995.supplement_19.16. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol. 1987 Oct;105(4):1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Cunha C., Custódio N., Carvalho C., Jordan P., Ferreira J., Parreira L. The topography of chromosomes and genes in the nucleus. Exp Cell Res. 1996 Dec 15;229(2):247–252. doi: 10.1006/excr.1996.0367. [DOI] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Ferreira J., Lamond A. I. Assembly of snRNP-containing coiled bodies is regulated in interphase and mitosis--evidence that the coiled body is a kinetic nuclear structure. J Cell Biol. 1993 Feb;120(4):841–852. doi: 10.1083/jcb.120.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapkin R., Le Roy G., Cho H., Akoulitchev S., Reinberg D. Human cyclin-dependent kinase-activating kinase exists in three distinct complexes. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6488–6493. doi: 10.1073/pnas.93.13.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Warren S. L. A functional interaction between the carboxy-terminal domain of RNA polymerase II and pre-mRNA splicing. J Cell Biol. 1997 Jan 13;136(1):5–18. doi: 10.1083/jcb.136.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakan S. Perichromatin fibrils are in situ forms of nascent transcripts. Trends Cell Biol. 1994 Mar;4(3):86–90. doi: 10.1016/0962-8924(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Ferreira J. A., Carmo-Fonseca M., Lamond A. I. Differential interaction of splicing snRNPs with coiled bodies and interchromatin granules during mitosis and assembly of daughter cell nuclei. J Cell Biol. 1994 Jul;126(1):11–23. doi: 10.1083/jcb.126.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J., Carmo-Fonseca M. The biogenesis of the coiled body during early mouse development. Development. 1995 Feb;121(2):601–612. doi: 10.1242/dev.121.2.601. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L., Gerard M., Chalut C., Lutz Y., Humbert S., Kanno M., Chambon P., Egly J. M. Cloning of the 62-kilodalton component of basic transcription factor BTF2. Science. 1992 Sep 4;257(5075):1392–1395. doi: 10.1126/science.1529339. [DOI] [PubMed] [Google Scholar]
- Frey M. R., Matera A. G. Coiled bodies contain U7 small nuclear RNA and associate with specific DNA sequences in interphase human cells. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):5915–5919. doi: 10.1073/pnas.92.13.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C. Relationships between DNA repair and transcription. Annu Rev Biochem. 1996;65:15–42. doi: 10.1146/annurev.bi.65.070196.000311. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Egly J. M., Vermeulen W. TFIIH: a key component in multiple DNA transactions. Curr Opin Genet Dev. 1996 Feb;6(1):26–33. doi: 10.1016/s0959-437x(96)90006-4. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Hassan A. B., Errington R. J., Cook P. R. Sites in human nuclei where damage induced by ultraviolet light is repaired: localization relative to transcription sites and concentrations of proliferating cell nuclear antigen and the tumour suppressor protein, p53. J Cell Sci. 1994 Jul;107(Pt 7):1753–1760. doi: 10.1242/jcs.107.7.1753. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Hassan A. B., Errington R. J., Cook P. R. Visualization of focal sites of transcription within human nuclei. EMBO J. 1993 Mar;12(3):1059–1065. doi: 10.1002/j.1460-2075.1993.tb05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-García L. F., Green S. R., Mathews M. B., Spector D. L. Organization of the double-stranded RNA-activated protein kinase DAI and virus-associated VA RNAI in adenovirus-2-infected HeLa cells. J Cell Sci. 1993 Sep;106(Pt 1):11–22. doi: 10.1242/jcs.106.1.11. [DOI] [PubMed] [Google Scholar]
- Jiménez-García L. F., Segura-Valdez M. L., Ochs R. L., Rothblum L. I., Hannan R., Spector D. L. Nucleologenesis: U3 snRNA-containing prenucleolar bodies move to sites of active pre-rRNA transcription after mitosis. Mol Biol Cell. 1994 Sep;5(9):955–966. doi: 10.1091/mbc.5.9.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P., Mannervik M., Tora L., Carmo-Fonseca M. In vivo evidence that TATA-binding protein/SL1 colocalizes with UBF and RNA polymerase I when rRNA synthesis is either active or inactive. J Cell Biol. 1996 Apr;133(2):225–234. doi: 10.1083/jcb.133.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond A. I., Carmo-Fonseca M. The coiled body. Trends Cell Biol. 1993 Jun;3(6):198–204. doi: 10.1016/0962-8924(93)90214-l. [DOI] [PubMed] [Google Scholar]
- Landberg G., Roos G. Antibodies to proliferating cell nuclear antigen as S-phase probes in flow cytometric cell cycle analysis. Cancer Res. 1991 Sep 1;51(17):4570–4574. [PubMed] [Google Scholar]
- Linné T., Jörnvall H., Philipson L. Purification and characterization of the phosphorylated DNA-binding protein from adenovirus-type-2-infected cells. Eur J Biochem. 1977 Jun 15;76(2):481–490. doi: 10.1111/j.1432-1033.1977.tb11618.x. [DOI] [PubMed] [Google Scholar]
- Maldonado E., Reinberg D. News on initiation and elongation of transcription by RNA polymerase II. Curr Opin Cell Biol. 1995 Jun;7(3):352–361. doi: 10.1016/0955-0674(95)80090-5. [DOI] [PubMed] [Google Scholar]
