Summary of recent advances
Transplant recipients exhibiting posttransplant antibodies are at a higher risk for acute and chronic antibody-mediated rejection (AMR). The primary alloantigens recognized by antibodies in recipients with AMR are the highly polymorphic HLA class I and class II molecules expressed on the surface of the endothelial cells (ECs) of the graft. Traditionally, anti-HLA antibodies were thought to mediate graft injury through complement-dependent mechanisms. However, recent studies indicate that antibodies can also contribute to alterations in EC function through complement independent mechanisms by transducing intracellular signals. Anti-HLA antibodies transduce signals that are both pro-inflammatory and pro-proliferative suggesting mechanistic roles in acute and chronic AMR.
Introduction
AMR is an important clinical problem following solid organ transplantation occurring in 10-15% of recipients of renal, heart, and lung allografts [1,2]. The production of anti-donor HLA class I and class II antibodies is a risk factor for development of chronic rejection, which manifests as transplant vasculopathy (TV) [3]. Recent data indicate that antibodies can contribute to the process of AMR through complement independent mechanisms by triggering signal transduction pathways in ECs. This review focuses on the molecular pathways activated through the binding of anti-MHC antibodies to the graft endothelium.
Anti-HLA antibody-mediated signaling promotes leukocyte recruitment
A hallmark of AMR is the accumulation of intravascular leukocytes and platelets in the capillaries of the graft [4]. This suggests that antibody-induced adhesion molecule expression on ECs may play an important role in transplant rejection. The endothelial cell harbors a range of bioactive molecules such as von Willebrand factor (VWF), P-selectin, IL-8, eotaxin-3, endothelin-1, CD63/lamp3, osteoprotegerin, and angiopoietin-2 in Weibel-Palade bodies [5]. Upon stimulation, Weibel-Palade bodies are exocytosed and these proteins are transported to the outside of the cell and control inflammation, thrombosis and atherogenesis. Evidence for anti-MHC antibody induced exocytosis of Weibel-Palade bodies has been provided from experiments in which donor specific antibodies were passively transferred into immunoglobulin knockout (IgKO) recipients of cardiac allografts [6] or severe combined immunodeficient/beige mice that were transplanted with human skin grafts [7••]. Transfer of anti-MHC antibodies stimulated Weibel-Palade body exocytosis and was accompanied by increased P-Selectin expression and von Willebrand Factor (vWF) release [7••]. Studies using F(ab’)2 fragments of anti-MHC antibodies demonstrated that endothelial cell exocytosis was complement- and FcR-independent [6,7••]. Leukocyte and platelet recruitment following HLA class I antibody-induced Weibel-Palade body exocytosis of P-Selectin is dependent upon N-Ethylmaleimide-Sensitive Factor (NSF) and calcium signaling [7••] which may be activated by class I antibody induced inositol triphosphate (IP3) [8] . Adherent platelets and leukocytes produce inflammatory mediators that can promote inflammation [9]. Additionally, chemokines such as monocyte chemotactic protein-1 (MCP-1) and KC (CXCL 1), and cytokines such as interleukin 6 (IL-6) and IL-1α are released from ECs following MHC class I ligation and contribute to monocyte infiltration [6,10]. These experiments imply that antibodies cause vascular inflammation via upregulation of adhesion molecules and/or production of chemokines and cytokines involved in leukocyte and platelet recruitment.
