Abstract
Sexually-selected communication signals can be used by competing males to settle contests without incurring the costs of fighting. Steroid regulation of these signals can render them as reliable indicators of a male's physiological state. We investigated how plasticity in electrocommunication signals is driven by social competition for mates, mediated by steroid hormones, and subject to the effects of past social experience. We measured the electric waveform's amplitude and duration and steroid hormone levels of male gymnotiform electric fish (Brachyhypopomus gauderio) following week-long periods of social isolation, and low or high social competition. To quantify the effect of social history on the modulation of the electric signal, six groups of six males experienced all the above three social conditions but in different order. We found that males differentially modulate their electric signals depending on the order they experienced these conditions. Thus, past social interactions affect both present and future social electric signals. Cortisol levels and the amplitude of the electric signal appeared to track the intensity of competition, while androgen levels and the duration of the electric signal only responded to the presence (low and high competition) or absence (isolation) of a social environment (low and high androgens respectively). In addition, cortisol levels were related to the body size of the males at high social competition. Taken together, these findings suggest that the capacity of males to modulate their signals in response to social competition is regulated by steroids.
Keywords: male competition, communication signal, steroid, androgen, cortisol, social history, gymnotiform, electric fish, electric organ discharge
Social experiences influence an animal's motivational state during present and future social interactions. Males compete fiercely or adopt alternative mating strategies when fewer mates are available, resources are limiting, and the reproductive period is short (Andersson, 1994). But contests between males consume time and energy in the best case, or can lead to injury in the worst case (Neat et al., 1998). Ritualized behaviors and reliable signals facilitate the resolution of contests while minimizing its costs (Grafen, 1990; Parker, 1974; Smith, 1973; Zahavi, 1975). Furthermore, male aggressive interactions regulate and are regulated by steroid hormones such as glucocorticosteroids and androgens (Abbott et al., 2003; Elofsson et al., 2000; Goymann and Wingfield, 2004; Oliveira et al., 2002; Overli et al., 1999; Summers and Winberg, 2006; Wingfield et al., 1987; Wingfield et al., 1990). Yet, this bidirectional relationship can result in collateral costs such as suppression of immune function, depletion of energy stores, and reduced parental care (Romero, 2004; Sapolsky et al., 2000; Wingfield et al., 1990).
Some organisms have evolved innovative adaptations to balance the benefits and costs of energetically-demanding signals and displays. Such is the case of the gymnotiform fish Brachyhypopomus gauderio1 (Giora and Malabarba, 2009), which, by coupling its electric signal waveform to endocrine systems with circadian, seasonal, and behavioral drivers, can direct its expensive signal displays to the times when it might derive the greatest benefit (Salazar and Stoddard, 2008). Four features of the life history of the nocturnal gymnotiform fish B. gauderio make it an excellent candidate to understand the adaptive role of the circadian regulation of communication signals. First, these fish generate an electric organ discharge (EOD) to navigate, locate prey, and communicate with conspecifics in the dark. During the breeding season, not only do males have larger amplitude and longer duration EODs, but they further enhance their EODs by increasing these sex differences at night while decreasing them during the day (Franchina and Stoddard, 1998; Stoddard et al., 2007b). Second, these sexually dimorphic characters are associated with reproductive success. Gravid female B. gauderio preferentially associate with bigger males with larger amplitude and longer duration EODs (Curtis and Stoddard, 2003). Third, EOD circadian rhythm plasticity is sensitive to changes in the social environment. Social isolation decreases the EOD circadian rhythm, and addition of a social companion to the tank of an isolated male restores the reduced EOD circadian rhythm of the isolated male to levels observed in males sampled from social groups. Nevertheless, this effect is sex-specific: a male social companion induces a bigger and faster effect than a female social companion (Franchina et al., 2001). Fourth, the EOD is modulated by melanocortins (Markham et al., 2009; Markham and Stoddard, 2005) and by steroid hormones (Mills and Zakon, 1991; Stoddard et al., 2006).
The EOD operates in the same modality as the nervous system's action potentials, making it a tractable system to investigate the role of hormones in the regulation of signal production mechanisms in the context of sexually-selected communication. The EOD can be deconstructed into its waveform amplitude (voltage in mV, Fig. 1A), its waveform duration (time in ms, Fig. 1A), and its repetition rate or frequency (EODs per second). The contributions of the central and the peripheral components of the electrocommunication neural network can be dissected out (Zakon, 1998): the EOD repetition rate is controlled by the medullary pacemaker nucleus (Dye and Meyer, 1986; Kawasaki and Heiligenberg, 1989; Kawasaki and Heiligenberg, 1990; Keller et al., 1991), while the EOD waveform's amplitude and duration are determined by the intrinsic properties of the peripheral electric organ (EO)'s electrocytes (Bennett, 1970; Bennett et al., 1967). Androgens can alter the EOD centrally (EOD repetition rate) and peripherally (EOD waveform) (Bass and Volman, 1987; Mills and Zakon, 1991; Mills et al., 1992; Stoddard et al., 2006; Zakon et al., 1991). The effects at either level are independent from each other (Few and Zakon, 2001). Furthermore, 11-ketotestosterone (11-KT) and cortisol are positively related to modulations of the EOD repetition rate in taxa with sex differences in this parameter (Dunlap, 2002; Dunlap et al., 2002).
Fig. 1.
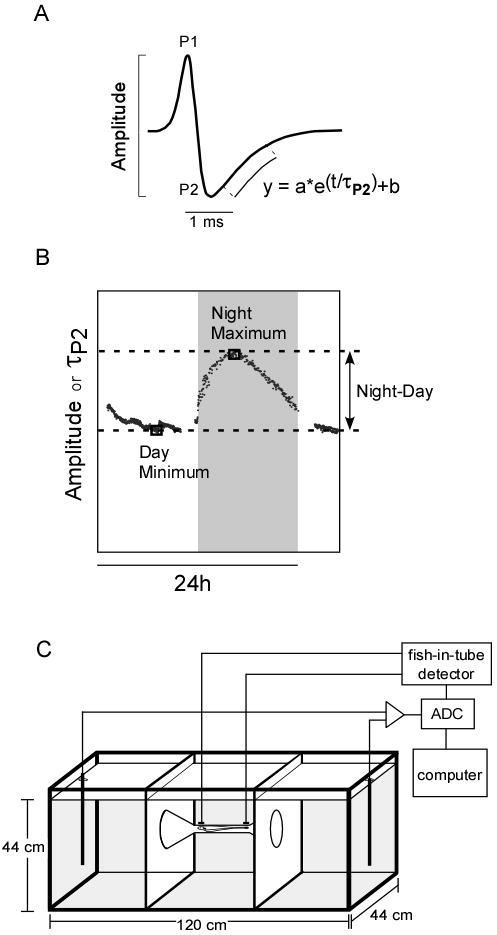
EOD data acquisition. A. The EOD of B. gauderio is a biphasic waveform composed of a positive phase (P1) and a negative phase (P2). We measured the peak-to-peak amplitude and the time constant of P2 repolarization (τP2). B. To determine changes in the EOD circadian rhythm, we measured daytime low, nighttime high, and the night-to-day difference for the amplitude and τP2. C. The tank set-up in the EOD machine automated system (Stoddard et al., 2003) allowed us to record the EOD of free-swimming fish continuously and accurately.
Increasing the density of males in a population has been shown to increase the incidence of aggressive encounters and the levels of androgens in teleost fish (Oliveira et al., 2002; Pankhurst and Barnett, 1993). Nevertheless, the effect of changing social group dynamics on steroid-regulated, condition-dependent communication signals is not well understood. In this study, we measured the EOD and the steroid hormone levels of isolated males, and males at low and high competition. Males experienced all three conditions but in different order which allowed us to quantify the effect of social history in the modulation of the EOD.