- McCracken S., Fong N., Yankulov K., Ballantyne S., Pan G., Greenblatt J., Patterson S. D., Wickens M., Bentley D. L. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature. 1997 Jan 23;385(6614):357–361. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]
- Meier U. T., Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol. 1994 Dec;127(6 Pt 1):1505–1514. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O. Principles of CDK regulation. Nature. 1995 Mar 9;374(6518):131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. Cyclin-dependent kinase 7: at the cross-roads of transcription, DNA repair and cell cycle control? Curr Opin Cell Biol. 1996 Jun;8(3):312–317. doi: 10.1016/s0955-0674(96)80003-2. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995 Jun;17(6):471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- O'Donovan A., Scherly D., Clarkson S. G., Wood R. D. Isolation of active recombinant XPG protein, a human DNA repair endonuclease. J Biol Chem. 1994 Jun 10;269(23):15965–15968. [PubMed] [Google Scholar]
- O'Keefe R. T., Henderson S. C., Spector D. L. Dynamic organization of DNA replication in mammalian cell nuclei: spatially and temporally defined replication of chromosome-specific alpha-satellite DNA sequences. J Cell Biol. 1992 Mar;116(5):1095–1110. doi: 10.1083/jcb.116.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raska I., Andrade L. E., Ochs R. L., Chan E. K., Chang C. M., Roos G., Tan E. M. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991 Jul;195(1):27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Raska I., Ochs R. L., Andrade L. E., Chan E. K., Burlingame R., Peebles C., Gruol D., Tan E. M. Association between the nucleolus and the coiled body. J Struct Biol. 1990 Jul-Sep;104(1-3):120–127. doi: 10.1016/1047-8477(90)90066-l. [DOI] [PubMed] [Google Scholar]
- Reardon J. T., Ge H., Gibbs E., Sancar A., Hurwitz J., Pan Z. Q. Isolation and characterization of two human transcription factor IIH (TFIIH)-related complexes: ERCC2/CAK and TFIIH. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6482–6487. doi: 10.1073/pnas.93.13.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebelo L., Almeida F., Ramos C., Bohmann K., Lamond A. I., Carmo-Fonseca M. The dynamics of coiled bodies in the nucleus of adenovirus-infected cells. Mol Biol Cell. 1996 Jul;7(7):1137–1151. doi: 10.1091/mbc.7.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R., Adamczewski J. P., Seroz T., Vermeulen W., Tassan J. P., Schaeffer L., Nigg E. A., Hoeijmakers J. H., Egly J. M. The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell. 1994 Dec 16;79(6):1093–1101. doi: 10.1016/0092-8674(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Sancar A. DNA excision repair. Annu Rev Biochem. 1996;65:43–81. doi: 10.1146/annurev.bi.65.070196.000355. [DOI] [PubMed] [Google Scholar]
- Shiekhattar R., Mermelstein F., Fisher R. P., Drapkin R., Dynlacht B., Wessling H. C., Morgan D. O., Reinberg D. Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature. 1995 Mar 16;374(6519):283–287. doi: 10.1038/374283a0. [DOI] [PubMed] [Google Scholar]
- Spector D. L. Macromolecular domains within the cell nucleus. Annu Rev Cell Biol. 1993;9:265–315. doi: 10.1146/annurev.cb.09.110193.001405. [DOI] [PubMed] [Google Scholar]
- Tassan J. P., Jaquenoud M., Fry A. M., Frutiger S., Hughes G. J., Nigg E. A. In vitro assembly of a functional human CDK7-cyclin H complex requires MAT1, a novel 36 kDa RING finger protein. EMBO J. 1995 Nov 15;14(22):5608–5617. doi: 10.1002/j.1460-2075.1995.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassan J. P., Schultz S. J., Bartek J., Nigg E. A. Cell cycle analysis of the activity, subcellular localization, and subunit composition of human CAK (CDK-activating kinase). J Cell Biol. 1994 Oct;127(2):467–478. doi: 10.1083/jcb.127.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinczek B., Robert-Nicoud M., Schwoch G. In situ localization of cAMP-dependent protein kinases in nuclear and chromosomal substructures: relation to transcriptional activity. Eur J Cell Biol. 1993 Feb;60(1):196–202. [PubMed] [Google Scholar]
- Tuma R. S., Stolk J. A., Roth M. B. Identification and characterization of a sphere organelle protein. J Cell Biol. 1993 Aug;122(4):767–773. doi: 10.1083/jcb.122.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Buratowski S., Svejstrup J. Q., Feaver W. J., Wu X., Kornberg R. D., Donahue T. F., Friedberg E. C. The yeast TFB1 and SSL1 genes, which encode subunits of transcription factor IIH, are required for nucleotide excision repair and RNA polymerase II transcription. Mol Cell Biol. 1995 Apr;15(4):2288–2293. doi: 10.1128/mcb.15.4.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Murphy C., Wu C. H., Tsvetkov A., Gall J. G. Snurposomes and coiled bodies. Cold Spring Harb Symp Quant Biol. 1993;58:747–754. doi: 10.1101/sqb.1993.058.01.082. [DOI] [PubMed] [Google Scholar]
- Zawel L., Reinberg D. Common themes in assembly and function of eukaryotic transcription complexes. Annu Rev Biochem. 1995;64:533–561. doi: 10.1146/annurev.bi.64.070195.002533. [DOI] [PubMed] [Google Scholar]