HLA class I ligation induces cyoskeleton reorganization
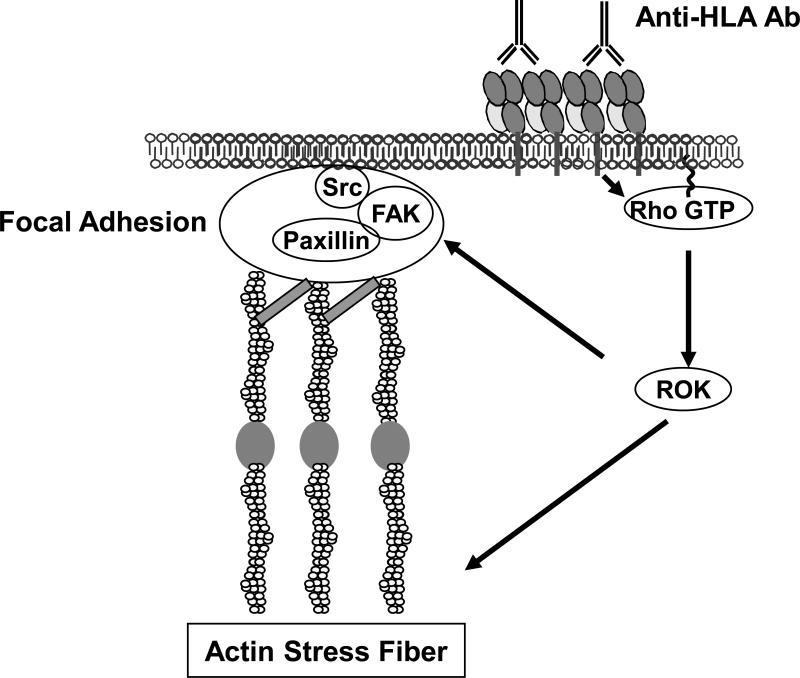
There is increasing evidence that HLA molecules are linked to the actin cytoskeleton and assembly of focal adhesions. Cross-linking of HLA class I by antibodies leads to the clustering of HLA molecules in a manner that can be super-imposed over stress fibers in human fibroblasts [11]. A stress fiber is a filament of actin connected to the focal adhesion complex (Fig. 1). Rho family proteins have been implicated in class I induced formation of stress fibers, cell contractibility and focal adhesions. Coupel et al. showed upregulation of the GTP-binding protein RhoA and stress fiber formation following antibody ligation of class I molecules on ECs [12]. They also reported that RhoA mediated PI3-kinase (PI3K) dependent EC proliferation [12]. Examination of class I induced EC cytoskeleton changes showed that Rho GTPase and Rho-kinase (ROK) are involved in class I-mediated stress fiber formation (Fig. 1) [13]. When Rho GTPase and ROK are blocked, class I-induced phosphorylation of focal adhesion kinase (FAK) and paxillin are inhibited [13]. Long-term exposure to a ROK inhibitor, suppressed development of TV in both human and murine cardiac transplants [14,15].
Figure 1.
Ligation of HLA class I molecules on ECs induces cytoskeleton rearrangement. Ligation of class I molecules by anti-HLA class I antibodies on the surface of ECs increases Rho-GTP activity, induces phosphorylation of ROK, and stimulates actin reorganization and assembly of stress fibers. RhoGTPase activation also triggers the assembly of FAK, Src and paxillin at the focal adhesions and subsequent phosphorylation of these proteins.
FAK is also an important mediator of cell survival, proliferation and migration and plays a critical role in wound repair, atherosclerosis and cancer. We have shown that ligation of class I molecules on ECs results in tyrosine phosphorylation of FAK, Src and paxillin [16]. The phosphorylation of Src and paxillin and the translocation of paxillin into focal adhesions following class I ligation were markedly decreased by small interfering RNA (siRNA) knockdown of FAK [17]. These results imply that the cytoskeleton is involved in the signaling process, because actin-dependent clustering of molecules might be necessary to elicit the class I signaling events. Since Rho family GTPases, ROK and other actin-regulating proteins are critically involved in the maintenance of endothelial barrier properties, it would be beneficial to determine whether HLA class I signaling affects EC permeability.
Anti-HLA antibodies elicit cell proliferation
Ligation of class I molecules on ECs and Smooth muscle cells results in cell proliferation [12,17, 18••, 19]. Capillary ECs in human and murine heart allografts with evidence of AMR displayed increased phosphorylation of S6 ribosomal protein (S6RP), a protein involved in cell proliferation [20••,21]. S6RP is a downstream target of mammalian target of rapamycin (mTOR) complex 1 (mTORC1). mTORC1 consists of mTOR, regulatory associated protein of mTOR (Raptor) and GβL. mTORC1 stimulates cell proliferation via the phosphorylation of eukaryotic initiation factor 4E binding protein1 (4E-BP1) and/or the activation of p70S6 kinase (S6K) and S6RP. Using siRNA technology, we explored the contribution of the mTOR pathway to anti-class I induced cell proliferation. Knockdown of mTOR, rictor or raptor blocked HLA class I-induced EC proliferation. HLA class I ligation led to the phosphorylation of several targets of mTORC1 including S6K at Thr389, S6RP at Ser235/236 and 4E-BP1 at Thr37/46 and the mTORC2 signaling target Akt at Ser473 (Fig. 2)[18••]. Knockdown of raptor inhibited the phosphorylation of S6K, but not Akt. In contrast, knockdown of rictor completed blocked class I induced Akt at Ser473, yet failed to alter S6K at Thr389. These results support the contention that mTORC1 is positioned upstream of S6K while mTORC2 is upstream of Akt in the MHC class I mediated signaling pathway.
