Abstract
Hyperlipidemia is one of the most important risk factors for atherosclerosis. This can be amplified by a localized inflammatory response mediated by macrophages. Macrophages are capable of taking up excess cholesterol, and it is well known that delivery of cholesterol to the mitochondria by steroidogenic acute regulatory (StAR) protein is the rate-limiting step for cholesterol degradation in the liver. It has also been shown that overexpression of StAR in hepatocytes dramatically increases the amount of regulatory oxysterols in the nucleus, which play an important role in the maintenance of intracellular lipid homeostasis. The goal of the present studywas to determine whether StAR plays a similar role in macrophages. We have found that overexpression of StAR in human THP-1 monocyte-derived macrophages decreases intracellular lipid levels, activates liver X receptor alpha (LXRα) and proliferation peroxysome activator receptor gamma (PPARγ), and increases ABCG1 and CYP27A1 expression. Furthermore, it reduces the secretion of inflammatory factors, and prevents apoptosis. These results suggest that StAR delivers cholesterol to mitochondria where regulatory oxysterols are generated. Regulatory oxysterols can in turn activate nuclear receptors, which increase expression of cholesterol efflux transporters, and decrease secretion of inflammatory factors. These effects can prevent macrophage apoptosis. These results imply a potential role of StAR in the prevention of atherosclerosis.
Keywords: Steroidogenic acute regulatory protein, Macrophage, Cholesterol, ABCG1, ABCA1, Mitochondria, Oxysterol
1. Introduction
Macrophages play a key role in both the initiation and progression of atherosclerosis. Macrophages in arterial walls uptake lipoproteins, especially oxidized low-density lipoprotein (ox-LDL), and accumulate cholesterol and other neutral lipids resulting in foam cell formation, which is considered a critical process in the development of atherosclerosis [1,2]. The factors regulating net accumulation or elimination of lipids from macrophages are critical in formation of the early atherosclerotic lesion and progression to the chronic stage of atherosclerosis. To stimulate the pathways involved in decreasing lipids in macrophages may be an effective way to prevent atherosclerosis.
Several studies have shown that the processes of uptake and/or efflux of cholesterol from macrophages are regulated by nuclear receptors, including liver X receptors (LXRs) and proliferation peroxysome activator receptors (PPARs). Expression of a number of ATP-binding cassette transporters (ABCs), such as ABCA1, ABCG1, and ABCG4 involved in lipid efflux are regulated through activation of LXRs [3]. ABC transporters have been implicated in macrophage reverse cholesterol transport (RCT) and atherosclerosis [4], while PPARs have been reported to coordinate the effect of LXRs [5,6] and play an important role in inflammatory response through interference with pro-inflammatory transcription factor pathways [7].
Oxysterols are well known as physiological ligands for LXRs [8]. 22-Hydroxycholesterol and 27-hydroxycholesterol synthesized by mitochondrial sterol 27-hydroxylase (CYP27A1) are able to activate LXRs as endogenous ligands in cholesterol-loaded cells [9,8]. CYP27A1 activity has been reported to play a fundamental role in the maintenance of intracellular lipid homeostasis in vitro and in vivo [10,11]. Recent studies have shown that mitochondrial cholesterol delivery by steroidogenic acute regulatory (StAR) protein is the rate-limiting step for CYP27A1 activity [12–14]. Overexpression of StAR can increase the activity of CYP27A1 to produce regulatory oxysterols that appear capable of regulating intracellular lipid homeostasis [13]. More recent studies have shown that overexpression of StAR in primary human and rat hepatocytes dramatically increases oxysterols in hepatocyte nuclei. These oxysterols are potent regulators involved in intracellular lipid metabolism [15–17]. Our previous work showed that StAR mRNA and protein also expressed in macrophages [18]. However, the physiological significance of StAR in maintenance of cholesterol homeostasis in macrophages remains unknown.
In the present study, we show that overexpression of StAR decreases intracellular lipid levels and the secretion of inflammatory factors. These results suggest that the mitochondrial cholesterol delivery protein StAR plays an important role in lipid metabolism and the inflammatory response in human THP-1 derived macrophages.
2. Materials and methods
All cell culture media and additives, TRIZOL reagent and SuperScript TMIII First-Strand Synthesis System for RT-PCR were purchased from Invitrogen (Carlsbad, CA). SYBR® Green real-time PCR Master Mix was from Toyobo Company (Osaka, JP). The primary antibodies against StAR and β-actin were purchased from Abcam (Cambridge, MA). ABCA1 and Histone H4 antibodies were from United States Biological (Swampscott, MA) and Cell Signaling Technology, Inc. (Boston, MA), respectively. ABCG1, CYP27A1, PPARγ and LXRα antibodies were purchased from SantaCruz Biotechnology, Inc. (Santa Cruz, CA). Secondary antibodies against rabbit, goat, and mouse IgG were obtained from Kirkegaard & Perry Laboratories (Guildford, UK). Cytokine ELISAassay and in situ apoptosis detection kits were from R&D Systems, Inc. (Minneapolis, MN). Lipoproteins were prepared from 80 ml of blood from healthy donors by gradient ultracentrifugation as previously described [19]. All other reagents were from Sigma-Aldrich Chemical Co (St. Louis, MO) unless otherwise indicated.