Methods
Animals
Our subjects were male B. gauderio, a gymnotiform pulse-type weakly electric fish native to marshes and slow waters of South America. Fish were selected randomly from a captive-reared, 11th generation breeding colony located at Florida International University, Miami, Florida. Males' body length ranged from 13.0 to 24.6 cm and females' body length ranged from 14.1 to 17.9 cm. We categorized juveniles by length (7 cm or smaller) and by the absence of sexually-mature characters (e.g, long and thick tails indicative of breeding males or swollen abdomens indicative of gravid females). Fish were reared and housed in 450-liter (185 × 95 × 26 cm) outdoor pools with water conductivity at 90±10 μS cm-1 and mean ambient temperature at 27±2 °C. The water surface of each pool was covered 80-100% with water hyacinths (Eichhornia crassipes). Each breeding pool contained 10-20 fish. All fish were fed live oligochaete blackworms (Gulfstream Tropical Aquarium, Dania, Florida) three times per week. Experiments took place during the reproductive months, typically from May to September.
Before the beginning of the experiment, we tagged male subjects with fluorescent visible implant elastomer (VIE, Northwest Marine Technology, Inc.) for individual identification. For individual tagging, we anesthetized each fish using 0.075% 2-phenoxyethanol for 2-3 min and injected the elastomer tags on the same side of each fish caudal to the pectoral fin. The elastomer tags were injected subcutaneously following a numerical code consisting on a combination of orange, yellow and green vertical and horizontal lines approx. 2-3 mm in length (Supplementary Materials). Experiments complied with NIH ‘Principles of Animal Care’ publication no. 86-23, rev. 1985, and were approved by the FIU IACUC (protocol approval no. 07-004).
The EOD machine
This method has been described in detail by (Stoddard et al., 2003). In brief, this automated system allowed us to record and perform online analysis of calibrated EODs in free-swimming fish. We placed male fish into one of the outer compartments of the recording tank (Fig. 1C) and recorded the fish's EOD only when it was positioned in the center of the tank. Every 60 seconds, the peak-to-peak amplitude and τP2 (time constant of repolarization of the 2nd phase, a measure of EOD duration) of nine consecutive EODs were recorded provided that the fish was in the center of the tank (Stoddard et al., 2003) (Fig. 1A). During the night, the EOD was sampled at irregular intervals since the fish were more active and did not necessarily swim through the center of the tank during all sampling intervals. Therefore, we fitted a smoothing cubic spline function using the MATLAB Spline Toolbox (Mathworks, Natick MA) to the selected 48h data block of each parameter to interpolate for any gaps in the data collection (Stoddard et al., 2007b). We used the fitted data to calculate the peak-to-peak amplitude and τP2 values at the day minimum and the night maximum (Fig. 1B). The night-day changes were calculated by subtracting the day minimum from the night maximum (Fig. 1B).
Design of social treatment groups
From May to August, thirty-six male fish were randomly sampled from our outdoor colony for inclusion in the experiment. Baseline social conditions in our outdoor pools during the experiment period consisted of groups of 10-20 fish with 2-4 males per group. Each group experienced each of the following three social conditions (Fig. 2A) but in different order: Isolation (1 male, 0 juveniles, and 0 females), Social 2 (low male competition: 2 males, 4 juveniles, and 6 females), and Social 6 (high male competition: 6 males, 0 juveniles, and 6 females). In the two competition treatments, juveniles were included to keep the total number of fish constant.
Fig. 2.
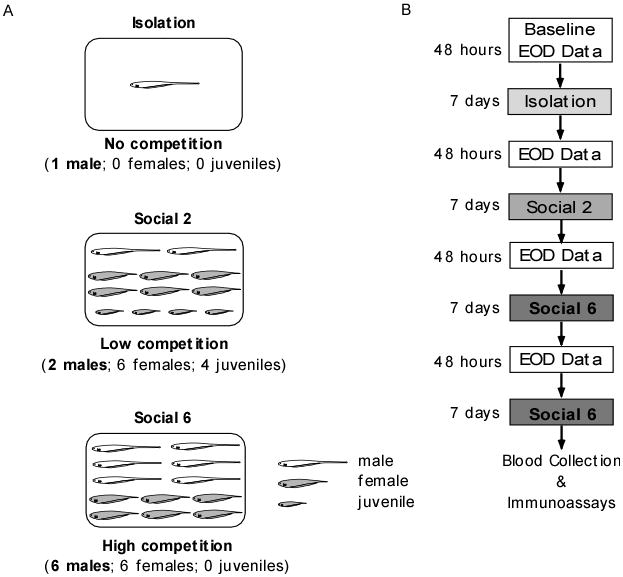
Social conditions and experimental design. A. We used three social conditions: Isolation, Social 2 and Social 6. For the Isolation condition, we housed males singly in a pool to deprive them of any social stimuli. For the Social 2 condition, we housed two males with six females and 4 juveniles in a pool to create a low competition social environment. For the Social 6 condition, we housed six males with six females in a pool to create a high competition social environment. B. We used a counterbalanced design where six groups of six males experienced all three social conditions but in different order. We chose this approach to evaluate the effect of social experience on the EOD changes displayed by the males at each social condition. For each group of six males, we recorded their EODs for 48 hours continuously at the beginning of each experimental order (baseline values) and after each social condition. Males remained at each social condition for a week. At the end of an experimental order, we chose one replicate for each condition, kept those males under this condition for another week and then bled them to assay their plasma for circulating levels of steroid hormones.
Before and after placement in each of the three social conditions, we weighed and measured each male, and recorded the circadian oscillation of his EOD for 48 hours in the EOD machine. Therefore, each group of fish alternated between being housed in outdoor pools under their respective social condition (isolation, Social 2, or Social 6) for seven days and being individually housed in tanks in the EOD machine for 48 hours (Fig. 2B). Since one group of six males went through one of the six possible combinations, at any time, males in the Social 6 group were together in one pool, males in the Social 2 group were housed in pairs in three pools, and socially isolated males were housed individually in six pools. At the end of the experiment, each social condition had two replicates (Fig. 2B). In all, the design had six groups of six males, counterbalanced by assigning subject males to six possible permutations of the three social treatments (Fig. 2B).
Blood collection
At the end of the experiment, fish were returned to their most recent social condition housing pools for another week (Fig. 2B). Then, six males from each social condition were quickly netted from their respective social pools in the late afternoon (15:00-16:00), lightly anesthetized by immersion for 2-3 min in a solution of 0.075% 2-phenoxyethanol, quickly bled from the ventral vertebral sinus, and returned back to their pools. We collected 50-200 μl of blood with a 10% EDTA-treated needle and syringe. The blood was transferred to a 10% EDTA-treated 0.5 ml polypropylene tube and kept on ice until centrifugation. Blood samples were centrifuged for 15 min at 7000 rpm using an Eppendorf MiniSpin centrifuge at 2-4 °C and the plasma was removed and stored at -80°C for later analysis. We also sampled blood from 12 females following the same protocol as with the males to quantify sex-specific differences in steroid hormone plasma levels. Females were sampled randomly from either Social 2 or Social 6 pools.