Figure 2.
Ligation of HLA class I molecules by anti-HLA antibodies activates signaling cascades that result in EC survival and proliferation. Class I mediated EC survival and proliferation involves the activation of two distinct signaling pathways depending upon the antibody concentration. Ligation of class I molecules with high titer antibodies stimulates intracellular signals that synergize with FGF receptors to stimulate cell proliferation via the MAPK signaling pathway. Ligation of class I molecules with low titer antibodies stimulates cell survival and cell proliferation through phosphorylation of Src, FAK and paxillin followed by downstream activation of mTORC1 and mTORC2. Class I ligation leads to the activation of several downstream targets of mTORC1 signaling including S6K at Thr389, S6RP at Ser235/236, and 4E-BP1 at Thr37/46 and increases cell proliferation. Class I mediated activation of mTORC2 promotes cell survival by activating Akt at Ser473 and upregulating Bcl-2 and Bcl-xL expression.
Based on our data showing that mTORC2 is the kinase for class I mediated phosphorylation of Akt at Ser473, and that knockdown of rictor inhibited class I mediated cell proliferation, we speculate that class I mediated alterations of the cytoskeleton affects cell proliferation via mTORC2. To explore the role of mTORC2 in cell proliferation we characterized the interactions between mTORC2 and ERK following stimulation with anti-class I antibodies [22•]. We showed that knockdown of rictor, but not raptor blocked class I -induced phosphorylation of ERK. These data underscore the differential roles of mTORC1 and mTORC2 in class I mediated EC proliferation [18••].
We further explored the significance of the MHC class I signaling pathway in AMR, using a mouse cardiac allograft model. B6.RAG1 knock-out hosts were transplanted with MHC incompatible BALB/c hearts and then transfused with anti-donor-MHC class I antibodies. Cardiac allografts of mice treated with anti-donor antibodies showed characteristic features of AMR including microvascular changes and C4d deposition. Phosphoproteomic analysis of signaling molecules involved in the MHC class I survival and proliferation pathways showed elevated levels in mice treated with anti-donor antibodies. Allografts harvested on day 30 showed increased phosphorylation of mTOR, S6RP, and S6K [20••] consistent with a phenotype of cell proliferation. In contrast, hearts harvested on day 15 showed prominent phosphorylation of Akt at Ser473 and increased Bcl-2 expression, consistent with survival/accommodation. We postulate that the duration of antibody exposure explains the differences in phosphorylation patterns seen in day 15 and 30 allografts. Recent studies support this hypothesis showing persistent exposure to anti-donor MHC antibodies is required to cause TV in a similar experimental model [23•]. This indicates that the duration of antibody exposure is a critical factor in the development of TV. In an analogous situation in human heart transplantation, we observed an increased risk of TV in recipients with sustained phosphorylation of S6RP Ser235/236 in patients with two or more episodes of AMR [13]. The mechanism by which these signaling cascades lead to the development of TV remains to be elucidated.
Further exploration of EC proliferation pathways revealed that following class I ligation, fibroblast growth factor receptor (FGFR) cell surface expression is upregulated [24] Increased FGFR expression is associated with augmented bFGF ligand binding and cell proliferation via the MAPK pathway. Importantly, ligation of class I molecules in ECs stimulates the redistribution of FGFR from intracellular stores to the plasma membrane in a dose-dependent fashion with the highest dose of antibody promoting the greatest degree of FGFR expression and cell proliferation. Exposure of ECs to interferon-γ results in upregulation of class I expression and is accompanied by an increased capacity to induce FGFR expression [19]. Disruption of actin polymerization by cytochalasin D attenuated class I-induced FGFR cell surface translocation [16]. Knockdown of FAK decreases class I-induced cell proliferation, but does not effect FGFR cell surface expression [17]. These data indicate that FGFR upregulation and the resulting cell proliferation is an alternative pathway from the class I induced-FAK-dependent cell proliferation pathway (Fig. 2).