2.1. Cell culture and macrophage differentiation
The human THP-1 monocytes were purchased from American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI 1640 media containing 10% (v/v) fetal calf serum, 100 U/ml of penicillin, and 100µg/ml of streptomycin, in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. THP-1 cells were induced to undergo macrophages differentiation by adding 160 nM phorbol 12-myristate 13-acetate (PMA) to the culture media and culturing for 48 h.
To illustrate the relationship of StAR and LXRα, cells were preincubated with or without LXRα antagonist 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS, 100µM) [20] for 30min followed by incubation with LXRα agonists 22(R)-hydroxycholesterol (22RHC, 10µM) or 27-hydroxycholesterol (27HC, 5µM) for various times as indicated in the text. Specific protein levels in the cell lysates were analyzed by Western blot analysis.
2.2. Infection of cells with adenovirus encoding human StAR
After macrophages differentiation, the cells were then infected with recombinant adenovirus encoding Adv-CMV-StAR at a multiplicity of 100 pfu/cell as previously described [21]. No virus control (Cont) and a virus encoding enhanced green-fluorescence protein (Adv-CMV-EGFP) were used as controls.
The recombinant adenoviruses encoding StAR were prepared as described [22]. The control adenoviruses expressing the enhanced green fluorescence protein (EGFP) were purchased from Vector Gene Technology Company Ltd. (Beijing, China).
2.3. Macrophage neutral lipid and total intracellular cholesterol analysis
Two hours post-infection, the media were replaced with fresh serum-free RPMI 1640 media containing 0.1% BSA with or without 50µg/ml of ox-LDL. After another 24 h, neutral lipids in the cells were either stained with oil-red O or extracted as previously described [23]. The relative lipid levels were examined by light microscopy and determined by spectrophotometer at OD518 nm after oil-red O was extracted into isopropanol. The total cellular cholesterol levels were determined by a commercial kit (Rongsheng Biotechnology Company Ltd., Shanghai, China) following the lipid extraction and the data was normalized by cellular total protein.
2.4. Determination of total mRNA by real-time fluorescent quantitative RT-PCR
Total RNA was extracted using the TRIZOL reagent according to the supplier’s instructions. Specific mRNA levels were determined by real-time RT-PCR as previously described [24]. The primer sets used in these assays are shown in Supplemental Table 1.
2.5. Determination of relative protein levels
After infection or treatment, cells were harvested in radioimmune precipitation assay (RIPA) lysis buffer, or subcellular fractions were separated and isolated by centrifugation as previously described [25]. Thirty micrograms of solubilized total protein were loaded onto a 6% SDS-PAGE to detect ABCG1 or ABCA1 and 10% SDS-PAGE to detect CYP27A1 and PPARγ, using β-actin as a protein loading control. Twenty micrograms of mitochondrial protein were loaded as above for detection of StAR, as well as 20µg nuclear protein for detection of activated LXRα using Histone H4 (HisH4) as a protein loading control, respectively. Western blots were performed as previously described [24].
2.6. ELISA assay for cytokines
The THP-1 derived macrophages were infected as described above and cell culture media were collected following addition of lipopolysaccharide (LPS, 10 ng/ml) for 16 h. Levels of IL-1β, TNFα, and IL-6 were determined by ELISA assay according to the manufacturer’s instructions.
2.7. Determination of apoptotic cells by terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling (TUNEL) assay
THP-1 cells were seeded on sterile coverslips in a 24-well plate at a density of 5 × 104 cells/well, differentiated, and infected as described above. Media were replaced with fresh serum-free media with or without 50µg/ml ox-LDL. After another 24 h, the in situ apoptotic cells were detected by TUNEL assay according to the manufacturer’s instructions. Five random fields per condition were photographed using a Leica, DM IL light microscopy (Wetzlar, Germany). For quantification purposes, an area containing approximately 100 cells under 40× magnification was selected and the positive-staining cells were counted.
2.8. Statistic analysis
Data are reported as mean ± standard deviation (S.D.) and subjected to one-way analysis of variance (ANOVA) analysis. Statistical significance is defined as P < 0.05.
