Abstract
To gain insight into the process of mitochondrial transmission in yeast, we directly labeled mitochondrial proteins and mitochondrial DNA (mtDNA) and observed their fate after the fusion of two cells. To this end, mitochondrial proteins in haploid cells of opposite mating type were labeled with different fluorescent dyes and observed by fluorescence microscopy after mating of the cells. Parental mitochondrial protein markers rapidly redistributed and colocalized throughout zygotes, indicating that during mating, parental mitochondria fuse and their protein contents intermix, consistent with results previously obtained with a single parentally derived protein marker. Analysis of the three-dimensional structure and dynamics of mitochondria in living cells with wide-field fluorescence microscopy indicated that mitochondria form a single dynamic network, whose continuity is maintained by a balanced frequency of fission and fusion events. Thus, the complete mixing of mitochondrial proteins can be explained by the formation of one continuous mitochondrial compartment after mating. In marked contrast to the mixing of parental mitochondrial proteins after fusion, mtDNA (labeled with the thymidine analogue 5-bromodeoxyuridine) remained distinctly localized to one half of the zygotic cell. This observation provides a direct explanation for the genetically observed nonrandom patterns of mtDNA transmission. We propose that anchoring of mtDNA within the organelle is linked to an active segregation mechanism that ensures accurate inheritance of mtDNA along with the organelle.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Hiraoka Y., Shaw P., Sedat J. W. Fluorescence microscopy in three dimensions. Methods Cell Biol. 1989;30:353–377. doi: 10.1016/s0091-679x(08)60986-3. [DOI] [PubMed] [Google Scholar]
- Azpiroz R., Butow R. A. Patterns of mitochondrial sorting in yeast zygotes. Mol Biol Cell. 1993 Jan;4(1):21–36. doi: 10.1091/mbc.4.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J. S. New alleles of mgm1: a gene encoding a protein with a GTP-binding domain related to dynamin. Curr Genet. 1995 Oct;28(5):499–501. doi: 10.1007/BF00310822. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J., Vöth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 1994 Feb 15;27(3):198–219. doi: 10.1002/jemt.1070270303. [DOI] [PubMed] [Google Scholar]