The development of post-transplant antibodies is associated with the onset of bronchiolitis obliterans syndrome (BOS) after lung transplantation. Cross-linking of airway epithelial cells with HLA class I antibody leads to cell proliferation, growth factor production and apoptosis; each of which may contribute to BOS [25]. Administration of anti-MHC class I antibodies into native lungs stimulated expression of the chemokines CXCL1, CXCL12 and CCL9, which signal through CCR2 and CXCR4, resulting in increased leukocyte infiltration in the lungs [26•].
Anti-HLA antibody-mediated survival signaling
Ligation of class I molecules by antibody activates the PI3K/Akt pathway and promotes EC survival by regulating levels of the antiapoptotic proteins Bcl-2 and Bcl-xL [27]. In contrast to class I mediated FGFR induction, maximum class I mediated increases in Bcl-2 and Bcl-xL expression were observed upon exposure of ECs to low concentrations of anti-class I antibodies. Similarly, at low concentrations of antibody, maximum class I mediated phosphorylation of AktSer473 was seen. HLA antibody mediated activation of Akt stimulates cell survival by phosphorylating Bad and preventing its interactions with Bcl-2 and Bcl-xL [28,29]. Preincubation of islet EC with subsaturating concentrations of anti-HLA antibodies up-regulated Bcl-2, Bcl-xL, and Heme Oxygenase-1 [30]. Patients with subsaturating levels of circulating donor-specific HLA antibodies showed increased Bcl-xL expression in the endothelium of renal allografts [31]. These observations linking antibody titer to expression of survival proteins on ECs are reminiscent of studies in xenogeneic and ABO incompatible transplants that showed increased expression of Bcl-xL, Bcl-2, A-20 and HO-1 on the graft endothelium and protection from apoptosis after exposure to low titers of anti-graft antibodies [32,33]. The phenomenon of resistance to the effects of antigraft antibodies has been termed graft accommodation [34]. The signaling mechanisms underlying how ABO and anti-Gal antibodies induce accommodation are unknown. It is tempting to speculate that like HLA antibodies, anti-carbohydrate antibodies crosslink glycoproteins that activate the Akt survival pathway.
It is debatable whether persistent class I mediated signaling by low concentrations of antibodies can provide long-term graft accommodation, since class I ligation activates both the survival and cell proliferation machinery at the same time. Instead we favor the model in which concomitant activation of mTORC1 and mTORC2 are synergistic in development of TV (Fig. 2). In this model, class I mediated activation of mTORC2 prevents apoptosis through the upregulation of survival proteins and enables chronic changes in the graft via the mTORC1 protein synthesis and proliferation signaling pathways. Support for this hypothesis comes from studies in non-human primates showing that allografts may initially survive episodes of acute AMR, but long-term, lack stable accommodation and develop chronic rejection [35,36]. In another model of murine transplantation, once the graft had been exposed to anti-MHC antibody long enough to reach a minimum threshold, chronic TV was inevitable [23•].
Anti-HLA class II antibody mediated signaling
Signal transduction via class II molecules has been extensively studied in B lymphocytes [37], yet little is known about class II signaling in endothelial cells. Using γ-interferon to upregulate the expression of class II molecules, Les Bas-Bernardet showed that in contrast to professional antigen presenting cells, ECs evade apoptosis mediated by HLA-DR ligation by activating protein kinase C-α/β and Akt [38]. We showed that ligation of HLA-class II molecules on ECs with antibodies stimulated increased phosphorylation of S6RP [21]. Additionally, development of anti-HLA class II antibodies and S6RP phosphorylation in the capillary endothelial cells of cardiac biopsies from recipients with AMR were strongly correlated [21]. The impact of class II antibodies on endothelial cells deserves further investigation.