3. Results
3.1. Recombinant StAR gene was successfully overexpressed by transfection of recombinant adenovirus
Infection of THP-1 derived macrophages with recombinant adenovirus containing a CMV-driven gene encoding StAR produced high StAR mRNA and protein levels without inducing any evidence of cell toxicity. Real-time RT-PCR analysis showed that infection with Adv-CMV-StAR increased StAR mRNA levels by 130-fold in the cells (Supplemental Fig. 1A). Western blot analysis of mitochondrial proteins showed one major immunoreactive band with a molecular weight of 30 kDa (lower bands) and a second band with the molecular weight of 37 kDa (upper bands) after infection, which is consistent with StAR mature protein as shown in the upper panel of Supplemental Fig. 1B. It showed that the StAR protein levels were increased about 35-fold by infecting with Adv-CMV-StAR. A summary of the data normalized to β-Actin is shown in the lower panel of Supplemental Fig. 1B.
3.2. Effect of StAR overexpression on intracellular lipid levels in THP-1 derived macrophages
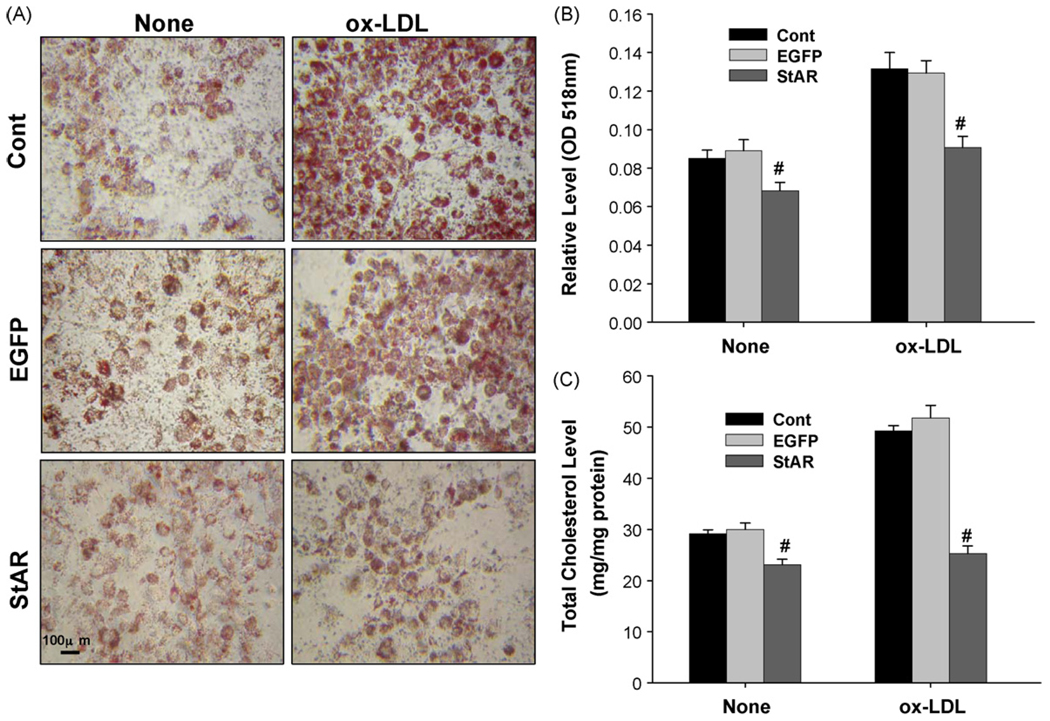
The effect of overexpressed StAR protein on intracellular neutral lipid levels was determined by oil-red O staining and total cell cholesterolwas determined by a commercial kit. Overexpression of StAR significantly decreased the neutral lipids levels after adding of 50µg/ml ox-LDL for 24 h following adenovirus Fig. 1A shows representative images for each condition, and Fig. 1B showed the quantitative levels of extracted oil-red O. Total cholesterol was also decreased by about 50% in StAR infected cells with ox-LDL compared to the controls (Fig. 1C).
Fig. 1.
Effect of StAR overexpression on intracellular neutral lipids in THP-1 derived macrophages. THP-1 derived macrophages were incubated with or without 50µg/ml ox-LDL for 24 h after 48 h following adenovirus infection, and the levels of intracellular neutral lipids were determined by oil-red O staining followed by spectrometric quantification as shown in Panels A and B. Panel A represents typical results of three separate experiments and Panel B shows the quantification of oil-red O staining. The total cellular cholesterol levels were determined after the lipids extracted as described in Section 2 and as shown in Panel C. Cont represents the cells were infected with null virus as control; EGFP, with recombinant adenovirus encoding CMV-EGFP as a negative gene control; StAR, with adenovirus encoding CMV-StAR; (#) represents P < 0.05 vs. Adv-CMV-EGFP. None represents cells incubated in serum-free media without ox-LDL; ox-LDL, with 50µg/ml ox-LDL in serum-free media.