- Burgess S. M., Delannoy M., Jensen R. E. MMM1 encodes a mitochondrial outer membrane protein essential for establishing and maintaining the structure of yeast mitochondria. J Cell Biol. 1994 Sep;126(6):1375–1391. doi: 10.1083/jcb.126.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Dabora S. L., Sheetz M. P. The microtubule-dependent formation of a tubulovesicular network with characteristics of the ER from cultured cell extracts. Cell. 1988 Jul 1;54(1):27–35. doi: 10.1016/0092-8674(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Dien B. S., Srienc F. Bromodeoxyuridine labeling and flow cytometric identification of replicating Saccharomyces cerevisiae cells: lengths of cell cycle phases and population variability at specific cell cycle positions. Biotechnol Prog. 1991 Jul-Aug;7(4):291–298. doi: 10.1021/bp00010a001. [DOI] [PubMed] [Google Scholar]
- Eakle K. A., Bernstein M., Emr S. D. Characterization of a component of the yeast secretion machinery: identification of the SEC18 gene product. Mol Cell Biol. 1988 Oct;8(10):4098–4109. doi: 10.1128/mcb.8.10.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K., Farh L., Marshall T. K., Deschenes R. J. Normal mitochondrial structure and genome maintenance in yeast requires the dynamin-like product of the MGM1 gene. Curr Genet. 1993 Jul-Aug;24(1-2):141–148. doi: 10.1007/BF00324678. [DOI] [PubMed] [Google Scholar]
- Hayashi J., Takemitsu M., Goto Y., Nonaka I. Human mitochondria and mitochondrial genome function as a single dynamic cellular unit. J Cell Biol. 1994 Apr;125(1):43–50. doi: 10.1083/jcb.125.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann G. J., King E. J., Shaw J. M. The yeast gene, MDM20, is necessary for mitochondrial inheritance and organization of the actin cytoskeleton. J Cell Biol. 1997 Apr 7;137(1):141–153. doi: 10.1083/jcb.137.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
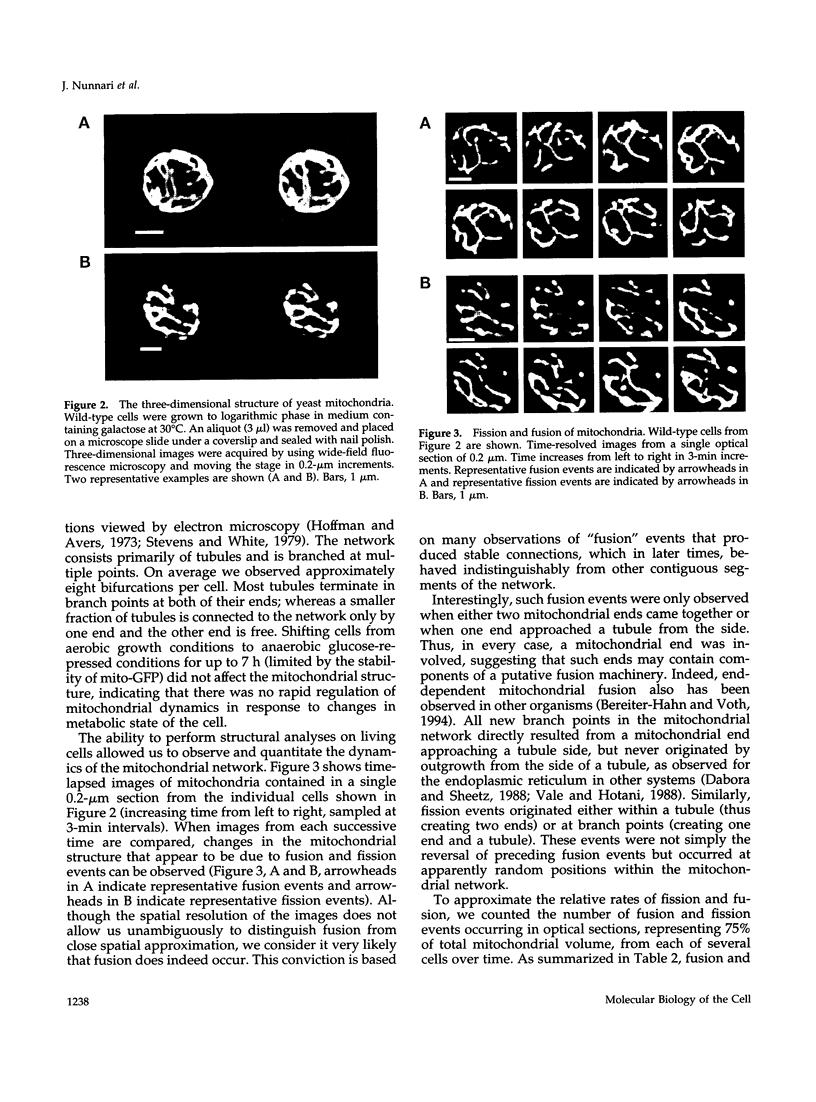
- Hoffmann H. P., Avers C. J. Mitochondrion of yeast: ultrastructural evidence for one giant, branched organelle per cell. Science. 1973 Aug 24;181(4101):749–751. doi: 10.1126/science.181.4101.749. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
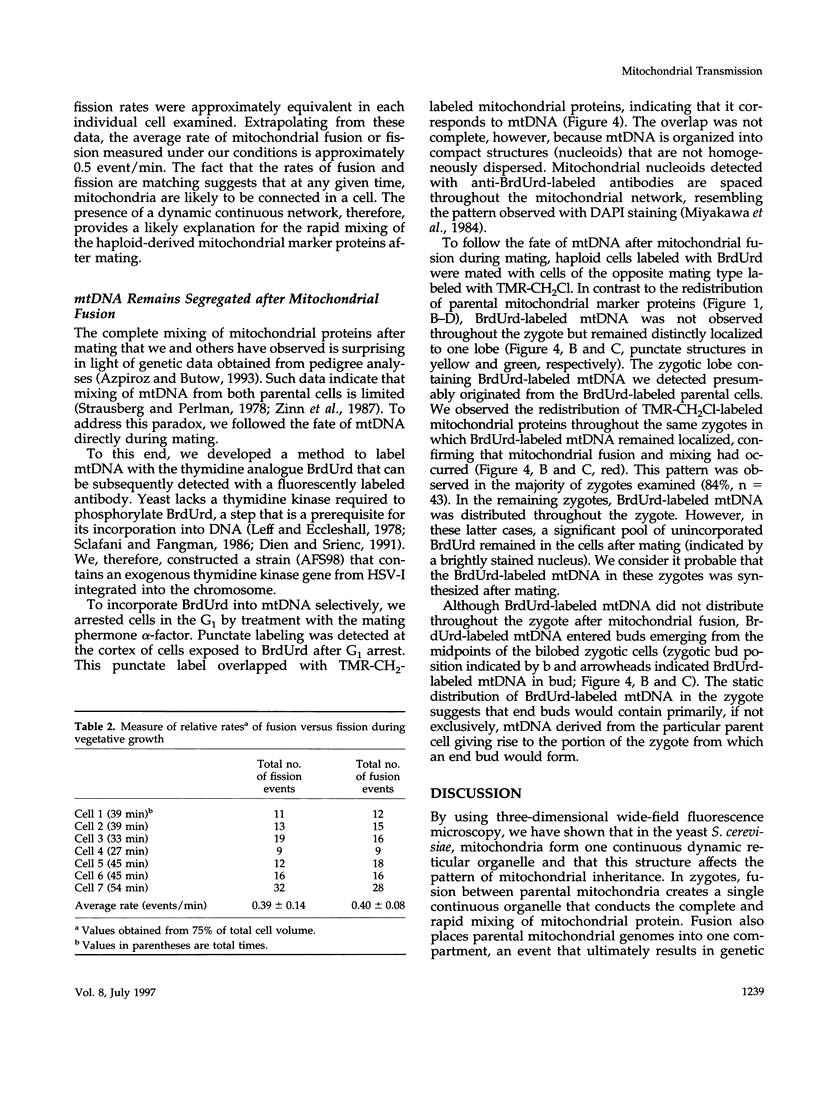
- Jones B. A., Fangman W. L. Mitochondrial DNA maintenance in yeast requires a protein containing a region related to the GTP-binding domain of dynamin. Genes Dev. 1992 Mar;6(3):380–389. doi: 10.1101/gad.6.3.380. [DOI] [PubMed] [Google Scholar]