Therapy
Evidence that HLA antibodies contribute to the process of TV supports the need for therapeutic intervention. Some promising therapies involve agents that target signal transduction pathways [39,40]. Rapamycin binds to FKB12 and inhibits mTORC1 [41] and possibly mTORC2 [42]. Since anti-HLA class I antibodies stimulate cell proliferation via mTORC1, this suggests that drugs such as rapamycin, that block class I mediated activation of both mTORC1 and mTORC2 may prevent EC proliferation and neoangiogenesis [18••,22•]. Indeed, clinical trials have shown the efficacy of rapamycin in preventing TV in cardiac transplantation [43]. The PKC inhibitor AEB071 (Sotrastaurin) may be effective in reducing inflammation by inhibiting antibody induced leukocyte and platelet recruitment by blocking calcium dependent Weibel-Palade body exocytosis and vWF and P-selectin expression on ECs [40,44]. Elucidating the MHC signaling pathways will permit the identification of new targeted therapies.
Conclusions
Antibodies binding to HLA class I molecules transduce inflammatory and proliferation signals that can promote development of chronic rejection. The capacity to transduce signals relates to the ability of antibodies to crosslink MHC molecules. Since MHC molecules lack endogenous kinase activity, crosslinking probably promotes molecular associations with other molecules that have the capacity to transduce signals. Future studies are needed to characterize the nature of the co-receptors that partner with HLA molecules to elicit signaling events. Characterization of the class I signaling pathway has the potential to identify novel therapeutic strategies to prevent TV and graft loss.
Acknowledgements
The NSF Graduate Research Fellowship to M.E.A, AHA Western States Postdoctoral Fellowship to F. L, R01 HL090995-01, NIH U01AI077821 and NIH AI042819 to E.F.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Reed EF, Demetris AJ, Hammond E, Itescu S, Kobashigawa JA, Reinsmoen NL, Rodriguez ER, Rose M, Stewart S, Suciu-Foca N, et al. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25:153–159. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Singh N, Pirsch J, Samaniego M. Antibody-mediated rejection: treatment alternatives and outcomes. Transplant Rev (Orlando) 2009;23:34–46. doi: 10.1016/j.trre.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trial. Am J Transplant. 2004;4:438–443. doi: 10.1111/j.1600-6143.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 4.Halloran PF, Wadgymar A, Ritchie S, Falk J, Solez K, Srinivasa NS. The significance of the anti-class I antibody response. I. Clinical and pathologic features of anti-class I-mediated rejection. Transplantation. 1990;49:85–91. doi: 10.1097/00007890-199001000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Goligorsky MS, Patschan D, Kuo MC. Weibel-Palade bodies--sentinels of acute stress. Nat Rev Nephrol. 2009;5:423–426. doi: 10.1038/nrneph.2009.87. [DOI] [PubMed] [Google Scholar]
- 6.Wasowska BA, Qian Z, Cangello DL, Behrens E, Van Tran K, Layton J, Sanfilippo F, Baldwin WM., 3rd Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation. 2001;71:727–736. doi: 10.1097/00007890-200103270-00007. [DOI] [PubMed] [Google Scholar]
- 7••.Yamakuchi M, Kirkiles-Smith NC, Ferlito M, Cameron SJ, Bao C, Fox-Talbot K, Wasowska BA, Baldwin WM, 3rd, Pober JS, Lowenstein CJ. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proc Natl Acad Sci U S A. 2007;104:1301–1306. doi: 10.1073/pnas.0602035104. [•• of outstanding interestThe ligation of class I molecules by anti-HLA class I antibodies activates endothelial exocytosis resulting in VWF release and P-selectin externalization in vitro. The authors also determined that, in vivo, anti-HLA class I antibodies induce EC exocytosis and promote leukocyte recruitment that may play a role in transplant rejection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bian H, Harris PE, Mulder A, Reed EF. Anti-HLA antibody ligation to HLA class I molecules expressed by endothelial cells stimulates tyrosine phosphorylation, inositol phosphate generation, and proliferation. Hum Immunol. 1997;53:90–97. doi: 10.1016/S0198-8859(96)00272-8. [DOI] [PubMed] [Google Scholar]