Steroid hormones analyses
For males in each of the three social treatments, circulating levels of unbound cortisol (F), testosterone (T), and 11-ketotestosterone (11-KT) were quantified in plasma using enzyme immunoassays (EIAs) specific for each hormone (Cayman Chemical Co.). The detection limits for these immunoassays were 1.2 × 10-2 ng/ml for cortisol, 1.3 × 10-3 ng/ml for testosterone and 6 × 10-3 ng/ml for 11-ketotestosterone. The Cayman EIA kits have sufficiently high sensitivity that after proper dilution (1:100 for isolated males and 1:500 for social males) one male plasma sample of 10 μl is sufficient for 3 triplicate immunoassays of three steroids.
We collected sufficient plasma from six females to assay 11-KT and T, but not cortisol, so we sampled six additional females to assay cortisol levels. From these additional plasma samples, four of the samples were sufficient to assay a second hormone so we measured circulating T levels a second time. To ensure that the hormone concentrations fell within the immunoassay detection range, at least two dilutions were tested in duplicates in pilot immunoassays. Because of their small body size, fish could not be bled more than once within a 2-3 weeks period, therefore all the fish in this experiment were only bled once. Fish were bled within the first 3 min after capture; therefore, we assumed that the plasma cortisol levels do not reflect the effects of handling during blood sampling (Fox et al., 1997; Pottinger and Moran, 1993), an assertion reinforced by uniformly low cortisol levels in our social isolates (see Results).
A pilot immunoassay using plasma aliquots from untreated fish detected assay interference. Therefore, we triple-extracted steroid hormones from the plasma using 4× sample volume of hexane:ethyl acetate (90:10 for samples tested for 11-KT and 70:30 for samples tested for T and F). Systematic pilot tests showed these solvent combinations and ratios yielded the best recoveries for all the hormone standards across the entire detection ranges of the kits. Extracted samples were evaporated in a vacuum centrifuge (Eppendorf Vacufuge, using the organic mode at 30°C) and reconstituted using the immunoassay kit's EIA buffer provided with Cayman's EIA kits. Standards of known concentrations were processed using the same extraction protocol applied to fish plasma samples to calculate percent recovery. Steroid hormones concentration values were adjusted to account for the percent recovery calculated for each assay.
Plasma samples were assayed in triplicate using two 96-well assay kits on the same day. The non-extracted and extracted standards were assayed in triplicate in both of the two 96-well plates. We used the extracted standard triplicates from the two plates for each hormone to calculate the intra-assay and inter-assay coefficients of variation. For the two cortisol plates, intra-assay variation was 2.6% and 1.9% and the inter-assay variation was 2.3%. For the two testosterone plates the intra-assay variation was 2.6% and 2.0% and the inter-assay variation was 2.3%. For the two 11-KT plates the intra-assay variation was 2.1% and 2.3% and the inter-assay variation was 2.2%. Cross-reactivities of the cortisol antiserum reported by the manufacturer were 100% for cortisol, 22% for prednisolone, 6.1% for cortexolone, 1.3% for corticosterone, 0.2% for DOC and 17-hydroxy-progesterone, and less than 0.01% for 18-hydroxy-DOC, progesterone, pregnenolone and 17-hydroxy-pregnenolone. The assays were highly specific for the steroids tested. Cross-reactivity of the testosterone antiserum was 100% for testosterone, 27.4% for 5α-dihydrotestosterone, 18.9% for 5β-dihydrotestosterone, 4.7% for methyltestosterone, 3.7% for androstenedione, 2.2% for 11-KT, 0.51% for 5-androstenediol, 0.2% for Epi-testosterone, 0.14% for progesterone, 0.11% for testosterone enanthane, 0.05% for androsterone, 0.04% for androsterone sulfate, 0.03% for testosterone sulfate, 0.02% for DHEA sulfate, and less than 0.01% for estradiol. The cross-reactivity for the 11-KT antiserum was 100% for 11-KT, 0.01 % for 4-androsten-11β,17b-diol-3-one, and less than 0.01% for testosterone, 5α-androstan-17β-ol-3-one and 5α-androsten-3β,17β-diol. We plated, incubated, and developed the samples following the kit manufacturer's instructions specific for each hormone tested. All developed plates were read at 405 nm with the ELx808 Ultramicroplate Reader (Biotek Instruments, Inc.) using the software interface KCJunior.
Data analyses
Plasma steroid levels were calculated against the standard curve (8 standards in triplicate) and the extraction recovery values using the Cayman Chemicals Analysis Tools (EIA tools available at http://www.caymanchem.com). We analyzed the effect of condition order versus the differences in EOD τP2 and amplitude across the social conditions using a two-way mixed ANOVA with repeated measures [GLM repeated measures procedure in SPSS v. 14.0, Model I – Fixed factors] with two factors: (1) social conditions [within-subjects factor with 4 levels = Baseline, Isolation, Social 2 and Social 6] and (2) condition order [between-subjects factor with 6 levels]. We log10 transformed all plasma steroid levels to fulfill the normality assumption. Whenever significant interactions were found between these 2 factors, we used one-way ANOVA for each social condition level and evaluated that social condition for each condition order. For non-significant interactions, we calculated the main effects' p-level across each variable. Fisher's LSD multiple comparison tests were calculated to determine significant pairwise differences. We used the GLM multivariate procedure in SPSS v.14.0 with social group as a fixed factor and steroid hormone as a covariate to evaluate the relationship between each steroid hormone with the dependent variables: day minima and night maxima for EOD τP2 and amplitude. We used multiple linear regression to evaluate the relationship between each steroid hormone with body length and mass. All statistical analyses were performed with MATLAB or SPSS v.14.0, α=0.05 two-tailed.
Results
Order of social experiences influences their effects on the EOD
The magnitude of the males' EOD τP2 in any particular social treatment condition depended on the order in which they experienced the three social treatments. Social condition treatment showed a significant order effect in daytime EOD τP2 (social condition × condition order interaction: F (15, 75) = 2.58, p = 0.004). Males that experienced isolation as their first treatment (Fig. 3, orders 3 & 6) depressed their daytime EOD τP2 significantly below baseline levels (LSD post-hoc tests, p < 0.001 and p = 0.011, respectively). Furthermore, the males in these orders did not recover their previous daytime EOD τP2 upon experiencing either the low or high competition conditions (Fig.3). By comparison, males in all other orders produced mean daytime EOD τP2 values during the low or high competition conditions that matched or exceeded their baseline levels (Fig. 3). Although we found a significant social condition × condition order interaction (GLM repeated measures: F (15, 66) = 2.12, p = 0.02) in daytime EOD amplitude, post hoc tests revealed no significant differences between social conditions at each condition order.
Fig. 3.
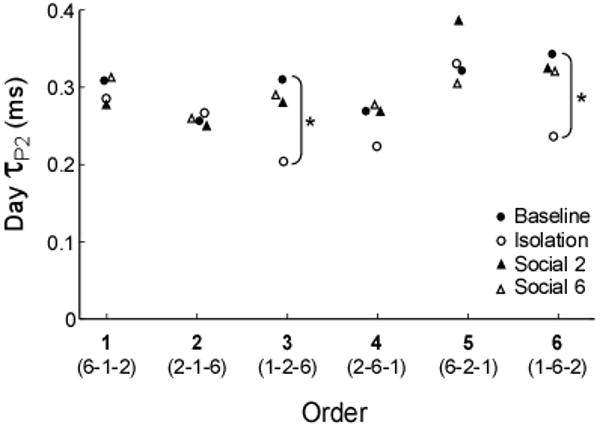
Males displayed differences in their EOD circadian rhythm plasticity according to the order they experienced the different social conditions. The magnitude of the day EOD τP2 was affected by social experience. Particularly, males that experienced the Isolation condition first in the order displayed significantly lower day EOD τP2 values than their prior baseline values. In addition, when compared to the day EOD τP2 of the males on the other orders, the day EOD τP2 of these males (orders 3 and 6) was lower than baseline levels during both the Social 2 and the Social 6 conditions.