3.3. Effect of StAR overexpression on the gene expression involved in cholesterol homeostasis in THP-1 derived macrophages
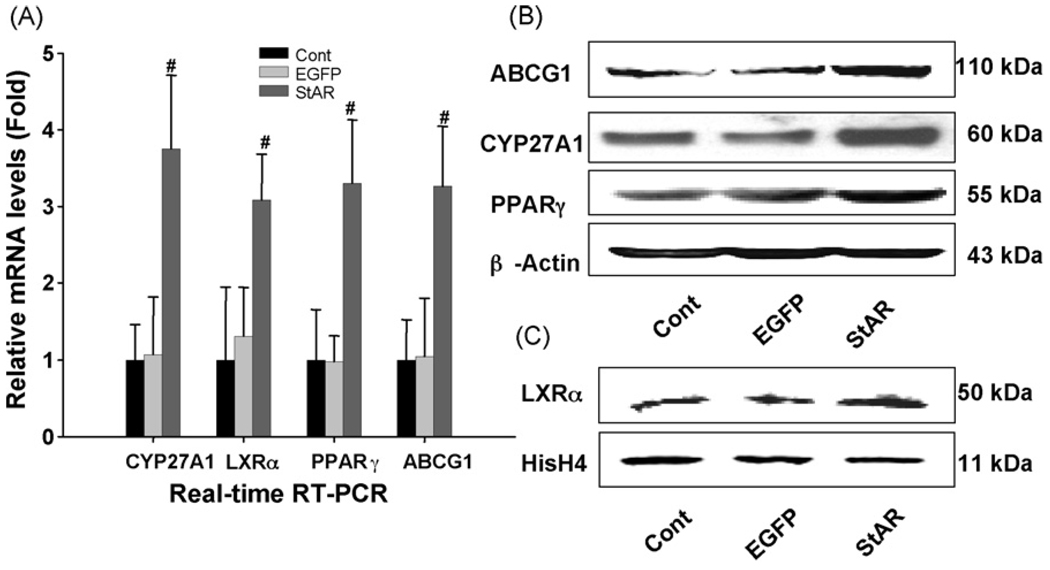
mRNA levels of the genes involved in cholesterol homeostasis were determined by real-time RT-PCR after the cells were infected with the recombinant adenovirus as indicated. CYP27A1, LXRα, PPARβ, and ABCG1 all increased by 3-fold, in the Adv-CMV-StAR transfected macrophages (Fig. 2A), whereas mRNA levels of apoE, sterol regulatory element binding protein 1 and 2 (SREBP-1/2), low-density lipoprotein receptor (LDLr) and ABCA1 did not change significantly (data not shown).
Fig. 2.
Effects of StAR overexpression on gene expression involved in lipid homeostasis. The cells were harvested after 48 h of adenovirus infection to isolate total RNA or proteins. Panel A: mRNA levels following StAR overexpression were determined by real-time Quantitative PCR. The data are normalized to the internal control GAPDH mRNA and are represented as the mean of results from three independent experiments ± S.D. Panel B: ABCG1, CYP27A1 and PPARγ protein expression were analyzed by Western blot using total cell lysates. β-Actin was used as the protein loading control. Panel C: LXRα protein expression was analyzed by Western blot of nuclear proteins extracts. Histone H4 (HisH4) was used as the protein loading control. (#) Represents P < 0.05 vs. Adv-CMV-EGFP.
3.4. Effect of StAR overexpression on CYP27A1, ABCG1, PPARγ and LXRα protein levels in THP-1 derived macrophages
The protein levels of CYP27A1, ABCG1, PPARγ and LXRα after StAR overexpression in THP-1 derived macrophages were detected by Western blot analysis as shown in Fig. 2B and C. Consistently, CYP27A1, ABCG1, PPARγ and LXRα proteins were increased by 2.8-, 2.5-, 2.2-, and 1.8-fold, respectively, in the Adv-CMV-StAR transfected macrophages. The expression of ABCA1 protein did not change significantly (data not shown).
To pursue the association between StAR and LXRα, the THP-1 derived macrophages were preincubated with or without LXRα antagonist DIDS for 30min followed by incubation with 10µM 22RHC for various times as indicated in Fig. 3a. The protein levels of ABCG1, StAR, and LXRα were detected by Western blot analysis after incubation with 22RHC. As shown in Fig. 3, the expression of ABCG1 (Fig. 3a(A)) and LXRα (Fig. 3a(B)) proteins were respectively increased by 6- and 3-fold after incubating with 22RHC. These increases could be blocked by preincubation with DIDS. On the other hand, the expression of StAR protein could also be increased by 22RHC but could not be blocked by preincubation with DIDS (Fig. 3a(C)). To confirm this result, a different physiological agonist of LXRα, 27-hydroxycholesterol (27HC, 5µM) was used for comparable studies. As shown in Fig. 3b, the results are similar to those with 22RHC.
Fig. 3.