- 9.Morrell CN, Murata K, Swaim AM, Mason E, Martin TV, Thompson LE, Ballard M, Fox-Talbot K, Wasowska B, Baldwin WM., 3rd In vivo platelet-endothelial cell interactions in response to major histocompatibility complex alloantibody. Circ Res. 2008;102:777–785. doi: 10.1161/CIRCRESAHA.107.170332. [DOI] [PubMed] [Google Scholar]
- 10.Lee CY, Lotfi-Emran S, Erdinc M, Murata K, Velidedeoglu E, Fox-Talbot K, Liu J, Garyu J, Baldwin WM, 3rd, Wasowska BA. The involvement of FcR mechanisms in antibody-mediated rejection. Transplantation. 2007;84:1324–1334. doi: 10.1097/01.tp.0000287457.54761.53. [DOI] [PubMed] [Google Scholar]
- 11.Huet C, Ash JF, Singer SJ. The antibody-induced clustering and endocytosis of HLA antigens on cultured human fibroblasts. Cell. 1980;21:429–438. doi: 10.1016/0092-8674(80)90479-1. [DOI] [PubMed] [Google Scholar]
- 12.Coupel S, Leboeuf F, Boulday G, Soulillou JP, Charreau B. RhoA activation mediates phosphatidylinositol 3-kinase-dependent proliferation of human vascular endothelial cells: an alloimmune mechanism of chronic allograft nephropathy. J Am Soc Nephrol. 2004;15:2429–2439. doi: 10.1097/01.ASN.0000138237.42675.45. [DOI] [PubMed] [Google Scholar]
- 13.Lepin EJ, Jin YP, Barwe SP, Rozengurt E, Reed EF. HLA class I signal transduction is dependent on Rho GTPase and ROK. Biochem Biophys Res Commun. 2004;323:213–217. doi: 10.1016/j.bbrc.2004.08.082. [DOI] [PubMed] [Google Scholar]
- 14.Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Kaibuchi K, Takeshita A. Long-term treatment with a specific Rho-kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res. 2004;94:46–52. doi: 10.1161/01.RES.0000107196.21335.2B. [DOI] [PubMed] [Google Scholar]
- 15.Ohki S, Iizuka K, Ishikawa S, Kano M, Dobashi K, Yoshii A, Shimizu Y, Mori M, Morishita Y. A highly selective inhibitor of Rho-associated coiled-coil forming protein kinase, Y-27632, prolongs cardiac allograft survival of the BALB/c-to-C3H/He mouse model. J Heart Lung Transplant. 2001;20:956–963. doi: 10.1016/s1053-2498(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 16.Jin YP, Singh RP, Du ZY, Rajasekaran AK, Rozengurt E, Reed EF. Ligation of HLA class I molecules on endothelial cells induces phosphorylation of Src, paxillin, and focal adhesion kinase in an actin-dependent manner. J Immunol. 2002;168:5415–5423. doi: 10.4049/jimmunol.168.11.5415. [DOI] [PubMed] [Google Scholar]
- 17.Jin YP, Korin Y, Zhang X, Jindra PT, Rozengurt E, Reed EF. RNA interference elucidates the role of focal adhesion kinase in HLA class I-mediated focal adhesion complex formation and proliferation in human endothelial cells. J Immunol. 2007;178:7911–7922. doi: 10.4049/jimmunol.178.12.7911. [DOI] [PubMed] [Google Scholar]
- ••.Jindra PT, Jin YP, Rozengurt E, Reed EF. HLA class I antibody-mediated endothelial cell proliferation via the mTOR pathway. J Immunol. 2008;180:2357–2366. doi: 10.4049/jimmunol.180.4.2357. [•• of outstanding interestKnockdown of mTOR, rictor, or raptor blocks HLA class I-induced endothelial cell proliferation. These results support the role of anti-HLA antibodies in the development of transplant vasculopathy and suggest that anti-HLA Abs may induce proliferation through the mTOR pathway.] [DOI] [PubMed] [Google Scholar]
- 19.Bian H, Reed EF. Alloantibody-mediated class I signal transduction in endothelial cells and smooth muscle cells: enhancement by IFN-gamma and TNF-alpha. J Immunol. 1999;163:1010–1018. [PubMed] [Google Scholar]