Differences in EOD circadian rhythm magnitudes across the different social conditions
Mean EOD amplitude tracked the differences in level of competition (Social 6 > Social 2 > Isolation), while mean EOD τP2 only tracked presence or absence of a social environment (Social > Isolation). Overall, social competition increased the magnitudes of minimum daytime values when the fish were at rest (amplitude: F = 11.07, p < 0.001; τP2 : F = 8.18, p < 0.001), maximum nighttime values when the fish were active (amplitude: F = 10.55, p < 0.001; τ P2 : F = 8.17, p < 0.001), and day-night differences reflecting the magnitudes of circadian rhythms (amplitude: F = 5.06, p < 0.001; τ P2 : F = 13.08, p < 0.001) (Fig. 4).
Fig. 4.
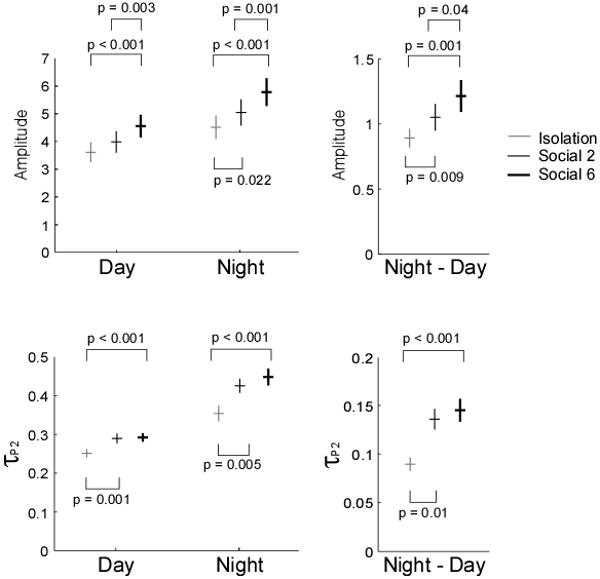
Raw data (dots) and mean±SEM are shown for the EOD parameters amplitude and τP2 across the social conditions. Daily minima, nightly maxima, and day-night differences follow the same trends wherein the highest competition (Social 6) promoted the highest values and social isolation promoted the smallest. P values are derived from post-hoc LSD pairwise tests following repeated-measures ANOVA.
Differences in plasma steroid levels across the different social conditions
Plasma concentrations of both androgens varied significantly with social condition and sex (T: F (3, 20) = 39.53, p < 0.001; 11-KT: F (3, 20) = 3.84, p = 0.025) (Fig. 5). Overall, males' androgen levels were higher in both social conditions than in social isolation, but did not differ significantly between high and low social competition conditions. Females sampled from Social 2 and Social 6 pools had plasma 11-KT levels comparable to those of isolated males, but surprisingly, had testosterone levels significantly higher than males in any of the three social treatments (Fig. 5).
Fig. 5.
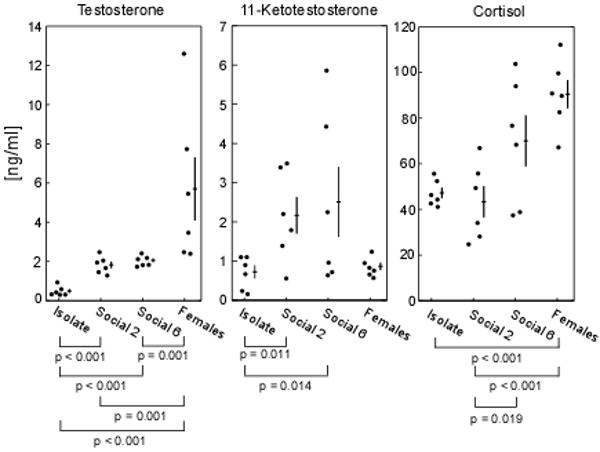
Plasma levels of T, 11-KT and cortisol (ng/ml), varied significantly with social condition and sex. Both T and 11-KT were lower in isolated males than in social males. Although females' T levels were higher than males', their 11-KT levels were similar to those of isolated males. High competition males' cortisol levels were higher than low competition males'. Females displayed higher cortisol levels than those of isolated and low competition males. Filled circles depict raw values while crosses depict mean ± SEM. Significant p-values from Fisher's LSD post-hoc pairwise comparisons are also shown.
The cortisol pattern followed the overall pattern of the androgens with one key difference – cortisol appeared to track the intensity of competition (number of males in the pool), whereas androgens tracked only the presence or absence of competition. Mean levels and variability of cortisol differed significantly between social conditions and sexes (F = 7.587; p = 0.001; Bartlett's test of variance = 8.95, p = 0.03), (Fig. 5). Cortisol levels among males in the high competition group, Social 6, were significantly higher than for males in the low competition group, Social 2, (p = 0.019, Fisher's LSD post-hoc test). While mean cortisol levels were comparable between isolation and Social 2, the latter was far more variable. Cortisol levels in females were high, comparable to those of males in the Social 6 treatment, and significantly higher than in the isolated and low competition males (p < 0.001 for both), (Fig. 5). Recall that females were sampled directly from Social 2 and Social 6 pools, a sex-specific competitive environment comparable to the Social 6 treatment that males received.
When looking at the variance in the steroid levels of males across the three social conditions, we found no significant differences in T levels (Levene statistic1,10: Isolation-Social2 = 2.09, p = 0.18, Isolation-Social6 = 0.15, p = 0.70, and Social2-Social6 = 1.76, p = 0.21). We found a significant difference in 11-KT levels for the Isolation-Social6 comparison (Levene statistic1,10 = 11.83, p = 0.006), but not for the Isolation-Social2 (Levene statistic1,10 = 4.58, p = 0.06) or Social2-Social6 (Levene statistic1,10 = 3.43, p = 0.09) comparisons. We also found a significant difference in cortisol levels for the Isolation-Social6 (Levene statistic1,10 = 8.53, p = 0.02) and Isolation-Social2 (Levene statistic1,10 = 11.97, p = 0.006) comparisons, but not for the Social2-Social6 (Levene statistic1,10 = 1.38, p = 0.27) comparison. In addition, we found no significant relationships between T, 11-KT and cortisol among the males at each social condition. For instance, T levels did not predict 11-KT levels (Isolation: F (1,5) = 1.93, R2 = 0.32, p (2-tailed) = 0.24, Social 2: F (1,5) = 1.13, R2 = 0.22, p (2-tailed) = 0.35, and Social 6: F (1,5) = 3.38, R2 = 0.46, p (2-tailed) = 0.14). Also, T levels did not predict cortisol levels (Isolation: F (1,5) = 0.26, R2 = 0.06, p (2-tailed) = 0.64, Social 2: F (1,5) = 0.40, R2 = 0.09, p (2-tailed) = 0.56, and Social 6: F (1,5) = 0.005, R2 = 0.001, p (2-tailed) = 0.94). In addition, 11-KT levels did not predict cortisol levels in males at each social condition (Isolation: F (1,5) = 0.54, R2 = 0.12, p (2-tailed) = 0.50, Social 2: F (1,5) = 0.34, R2 = 0.08, p (2-tailed) = 0.59, and Social 6: F (1,5) = 0.27, R2 = 0.06, p (2-tailed) = 0.63).