(a) Effects of LXR agonist and antagonist on ABCG1, LXRα and StAR proteins expression. The macrophages were preincubated with DMSO (without DIDS) (upper panels) or with 100µM DIDS (lower panels) for 30min and followed by incubation with 10µM 22RHC for various times as indicated. Total cell lysates were extracted for determination of ABCG1 protein levels (A), nuclear fractions for LXRα protein levels (B) and mitochondrial fractions for StAR protein levels (C) by Western blot analysis. Summaries of three experiments were shown in the bottom panels. The data are shown as the mean of three independent experiments ± S.D. (#) Represents P < 0.05 vs. 0 h. (b) Effects of LXR agonist and antagonist on ABCG1, LXRα and StAR proteins expression. The macrophages were preincubated with DMSO (without DIDS) (upper panels) or with 100µM DIDS (lower panels) for 30min and followed by incubation with 5µM 27HC for various times as indicated. Total cell lysates were extracted for determination of ABCG1 protein levels (A), nuclear fractions for LXRα protein levels (B) and mitochondrial fractions for StAR protein levels (C) by Western blot analysis. Summaries of three experiments were shown in the bottom panels. The data are shown as the mean of three independent experiments ± S.D. (#) Represents P < 0.05 vs. 0 h.
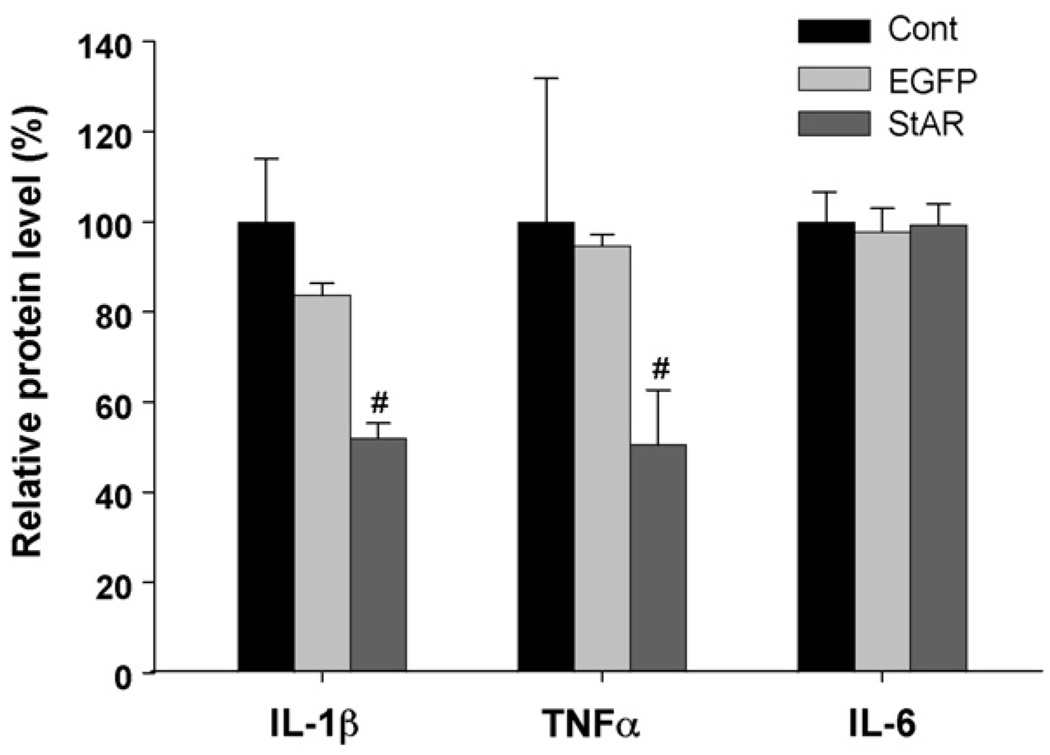
3.5. Effect of StAR overexpression on the levels of cytokines secreted from THP-1 derived macrophages
The levels of secreted IL-1β, TNFα, and IL-6 induced by LPS in the cultured media were determined by ELISA following StAR overexpression in the macrophages. As shown in Fig. 4, overexpression of the gene encoding StAR protein significantly decreased the levels of IL-1β and TNFα secreted from the macrophages while the levels of IL-6 did not change significantly after 48 h following the StAR transfection.
Fig. 4.
Effects of StAR overexpression on the cytokines secretion. The cells were incubated with LPS (10 ng/ml) for 16 h after 48 h following adenovirus infection. The cell culture supernatant was collected and the levels of cytokines such as IL-1β, TNFα, and IL-6 were determined by ELISA analysis. (#) Represents P < 0.05 vs. Adv-CMV-EGFP.