- 20••.Jindra PT, Hsueh A, Hong L, Gjertson D, Shen XD, Gao F, Dang J, Mischel PS, Baldwin WM, 3rd, Fishbein MC, et al. Anti-MHC class I antibody activation of proliferation and survival signaling in murine cardiac allografts. J Immunol. 2008;180:2214–2224. doi: 10.4049/jimmunol.180.4.2214. [•• of outstanding interestThe authors developed a murine vascularized heterotopic cardiac allograft model in which B6.RAG1 KO hosts received a fully MHC incompatible BALB/c heart transplants and were passively transfused with anti-donor MHC class I antibody. Allografts from antibody treated mice showed characteristic features of antibody-mediated rejection and increased phosphorylation of signaling proteins involved in cell survival and proliferation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepin EJ, Zhang Q, Zhang X, Jindra PT, Hong LS, Ayele P, Peralta MV, Gjertson DW, Kobashigawa JA, Wallace WD, et al. Phosphorylated S6 ribosomal protein: a novel biomarker of antibody-mediated rejection in heart allografts. Am J Transplant. 2006;6:1560–1571. doi: 10.1111/j.1600-6143.2006.01355.x. [DOI] [PubMed] [Google Scholar]
- 22•.Jindra PT, Jin YP, Jacamo R, Rozengurt E, Reed EF. MHC class I and integrin ligation induce ERK activation via an mTORC2-dependent pathway. Biochem Biophys Res Commun. 2008;369:781–787. doi: 10.1016/j.bbrc.2008.02.093. [• of special interestThese data show, for the first time, that ligation of either MHC class I or integrin on the surface of EC leads to ERK activation through an mTORC2-dependent pathway.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Uehara S, Chase CM, Cornell LD, Madsen JC, Russell PS, Colvin RB. Chronic cardiac transplant arteriopathy in mice: relationship of alloantibody, C4d deposition and neointimal fibrosis. Am J Transplant. 2007;7:57–65. doi: 10.1111/j.1600-6143.2006.01599.x. [• of special interestUsing a murine heterotopic cardiac allograft model, the authors present evidence that alloantibody is sufficient to cause arterial neointimal proliferation. If antibody treatment was stopped early, lesions showed little progression, whereas prolonged treatment for 28 days when the lesions were fully developed, no regression occurred even though C4d deposition and circulating antibody became undetectable. Therefore, a minimum threshold of antibody exposure is needed to cause neointimal proliferation.] [DOI] [PubMed] [Google Scholar]
- 24.Harris PE, Bian H, Reed EF. Induction of high affinity fibroblast growth factor receptor expression and proliferation in human endothelial cells by anti-HLA antibodies: a possible mechanism for transplant atherosclerosis. J Immunol. 1997;159:5697–5704. [PubMed] [Google Scholar]
- 25.Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol. 2003;64:521–529. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 26•.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–318. doi: 10.4049/jimmunol.182.1.309. [• of special interestAdministration of anti- MHC class I antibodies into native murine lungs resulted in the marked cellular infiltration and epithelial hyperplasia, fibrosis, and occlusion of the distal airways. Treated lungs show increased expression of chemokines, their receptors, and growth factors, and induced IL-17. These results indicate that antibodies to donor MHC can induce autoimmunity which plays a crucial role in chronic rejection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayanan K, Jendrisak MD, Phelan DL, Mohanakumar T. HLA class I antibody mediated accommodation of endothelial cells via the activation of PI3K/cAMP dependent PKA pathway. Transpl Immunol. 2006;15:187–197. doi: 10.1016/j.trim.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Narayanan K, Jaramillo A, Phelan DL, Mohanakumar T. Pre-exposure to sub-saturating concentrations of HLA class I antibodies confers resistance to endothelial cells against antibody complement-mediated lysis by regulating Bad through the phosphatidylinositol 3-kinase/Akt pathway. Eur J Immunol. 2004;34:2303–2312. doi: 10.1002/eji.200324843. [DOI] [PubMed] [Google Scholar]