Relationship between steroid hormone levels and the EOD circadian rhythm across the different social conditions
Cortisol plasma levels covaried significantly and strongly with the EOD circadian rhythm across the different social conditions (Wilks' λ = 7.94, p=0.003, eta-squared = 0.74, observed power = 0.97). Plasma levels of cortisol were significantly and strongly related to daytime and nighttime EOD amplitude (univariate between-subjects tests; day amplitude: p = 0.001, partial eta-squared = 0.57, observed power = 0.98 and night amplitude: p < 0.001, partial eta-squared = 0.60, observed power = 0.99). In contrast, plasma levels of cortisol were not significantly related to daytime or nighttime EOD τP2 (Univariate between-subjects tests; day τP2: p = 0.874, partial eta-squared = 0.002, observed power = 0.05 and night τP2: p = 0.25, partial eta-squared = 0.09, observed power = 0.20). When evaluating these relationships across each social group, we found that plasma cortisol levels positively predicted EOD amplitude in males under both low and high competition treatments but not in the isolated males, which showed almost no variance in cortisol (Isolates: day R2 = 0.24, p = 0.32, night R2 = 0.26, p = 0.30; Social 2: day R2 = 0.71, p = 0.035, night R2 = 0.70, p = 0.038; Social 6: day R2 = 0.90, p = 0.004, night R2 = 0.90, p = 0.004) (Fig. 6). Plasma cortisol levels and EOD τP2 showed no apparent relationship (Fig. 6).
Fig. 6.
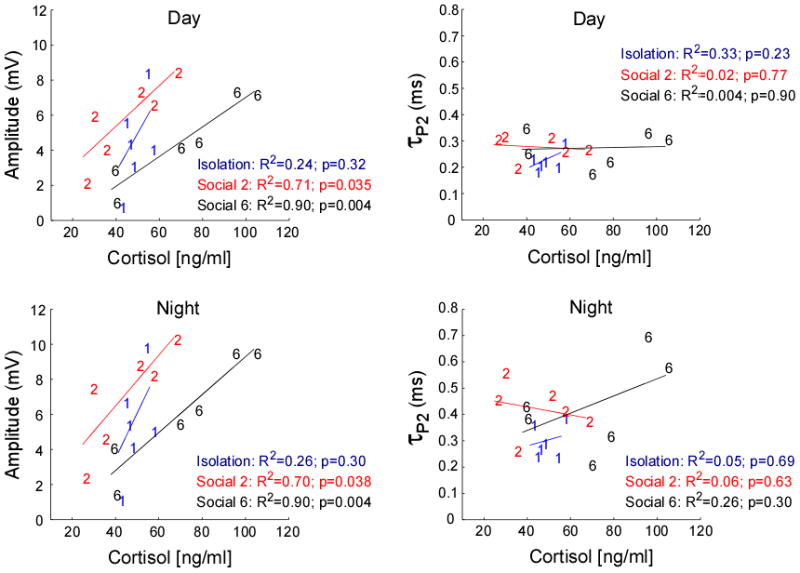
As plasma cortisol levels (ng/ml) increase, EOD amplitude (mV) increases for the low and high competition males but not for the isolated males (low competition; day: y = 0.12x + 0.77 & night: y = 0.15x + 0.70 and high competition; day: y = 0.09x − 1.44 & night: y = 0.11x − 1.52). EOD τP2 and cortisol were not related in our dataset.
Neither T nor 11-KT plasma levels covaried with the EOD circadian rhythm across the different social conditions (11-KT: Wilks' λ = 1.68, p = 0.22, partial eta-squared = 0.38, observed power = 0.36, and T: Wilks' λ = 0.173, p = 0.95, partial eta-squared = 0.06, observed power = 0.08). Even though we saw no significant relationship between either androgen and EOD amplitude or τP2 at each social treatment (Fig. 7 & 8), when the three social treatments were pooled, T levels positively predicted EOD τP2 at night (R2 = 0.22, p = 0.05) (Supplementary Materials). We found no significant relationship between plasma T levels and the EOD amplitude when all social treatments were pooled. We also found no significant relationship between 11-KT and the EOD amplitude or EOD τP2 on pooled analysis (Fig. 8).
Fig. 7.
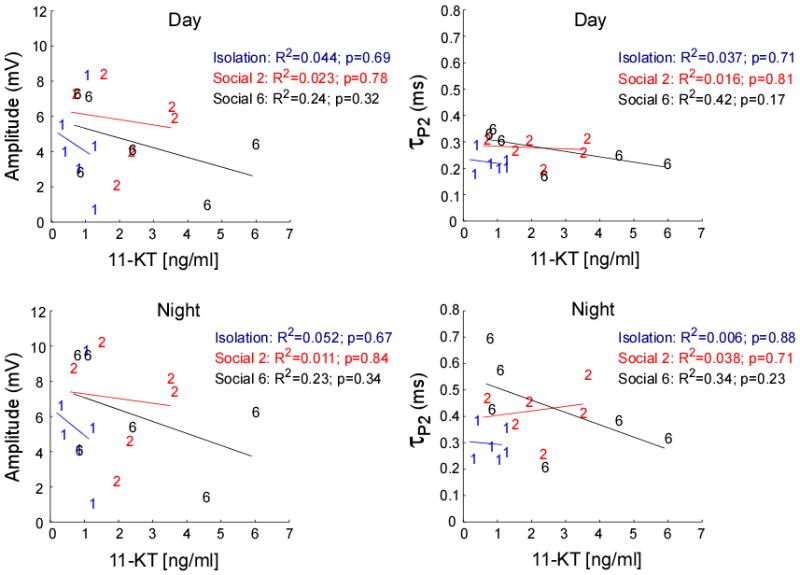
Testosterone plasma levels were not significantly related to EOD amplitude or τP2 at any of the three conditions, isolation, Social 2 or Social 6.
Fig. 8.
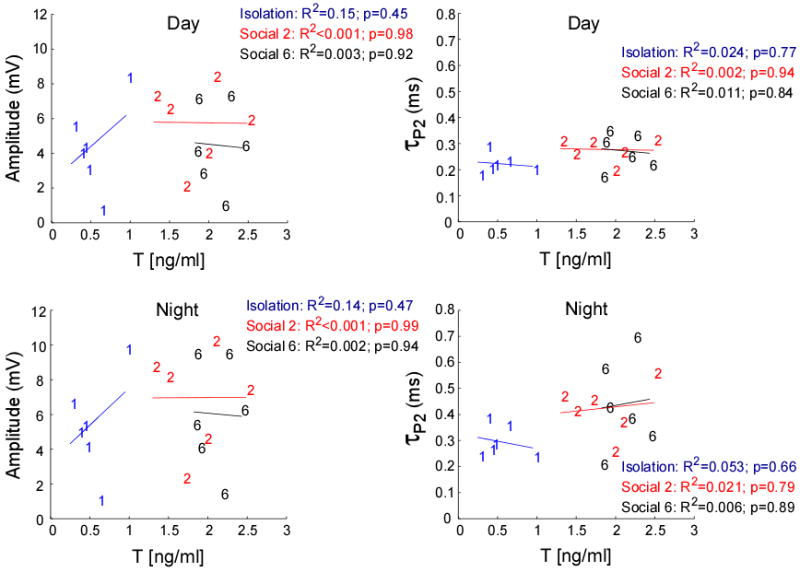
11-ketotestosterone plasma levels were not significantly related to EOD amplitude or τP2 at any of the three conditions, isolation, Social 2 or Social 6.
We found significant relationships between the two key EOD parameters amplitude and τP2. Day-night change in EOD amplitude positively predicted EOD τP2 (R2 = 0.23, p = 0.046), whereas the day minima and night maxima were not associated in either parameter (day: R2 = 0.02, p = 0.63; night: R2 = 0.09, p = 0.24) (data not shown).