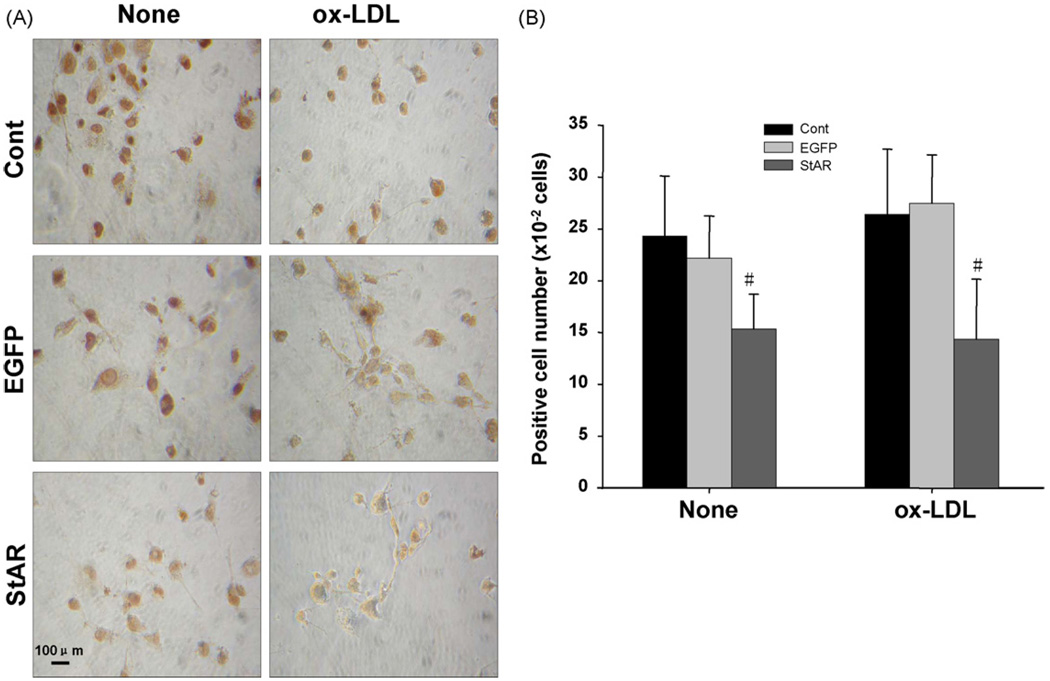
3.6. Effect of StAR overexpression on the apoptosis in THP-1 derived macrophages induced by serum-free media or ox-LDL
The in situ apoptosis were detected by TUNEL and the pictures were taken under microscope as shown in Fig. 5A and the positive-staining cell numbers are shown in Fig. 5B. The cells infected with adenovirus encoding StAR showed decreased susceptibility to apoptosis induced by serum-free media or ox-LDL.
Fig. 5.
Effects of StAR overexpression on macrophage apoptosis. Cells were cultured in fresh serum-free media with or without 50µg/ml ox-LDL for another 24 h after 48 h following adenovirus infection. Panel A: Representative images of apoptotic cells with nuclei stained brown. Panel B: The positive-staining cell numbers were counted per 100 total cells. The experiment was repeated three times with similar results and the data are shown as mean ± S.D. None represents cells were incubated in serum-free media without ox-LDL; ox-LDL, with 50µg/ml ox-LDL in serum-free media. (#) Represents P < 0.05 vs. Adv-CMV-EGFP.
4. Discussion
Hyperlipidemia is one of the most important risk factors for atherosclerosis, in which macrophages are important cells that can uptake extra cholesterol and enhance the local inflammatory response, which promotes the progression of atherosclerosis [26]. In the current study, we overexpressed StAR in THP-1 derived macrophages by recombinant adenovirus infection. After successful infection, intracellular neutral lipid levels in the macrophages were significantly decreased and total cholesterol was reduced about 50%. Concomitantly, secretion of pro-inflammatory cytokines and the number of apoptotic cells were significantly reduced.
To better understand the signal transduction events induced by StAR overexpression in macrophages, we determined the expression of genes involved in cholesterol homeostasis by real-time RT-PCR. We found that the expression of ABCG1 and CYP27A1 mRNA were increased by 2.2- and 2.7-fold, respectively, but ABCA1 expression did not change significantly, suggesting that ABCG1 and ABCA1 may be regulated by a different mechanism [27–29]. The details of said mechanism are unclear at present time. We also found that the mRNA and protein levels of two nuclear receptors PPARγ and LXRα were also increased significantly. This suggests that overexpression of StAR may decrease intracellular lipid levels by activating these nuclear receptors. The possible reason is that overexpression of StAR delivered cholesterol to mitochondria where regulatory oxysterols were synthesized. The regulatory oxysterols in turn activated the nuclear receptors, which then increase the expression of cholesterol efflux transporters and effectively decrease intracellular lipid levels. Previous works have reported that overexpression of StAR could increase cholesterol oxygenation resulting in synthesis of regulatory oxysterols in hepatocytes, which plays an important role in lipid metabolism [16]. These results support our hypothesis that the regulatory oxysterols can regulate expression of key enzymes involved in lipid metabolism and subsequently decrease intracellular lipid levels [17]. More work is needed to elucidate the mechanism.