- Latterich M., Fröhlich K. U., Schekman R. Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell. 1995 Sep 22;82(6):885–893. doi: 10.1016/0092-8674(95)90268-6. [DOI] [PubMed] [Google Scholar]
- Leff J., Eccleshall T. R. Replication of bromodeoxyuridylate-substituted mitochondrial DNA in yeast. J Bacteriol. 1978 Aug;135(2):436–444. doi: 10.1128/jb.135.2.436-444.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell S. J., Stewart L. C., Talin A., Yaffe M. P. Temperature-sensitive yeast mutants defective in mitochondrial inheritance. J Cell Biol. 1990 Sep;111(3):967–976. doi: 10.1083/jcb.111.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell S. J., Yaffe M. P. Intermediate filament formation by a yeast protein essential for organelle inheritance. Science. 1993 Apr 30;260(5108):687–689. doi: 10.1126/science.8480179. [DOI] [PubMed] [Google Scholar]
- McConnell S. J., Yaffe M. P. Nuclear and mitochondrial inheritance in yeast depends on novel cytoplasmic structures defined by the MDM1 protein. J Cell Biol. 1992 Jul;118(2):385–395. doi: 10.1083/jcb.118.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa I., Aoi H., Sando N., Kuroiwa T. Fluorescence microscopic studies of mitochondrial nucleoids during meiosis and sporulation in the yeast, Saccharomyces cerevisiae. J Cell Sci. 1984 Mar;66:21–38. doi: 10.1242/jcs.66.1.21. [DOI] [PubMed] [Google Scholar]
- Nunnari J., Fox T. D., Walter P. A mitochondrial protease with two catalytic subunits of nonoverlapping specificities. Science. 1993 Dec 24;262(5142):1997–2004. doi: 10.1126/science.8266095. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., Gull K. Basal body movements as a mechanism for mitochondrial genome segregation in the trypanosome cell cycle. Nature. 1991 Aug 22;352(6337):731–733. doi: 10.1038/352731a0. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., Sherwin T., Ploubidou A., Byard E. H., Gull K. Microtubule polarity and dynamics in the control of organelle positioning, segregation, and cytokinesis in the trypanosome cell cycle. J Cell Biol. 1995 Mar;128(6):1163–1172. doi: 10.1083/jcb.128.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena M., Picard D., Yamamoto K. R. Vectors for constitutive and inducible gene expression in yeast. Methods Enzymol. 1991;194:389–398. doi: 10.1016/0076-6879(91)94029-c. [DOI] [PubMed] [Google Scholar]