Relationship between steroid hormone levels and body size
Both body length and mass positively predicted plasma cortisol levels in the high competition condition only (length: R2 = 0.82, p = 0.045; mass: R2 = 0.65, p = 0.05) (Fig. 9). Body length was associated with mass (Fig. 9) although the effect was weakest and thus not significant in isolated males (isolates: R2 = 0.39, p =0.19; Social 2: R2 = 0.79, p = 0.018; Social 6: R2 = 0.97, p < 0.001). In addition, although the mean mass of all the males was similar across all three social conditions, a high proportion of Social 2 males lost weight (66%) (χ2 = 19; df = 2; p < 0.001).
Fig. 9.
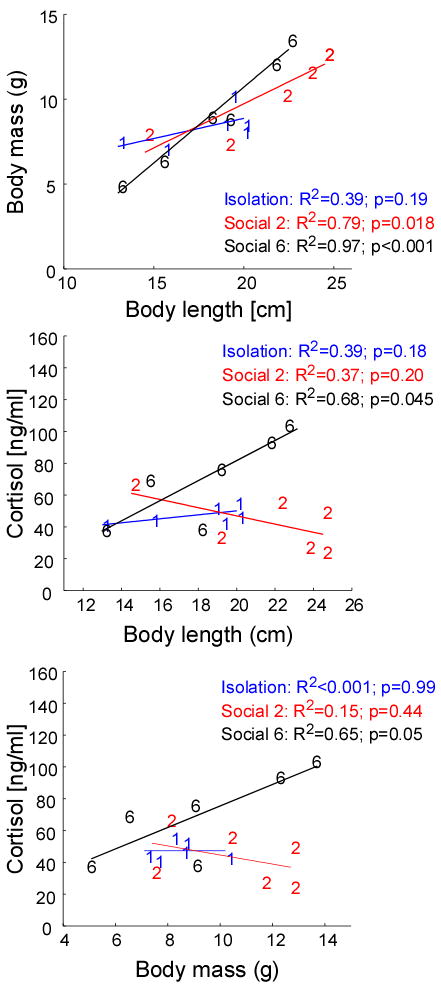
Body length (cm) predicts body mass (g) in low and high competition males butnot in isolated males (low competition; y = 0.52x − 0.67 and high competition; y = 0.89x − 7.15). Only under high competition (Social 6), the body length (cm) and body mass (g) of the males predicts their circulating plasma cortisol levels (ng/ml). The regression models are y = 6.21x − 43.24 for body length vs. cortisol and y = 6.75x + 8.40 for body mass vs. cortisol.
Discussion
Our results demonstrate that changes in the social environment of the gymnotiform fish B. gauderio males are accompanied by changes in both their electric signals and their steroid hormone profiles. By increasing the number of male competitors in a social group, we show that the EOD amplitude, but not the EOD τP2, is responsive to these changes. In addition, we also show an increase in the plasma cortisol levels, but not androgens, of these males as a function of the increase in the number of male competitors in the social group. In contrast, the EOD τP2 and the plasma androgen levels only increased significantly when the males transition from social isolation to social conditions (either low or high competition). Our findings also suggest that altering the order in which males experienced isolation versus social competition had dramatic effects on their ability to fully enhance their electric signals during periods of intense competition for mates.
Etho-ecological validity of our study
Although both male competition groups included six females, the sex ratio between these two social conditions varied (low competition: 1 male: 3 females versus high competition: 1 male: 1 female). Three weeks into the breeding season, a field study of B. gauderio populations in Uruguay found a 1:4 female-biased operational sex ratio across the multiple sites (Miranda et al., 2008). Males were spaced out in their habitat and displayed non-overlapping home ranges, while females occupied much larger ranges overlapping other females and the home ranges of multiple males (Miranda et al., 2008). B. gauderio spatially aggregated by day and night, keeping a meter or less of distance between each other in an uniform habitat (Miranda et al., 2008). Based on these findings, we suggest that of the three pool conditions, the low competition condition (Social 2) is closest to the densities and sex ratios observed in wild B. gauderio populations partway through the breeding season (initial conditions have not been observed). It is important to note that although the males in the high competition group were potentially subjected to fewer reproductive opportunities, we collected comparable numbers of eggs from both the low and high competition pools throughout the experiment indicating that our experimental design and manipulations did not disrupt breeding success in our test fish.
Social environment affects the EOD plasticity and steroid levels
Our experimental design revealed an effect of past social environment on the outcome of present and future social interactions. The males that experienced social competition as their first treatment were more resilient to reduction of their basal (daytime) EOD τP2 when subsequently isolated (orders 1, 2, 4 and 5; Fig. 3). It is important to note that although all the fish in this experiment were randomly sampled from social groups of 10-20 fish with 2-4 males per group (baseline social conditions), and that these conditions are equivalent to what they experienced in the experimental social conditions, the baseline social conditions were sustained, stable conditions which adult fish had experienced since they were juveniles. We believe that it is the combined effect of initial absence of non-specific social stimuli followed by a continually-changing social environment (fish were moved every 7 days to a different social condition for almost a month) what drives the differences observed in the isolation treatment for those condition orders where isolation was experienced first (orders 3 & 6; Fig. 3).
Social isolation suppressed both EOD parameters and androgen levels of male B. gauderio. Isolated males had the lower EOD circadian rhythm magnitudes than males in either the low or the high competition conditions (Fig. 4). These results agree with the findings reported by Franchina and her colleagues (2001), wherein males isolated for 3-5 days displayed a significant reduction in the circadian modulation of their EOD amplitude and duration. In our study, isolated males reduced their day and night EOD waveforms (Fig. 4) along with circulating plasma levels of the androgens T and 11-KT (Fig. 5, 7 and 8). Our isolation treatment not only removed all potential male competitors but it also removed all potential mates and non-sexual social stimuli (i.e., juveniles). Therefore, we cannot rule out the possibility that the reductions observed in the EOD and androgens are due to the lack of complete non-specific social stimuli rather than specifically due to the lack of male competitors. Cortisol levels were significantly higher under high competition than low competition, but were not higher in low competition than isolation, though isolates had significantly less variance (Fig. 5). Nevertheless, gymnotiform Apteronotus leptorhynchus size-matched male pairs displayed significantly higher circulating levels of cortisol and more EOD chirps, an agonistic EOD frequency modulation, than isolated males (Dunlap et al., 2002). Even though males were tested under isolation for 7 days in both studies, Dunlap and his colleagues (2002) kept their males isolated for 2-weeks prior to their experiments, while our fish were under either baseline social conditions or the experimental social conditions (Social 2 and Social 6) before they were isolated. In addition, when compared to Apteronotus leptorhynchus, B. gauderio's baseline cortisol levels are much higher (Dunlap et al., 2002) (Fig. 5 and Supplementary Materials). Elevated cortisol levels during the breeding season is a pervasive adaptive strategy among vertebrates (Landys et al., 2006), a mechanism to deal with high metabolic demands associated with reproduction (Wingfield and Kitaysky, 2002). Either of these factors can account for the lack of difference in cortisol levels between isolated and social males in our study.