Accumulation of cholesterol and other neutral lipids resulting in foam cell formation is considered to be a critical process in the development of atherosclerosis. Progressive lipid accumulation leads to increases in the secretion of pro-inflammatory cytokines and the infiltration of inflammatory cells. The present study shows that the decreases in secretion of inflammatory factors by overexpression of StAR may be the result of increased PPARγ levels [30,31], and that activation of nuclear receptors by regulatory oxysterols decreases the secretion of inflammatory factors, which can prevent macrophages from apoptosis. Our results implied a potential role of StAR in the prevention of atherosclerosis.
To confirm our hypothesis that mitochondrial-derived oxysterols play an important role in activation of the nuclear receptors, we studied the effects of oxysterols on LXRα activation. These results showed that addition of 22RHC and 27HC increases LXRα activation and the expression of some of its known target genes, which can be blocked by an antagonist of LXRα.
In summary, overexpression of StAR in THP-1 derived macrophages can reduce the intracellular lipid levels by increasing the expression of cholesterol transporters and reduce inflammatory response by decreasing pro-inflammatory cytokines secretion, blocking macrophages from apoptosis. Further studies are in progress to elucidate the mechanism behind the role of this mitochondrial cholesterol delivery protein in the prevention of atherosclerosis.
Supplementary Material
Acknowledgements
This work was supported by Grant (30470689) from the National Foundation for Natural Science, PR China for Yin, L., and by National Institute of Health, USA, Grant R01 HL078898 and VA Merit Review Grant for Ren, S.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.atherosclerosis.2008.09.006.
References
- 1.Bobryshev YV. Monocyte recruitment and foam cell formation in atherosclerosis. Micron. 2006;37:208–222. doi: 10.1016/j.micron.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. NEngl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy MA, Venkateswaran A, Tarr PT, et al. Characterization of the human ABCG1 gene: liver X receptor activates an internal promoter that produces a novel transcript encoding an alternative form of the protein. J Biol Chem. 2001;276:39438–39447. doi: 10.1074/jbc.M105863200. [DOI] [PubMed] [Google Scholar]
- 4.Van EM, Pennings M, Hoekstra M, Out R, Van Berkel TJ. Scavenger receptor BI and ATP-binding cassette transporter A1 in reverse cholesterol transport and atherosclerosis. Curr Opin Lipidol. 2005;16:307–315. doi: 10.1097/01.mol.0000169351.28019.04. [DOI] [PubMed] [Google Scholar]
- 5.Chawla A, Boisvert WA, Lee CH, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 6.Chinetti G, Lestavel S, Bocher V, et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 7.Chinetti G, Fruchart JC, Staels B. Transcriptional regulation of macrophage cholesterol trafficking by PPARalpha and LXR. Biochem Soc Trans. 2006;34:1128–1131. doi: 10.1042/BST0341128. [DOI] [PubMed] [Google Scholar]
- 8.Repa JJ, Mangelsdorf DJ. The liver X receptor gene team: potential new players in atherosclerosis. Nat Med. 2002;8:1243–1248. doi: 10.1038/nm1102-1243. [DOI] [PubMed] [Google Scholar]
- 9.Fu X, Menke JG, Chen Y, et al. 27-Hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001;276:38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 10.Gueguen Y, Ferrari L, Souidi M, et al. Compared effect of immunosuppressive drugs cyclosporine A and rapamycin on cholesterol homeostasis key enzymes CYP27A1 and HMG-CoA reductase. Basic Clin Pharmacol Toxicol. 2007;100:392–397. doi: 10.1111/j.1742-7843.2007.00066.x. [DOI] [PubMed] [Google Scholar]
- 11.Dubrac S, Lear SR, Ananthanarayanan M, et al. Role of CYP27A in cholesterol and bile acid metabolism. J Lipid Res. 2005;46:76–85. doi: 10.1194/jlr.M400219-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Sugawara T, Lin D, Holt JA, et al. Structure of the human steroidogenic acute regulatory protein (StAR) gene: StAR stimulates mitochondrial cholesterol 27-hydroxylase activity. Biochemistry. 1995;34:12506–12512. doi: 10.1021/bi00039a004. [DOI] [PubMed] [Google Scholar]