- 29.Jin YP, Fishbein MC, Said JW, Jindra PT, Rajalingam R, Rozengurt E, Reed EF. Anti-HLA class I antibody-mediated activation of the PI3K/Akt signaling pathway and induction of Bcl-2 and Bcl-xL expression in endothelial cells. Hum Immunol. 2004;65:291–302. doi: 10.1016/j.humimm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Bharat A, Saini D, Benshoff N, Goodman J, Desai NM, Chapman WC, Mohanakumar T. Role of intra-islet endothelial cells in islet allo-immunity. Transplantation. 2007;84:1316–1323. doi: 10.1097/01.tp.0000288192.11396.70. [DOI] [PubMed] [Google Scholar]
- 31.Salama AD, Delikouras A, Pusey CD, Cook HT, Bhangal G, Lechler RI, Dorling A. Transplant accommodation in highly sensitized patients: a potential role for Bcl-xL and alloantibody. Am J Transplant. 2001;1:260–269. doi: 10.1034/j.1600-6143.2001.001003260.x. [DOI] [PubMed] [Google Scholar]
- 32.Bach FH, Ferran C, Hechenleitner P, Mark W, Koyamada N, Miyatake T, Winkler H, Badrichani A, Candinas D, Hancock WW. Accommodation of vascularized xenografts: expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nat Med. 1997;3:196–204. doi: 10.1038/nm0297-196. [DOI] [PubMed] [Google Scholar]
- 33.Chopek MW, Simmons RL, Platt JL. ABO-incompatible kidney transplantation: initial immunopathologic evaluation. Transplant Proc. 1987;19:4553–4557. [PubMed] [Google Scholar]
- 34.Tang AH, Platt JL. Accommodation of grafts: implications for health and disease. Hum Immunol. 2007;68:645–651. doi: 10.1016/j.humimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, Colvin RB. Chronic antibody mediated rejection of renal allografts: pathological, serological and immunologic features in nonhuman primates. Am J Transplant. 2006;6:1790–1798. doi: 10.1111/j.1600-6143.2006.01351.x. [DOI] [PubMed] [Google Scholar]
- 36.Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, Colvin RB. Four stages and lack of stable accommodation in chronic alloantibody-mediated renal allograft rejection in Cynomolgus monkeys. Am J Transplant. 2008;8:1662–1672. doi: 10.1111/j.1600-6143.2008.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang HY, Kim J, Chung GH, Lee JC, Jang YS. Cross-linking of MHC class II molecules interferes with phorbol 12,13-dibutyrate-induced differentiation of resting B cells by inhibiting Rac-associated ROS-dependent ERK/p38 MAP kinase pathways leading to NF-kappaB activation. Mol Immunol. 2007;44:1577–1586. doi: 10.1016/j.molimm.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Le Bas-Bernardet S, Coupel S, Chauveau A, Soulillou JP, Charreau B. Vascular endothelial cells evade apoptosis triggered by human leukocyte antigen-DR ligation mediated by allospecific antibodies. Transplantation. 2004;78:1729–1739. doi: 10.1097/01.tp.0000147339.31581.99. [DOI] [PubMed] [Google Scholar]
- 39.Ghoneim MA, Refaie AF. Is matching for human leukocyte antigen-DR beneficial in pediatric kidney transplantation? Nat Clin Pract Nephrol. 2009;5:70–71. doi: 10.1038/ncpneph1017. [DOI] [PubMed] [Google Scholar]
- 40.Vincenti F, Kirk AD. What's next in the pipeline. Am J Transplant. 2008;8:1972–1981. doi: 10.1111/j.1600-6143.2008.02403.x. [DOI] [PubMed] [Google Scholar]
- 41.Delgado JF, Manito N, Segovia J, Almenar L, Arizon JM, Camprecios M, Crespo-Leiro MG, Diaz B, Gonzalez-Vilchez F, Mirabet S, et al. The use of proliferation signal inhibitors in the prevention and treatment of allograft vasculopathy in heart transplantation. Transplant Rev (Orlando) 2009;23:69–79. doi: 10.1016/j.trre.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 43.Pinney SP, Mancini D. Cardiac allograft vasculopathy: advances in understanding its pathophysiology, prevention, and treatment. Curr Opin Cardiol. 2004;19:170–176. doi: 10.1097/00001573-200403000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Lorenzi O, Frieden M, Villemin P, Fournier M, Foti M, Vischer UM. Protein kinase C-delta mediates von Willebrand factor secretion from endothelial cells in response to vascular endothelial growth factor (VEGF) but not histamine. J Thromb Haemost. 2008;6:1962–1969. doi: 10.1111/j.1538-7836.2008.03138.x. [DOI] [PubMed] [Google Scholar]