EOD power (i.e., voltage squared or the voltage area under both waveform's phases at a constant resistance) perfectly matches the energy expended on electrogenesis (Salazar and Stoddard, 2008). Thus for isolated males to reduce both basal EOD parameters and their circadian augmentation appears to be an adaptive strategy to minimize energetic costs when social benefits due to the enhanced signal parameters are absent. The EOD of B. gauderio males seems to be a condition-dependent trait (Salazar and Stoddard, 2008). Male B. gauderio, like male orthopterans, frogs, and some birds, invest a considerable fraction of their metabolic energy into signal production (Bucher et al., 1982; Eberhardt, 1994; Hoback and Wagner, 1997; Kavanagh, 1987; Prestwich et al., 1989; Prestwich and Walker, 1981; Taigen and Wells, 1985). While androgens typically facilitate male signaling behavior, elevated glucocorticoids would be expected to release energy stores necessary to sustain signaling for high-energy signalers. Although high glucocorticoid levels are typically related to the suppression of androgen levels (Sapolsky, 1994; Sapolsky et al., 2000), in many species, both glucocorticoid and androgen levels are increased during reproduction (Beletsky et al., 1989; Creel et al., 1996; Emerson and Hess, 2001; Remage-Healey and Bass, 2005).
Social competition affects EOD amplitude's circadian plasticity and cortisol levels
Cortisol and EOD amplitude covary positively with density of males in the social group (Figs. 4-6), suggesting that cortisol may be a modulator of the EOD amplitude. We do not know if this potential modulatory effect is direct or indirect. Activating the hypothalamic-pituitary-interrenal (HPI) axis at various levels enhances the EOD amplitude and τP2 in B. gauderio males (Markham et al., 2009; Markham and Stoddard, 2005). But only the melanocortins, adrenocorticotropic hormone (ACTH) and alpha-melanocyte-stimulating hormone (α-MSH), exert rapid effects directly at the electrocytes (Markham et al., 2009; Markham and Stoddard, 2005). In this study, we measured cortisol levels several days after the males had been housed at their specific social conditions. Although cortisol could directly initiate slow transcriptional effects that can drive the enhancement of the EOD amplitude within this period of time, ACTH and/or α-MSH can increase both the EOD waveform and cortisol levels independently within the same period of time. Therefore, we cannot rule out the possibility that the relationship that we observed between cortisol and EOD amplitude could be mediated by the effect of social experience on melanocortins. Beyond that, cortisol may support the enhanced EOD by increasing availability of glucose and lipids to support energetically costly signaling and swimming behaviors associated with territoriality and courtship (Landys et al., 2006; Sapolsky et al., 2000).
Increases in the density of competing males or in the number of perceived competitors in a population have been shown to increase androgen levels in other teleost fish (Carlson et al., 2000; Oliveira et al., 2001; Pankhurst and Barnett, 1993; Remage-Healey and Bass, 2005). As discussed by Oliveira and his colleagues (2002) and as predicted by the Challenge Hypothesis (Wingfield et al., 1990), an increase in potential competitors could intensify agonistic interactions leading to the activation of the hypothalamic-pituitary-gonadal (HPG) axis and an increase in circulating androgen levels. Unexpectedly, circulating androgen levels did not differ for males in the low and high competition groups (Fig. 5). However, if only one male dominates in the low competition pool and one or two males dominate in the high competition pools, they alone might show elevated androgens while suppressing androgen levels in the subordinates. Thus variance would increase but not group mean, a hypothesis consistent with the obtained distribution of 11-KT measurements (Fig. 5). Nevertheless, we only found a significant difference in the variance of plasma levels of 11-KT between the isolated males and the males in the low competition group. Carlson and his colleagues (2000) found in the mormyrid fish Brienomyrus brachyistius, an African electric fish, that the dominance status of males housed in social groups determined the levels of 11-KT and the changes on their EOD total duration. We did not assess the dominance status of the males in our study. Body size is usually a good predictor of dominance status in size-mismatched male-male contests (Maynard Smith, 1982; Parker, 1974). We randomly paired the males in the low competition (Social 2) condition rather than matching them for size. Eleven of the eighteen Social 2 pairs were size-mismatched, and all three Social 2 pairs assayed for steroid levels were also size-mismatched. Therefore, it is possible that by looking at the males as a group in each social condition rather than as subgroups (i.e., alpha males, beta males and omega males) based on their dominance status (Carlson et al., 2000), we were not able to identify the link between 11-KT levels and male-male competition as we increased the number of competing males.
In both gymnotiform and mormyrid electric fish species, the electric organ (EO) that generates the EOD has been shown to be a direct androgen-target tissue (Few and Zakon, 2001), and androgen receptors have been identified in the nuclei of the electrocytes that composed the EO (Bass et al., 1986; Dunlap and Zakon, 1998). Furthermore, androgens directly change the ion conductances of the electrocytes leading to changes in the EOD waveform (Zakon et al., 1999). Androgen implants to male and female B. gauderio (Allee et al., 2009; Silva et al., 2002; Stoddard et al., 2007a) and its congener B. occidentalis (Hagedorn and Carr, 1985) enhance the duration of the 2nd phase of the EOD but have little or no effect on EOD amplitude. Androgen implants increase the effect of melanocortins on the EOD of B. gauderio (Allee et al., 2009; Stoddard et al., 2007a). Therefore, we speculate that during the breeding season, in B. gauderio, interaction with competing males and prospective mates stimulates the HPG axis leading to an increase in baseline circulating androgen levels. Higher level of circulating androgens would enhance the EOD τP2. The additional metabolic cost associated with the enhancement of the EOD via androgens and melanocortins would be sustained by increasing cortisol to release energy.
Sex differences in steroid hormone levels across gymnotiform species
In teleost fish, 11-KT and T are the major androgens in circulation in breeding males (Kime, 1993). In many species, females have similar or higher levels of the aromatizable androgen, testosterone (Borg, 1994). In our study, B. gauderio females had significantly higher plasma levels of T than the males irrespective of their experimental condition (Fig. 5). Females tend to have much lower levels of 11-KT than breeding males (Borg, 1994). Accordingly, the plasma 11-KT levels of B. gauderio females were very similar to those observed in isolated males and were in the lower end of the range of plasma 11-KT levels observed in the males under social conditions (Fig. 5). The androgen profiles of B. gauderio males and females follow a similar trend as in other gymnotiform species tested, where females have lower 11-KT levels than males but similar or higher levels of T (Supplementary Materials).
Although females had similar plasma cortisol levels than the males in the high competition group, they displayed higher levels of cortisol when compared to isolated males and the males in the low competition group (Fig. 5). To our knowledge, this is the first time that circulating plasma cortisol levels for female gymnotiform fish have been reported. As mentioned earlier, B. gauderio baseline cortisol levels are much higher than the cortisol levels reported for one other gymnotiform species (Dunlap et al., 2002).
In conclusion, social environment regulates the enhanced male EOD potentially via 11-ketotestosterone and cortisol. Social competition further enhances the day to night changes in male EOD suggesting that circadian regulation of the EOD plays a role in male-male interactions. Cortisol levels increase in males as the level of competition increases, and cortisol is related to the social males' body size. Since the male EOD is an energetically expensive trait, we speculate that cortisol may be regulating EOD amplitude directly as well indirectly by providing the fuel to sustain it.
Supplementary Material
Acknowledgments
We thank S. Allee, A. Goldina, M. Markham and C. Muñoz for help with data collection. We thank M. Perez and F. Noriega for help with some of the validation steps for the immunoassays and for use of the plate reader. Dr. Andrew H. Bass provided valuable comments on an earlier version of the manuscript. This work was supported by NIH-MBRS grant GM08205 to P.K.S., and a Graduate Students Association's Research Grant and a University Graduate School's Dissertation Year Fellowship at Florida International University to V.L.S. This paper is contribution no. xxx of the FIU Tropical Biology Program.