- 13.Hall EA, Ren S, Hylemon PB, et al. Detection of the steroidogenic acute regulatory protein, StAR, in human liver cells. Biochim Biophys Acta. 2005;1733:111–119. doi: 10.1016/j.bbalip.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Pandak WM, Ren S, Marques D, et al. Transport of cholesterol into mitochondria is rate-limiting for bile acid synthesis via the alternative pathway in primary rat hepatocytes. J Biol Chem. 2002;277:48158–48164. doi: 10.1074/jbc.M205244200. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Pandak WM, Erickson SK, et al. Biosynthesis of the regulatory oxysterol, 5-cholesten-3beta,25-diol 3-sulfate, in hepatocytes. J Lipid Res. 2007;48:2587–2596. doi: 10.1194/jlr.M700301-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Ren S, Hylemon P, Zhang ZP, et al. Identification of a novel sulfonated oxysterol, 5-cholesten-3beta,25-diol 3-sulfonate, in hepatocyte nuclei and mitochondria. J Lipid Res. 2006;47:1081–1090. doi: 10.1194/jlr.M600019-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Ren S, Li X, Rodriguez-Agudo D, et al. Sulfated oxysterol, 25HC3S, is a potent regulator of lipid metabolism in human hepatocytes. Biochem Biophys Res Commun. 2007;360:802–808. doi: 10.1016/j.bbrc.2007.06.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, Ren S, Pandak W, et al. The effects of inflammatory cytokines on steroidogenic acute regulatory protein expression in macrophages. Inflamm Res. 2007;56:495–501. doi: 10.1007/s00011-007-6133-3. [DOI] [PubMed] [Google Scholar]
- 19.Schulz T, Schiffl H, Scheithe R, Hrboticky N, Lorenz R. Preserved antioxidative defense of lipoproteins in renal failure and during hemodialysis. Am J Kidney Dis. 1995;25:564–571. doi: 10.1016/0272-6386(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 20.Tang C, He X, Yi G, et al. The action of Liver X receptor alpha on cholesterol efflux in THP-1 macrophage derived foam cells. Prog Biochem Biophys. 2003;30:940–944. [Google Scholar]
- 21.Ren S, Hylemon P, Marques D, et al. Effect of increasing the expression of cholesterol transporters (StAR, MLN64, and SCP-2) on bile acid synthesis. J Lipid Res. 2004;45:2123–2131. doi: 10.1194/jlr.M400233-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Pandak WM, Bohdan P, Franklund C, et al. Expression of sterol 12alpha-hydroxylase alters bile acid pool composition in primary rat hepatocytes and in vivo. Gastroenterology. 2001;120:1801–1809. doi: 10.1053/gast.2001.24833. [DOI] [PubMed] [Google Scholar]
- 23.Guo GL, Santamarina-Fojo S, Akiyama TE, et al. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim Biophys Acta. 2006;1761:1401–1409. doi: 10.1016/j.bbalip.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ning Y, Chen S, Li X, et al. Cholesterol, LDL, and 25-hydroxycholesterol regulate expression of the steroidogenic acute regulatory protein in microvascular endothelial cell line (bEnd.3) Biochem Biophys Res Commun. 2006;342:1249–1256. doi: 10.1016/j.bbrc.2006.02.093. [DOI] [PubMed] [Google Scholar]
- 25.Hall E, Hylemon P, Vlahcevic Z, et al. Overexpression of CYP27 in hepatic and extrahepatic cells: role in the regulation of cholesterol homeostasis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G293–G301. doi: 10.1152/ajpgi.2001.281.1.G293. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz G, Grandl M. Lipid homeostasis in macrophages—implications for atherosclerosis. Rev Physiol Biochem Pharmacol. 2008;160:93–125. doi: 10.1007/112_2008_802. [DOI] [PubMed] [Google Scholar]
- 27.Mauldin JP, Srinivasan S, Mulya A, et al. Reduction in ABCG1 in Type 2 diabetic mice increases macrophage foam cell formation. J Biol Chem. 2006;281:21216–21224. doi: 10.1074/jbc.M510952200. [DOI] [PubMed] [Google Scholar]
- 28.Venkateswaran A, Laffitte BA, Joseph SB, et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci USA. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walczak R, Joseph SB, Laffitte BA, et al. Transcription of the vascular endothelial growth factor gene in macrophages is regulated by liver X receptors. J Biol Chem. 2004;279:9905–9911. doi: 10.1074/jbc.M310587200. [DOI] [PubMed] [Google Scholar]
- 30.Posokhova EN, Khoshchenko OM, Chasovskikh MI, Pivovarova EN, Dushkin MI. Lipid Synthesis in Macrophages during Inflammation in vivo: effect of agonists of peroxisome proliferator activated receptors alpha and gamma and of retinoid X receptors. Biochemistry (Mosc) 2008;73:296–304. doi: 10.1134/s0006297908030097. [DOI] [PubMed] [Google Scholar]
- 31.Duan SZ, Usher MG, Mortensen RM. Peroxisome proliferator-activated receptor-gamma-mediated effects in the vasculature. Circ Res. 2008;102:283–294. doi: 10.1161/CIRCRESAHA.107.164384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.