- Sclafani R. A., Fangman W. L. Thymidine utilization by tut mutants and facile cloning of mutant alleles by plasmid conversion in S. cerevisiae. Genetics. 1986 Nov;114(3):753–767. doi: 10.1093/genetics/114.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon V. R., Swayne T. C., Pon L. A. Actin-dependent mitochondrial motility in mitotic yeast and cell-free systems: identification of a motor activity on the mitochondrial surface. J Cell Biol. 1995 Jul;130(2):345–354. doi: 10.1083/jcb.130.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo L. F., Yaffe M. P. Regulation of mitochondrial morphology and inheritance by Mdm10p, a protein of the mitochondrial outer membrane. J Cell Biol. 1994 Sep;126(6):1361–1373. doi: 10.1083/jcb.126.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B. J., White J. G. Computer reconstruction of mitochondria from yeast. Methods Enzymol. 1979;56:718–728. doi: 10.1016/0076-6879(79)56064-9. [DOI] [PubMed] [Google Scholar]
- Strausberg R. L., Perlman P. S. The effect of zygotic bud position on the transmission of mitochondrial genes in Saccharomyces cerevisiae. Mol Gen Genet. 1978 Jul 11;163(2):131–144. doi: 10.1007/BF00267404. [DOI] [PubMed] [Google Scholar]
- Thorsness P. E., White K. H., Ong W. C. AFG2, an essential gene in yeast, encodes a new member of the Sec18p, Pas1p, Cdc48p, TBP-1 family of putative ATPases. Yeast. 1993 Nov;9(11):1267–1271. doi: 10.1002/yea.320091114. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Hotani H. Formation of membrane networks in vitro by kinesin-driven microtubule movement. J Cell Biol. 1988 Dec;107(6 Pt 1):2233–2241. doi: 10.1083/jcb.107.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Wickner W. Organelle inheritance. Cell. 1996 Feb 9;84(3):395–400. doi: 10.1016/s0092-8674(00)81284-2. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Wilcox C. A., Flynn G. C., Chen E., Kuang W. J., Henzel W. J., Block M. R., Ullrich A., Rothman J. E. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989 Jun 1;339(6223):355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- Zinn A. R., Pohlman J. K., Perlman P. S., Butow R. A. Kinetic and segregational analysis of mitochondrial DNA recombination in yeast. Plasmid. 1987 May;17(3):248–256. doi: 10.1016/0147-619x(87)90033-3. [DOI] [PubMed] [Google Scholar]