Footnotes
Recently, B. pinnicaudatus was divided into two species. Specimens found in the northern range of its distribution remain as B. pinnicaudatus, while those found in the southern range are now classified as B. gauderio. Drs. William Crampton and David de Santana have confirmed that our laboratory colony originated from the southern species B. gauderio.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Allee SJ, Markham MR, Stoddard PK. Androgens enhance plasticity of an electric communication signal in female knifefish, Brachyhypopomus pinnicaudatus. Horm Behav. 2009 doi: 10.1016/j.yhbeh.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M. Sexual selection. Princeton University Press; Princeton: 1994. [Google Scholar]
- Bass AH, Segil N, Kelley DB. Androgen binding in the brain and electric organ of a mormyrid fish. J Comp Physiol A. 1986;159:535–44. doi: 10.1007/BF00604173. [DOI] [PubMed] [Google Scholar]
- Bass AH, Volman SF. From behavior to membranes: testosterone-induced changes in action potential duration in electric organs. Proc Natl Acad Sci U S A. 1987;84:9295–8. doi: 10.1073/pnas.84.24.9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletsky L, Orians G, Wingfield J. Relationships of steroid hormones and polygyny to territorial status, breeding experience, and reproductive success in male red-winged blackbirds. Auk. 1989;106:107–117. [Google Scholar]
- Bennett MV. Comparative physiology: electric organs. Annu Rev Physiol. 1970;32:471–528. doi: 10.1146/annurev.ph.32.030170.002351. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Pappas GD, Gimenez M, Nakajima Y. Physiology and ultrastructure of electrotonic junctions. IV. Medullary electromotor nuclei in gymnotid fish. J Neurophysiol. 1967;30:236–300. doi: 10.1152/jn.1967.30.2.236. [DOI] [PubMed] [Google Scholar]
- Borg B. Androgens in teleost fishes. Comp Biochem Physiol C. 1994;109:219–245. [Google Scholar]
- Bucher TL, Ryan MJ, Bartholomew GA. oxygen consumption during resting, calling, and nest building in the frog Physalaemus pustulosus. Physiol Zool. 1982;55:10–22. [Google Scholar]
- Carlson BA, Hopkins CD, Thomas P. Androgen correlates of socially induced changes in the electric organ discharge waveform of a mormyrid fish. Horm Behav. 2000;38:177–86. doi: 10.1006/hbeh.2000.1613. [DOI] [PubMed] [Google Scholar]
- Creel S, Creel N, Monfort S. Social stress and dominance. Nature. 1996;379:212. [Google Scholar]
- Curtis CC, Stoddard PK. Mate preference in female electric fish, Brachyhypopomus pinnicaudatus. Anim Behav. 2003;66:329–336. [Google Scholar]
- Dunlap KD. Hormonal and body size correlates of electrocommunication behavior during dyadic interactions in a weakly electric fish, Apteronotus leptorhynchus. Horm Behav. 2002;41:187–94. doi: 10.1006/hbeh.2001.1744. [DOI] [PubMed] [Google Scholar]
- Dunlap KD, Pelczar PL, Knapp R. Social interactions and cortisol treatment increase the production of aggressive electrocommunication signals in male electric fish, Apteronotus leptorhynchus. Horm Behav. 2002;42:97–108. doi: 10.1006/hbeh.2002.1807. [DOI] [PubMed] [Google Scholar]
- Dunlap KD, Zakon HH. Behavioral actions of androgens and androgen receptor expression in the electrocommunication system of an electric fish, Eigenmannia virescens. Horm Behav. 1998;34:30–8. doi: 10.1006/hbeh.1998.1460. [DOI] [PubMed] [Google Scholar]
- Dye JC, Meyer JH. Central control of the electric organ discharge in weakly electric fish. In: Bullock TH, Heiligenberg W, editors. Electroreception. Wiley; New York: 1986. pp. 71–102. [Google Scholar]
- Eberhardt LS. Oxygen consumption during singing by male Carolina wrens (Thryothorus ludovicianus) Auk. 1994;111:124–130. [Google Scholar]
- Elofsson UO, Mayer I, Damsgard B, Winberg S. Intermale competition in sexually mature arctic charr: effects on brain monoamines, endocrine stress responses, sex hormone levels, and behavior. Gen Comp Endocrinol. 2000;118:450–60. doi: 10.1006/gcen.2000.7487. [DOI] [PubMed] [Google Scholar]
- Emerson SB, Hess DL. Glucocorticoids, androgens, testis mass, and the energetics of vocalization in breeding male frogs. Horm Behav. 2001;39:59–69. doi: 10.1006/hbeh.2000.1635. [DOI] [PubMed] [Google Scholar]
- Few WP, Zakon HH. Androgens alter electric organ discharge pulse duration despite stability in electric organ discharge frequency. Horm Behav. 2001;40:434–42. doi: 10.1006/hbeh.2001.1709. [DOI] [PubMed] [Google Scholar]
- Fox HE, White SA, Kao MH, Fernald RD. Stress and dominance in a social fish. J Neurosci. 1997;17:6463–9. doi: 10.1523/JNEUROSCI.17-16-06463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchina CR, Salazar VL, Volmar CH, Stoddard PK. Plasticity of the electric organ discharge waveform of male Brachyhypopomus pinnicaudatus. II. Social effects. J Comp Physiol A. 2001;187:45–52. doi: 10.1007/s003590000176. [DOI] [PubMed] [Google Scholar]
- Franchina CR, Stoddard PK. Plasticity of the electric organ discharge waveform of the electric fish Brachyhypopomus pinnicaudatus. I. Quantification of day-night changes. J Comp Physiol A. 1998;183:759–68. doi: 10.1007/s003590050299. [DOI] [PubMed] [Google Scholar]
- Giora J, Malabarba LR. Brachyhypopomus gauderio, new species, a new example of underestimated species diversity of electric fishes in the southern South America (Gymnotiformes: Hypopomidae) Zootaxa. 2009;2093:60–68. [Google Scholar]
- Goymann W, Wingfield JC. Allostatic load, social status and stress hormones: the costs of social status matter. Anim Behav. 2004;67:591–602. [Google Scholar]
- Grafen A. Biological signals as handicaps. J Theor Biol. 1990;144:517–46. doi: 10.1016/s0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Carr C. Single electrocytes produce a sexually dimorphic signal in South American electric fish, Hypopomus occidentalis (Gymnotiformes, Hypopomidae) J Comp Physiol A. 1985;156:511–523. [Google Scholar]
- Hoback WW, Wagner WEJ. The energetic cost of calling in the variable field cricket, Gryllus lineaticeps. Physiol Entomol. 1997;22:286–290. [Google Scholar]
- Kavanagh MW. The efficiency of sound production in two cricket species, Gryllotalpa australis and Teleogryllus commodus (Orthoptera: Grylloidea) J exp Biol. 1987;130:107–119. [Google Scholar]
- Kawasaki M, Heiligenberg W. Distinct mechanisms of modulation in a neuronal oscillator generate different social signals in the electric fish Hypopomus. J Comp Physiol A. 1989;165:731–41. doi: 10.1007/BF00610872. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Heiligenberg W. Different classes of glutamate receptors and GABA mediate distinct modulations of a neuronal oscillator, the medullary pacemaker of a gymnotiform electric fish. J Neurosci. 1990;10:3896–904. doi: 10.1523/JNEUROSCI.10-12-03896.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CH, Kawasaki M, Heiligenberg W. The control of pacemaker modulations for social communication in the weakly electric fish Sternopygus. J Comp Physiol A. 1991;169:441–50. doi: 10.1007/BF00197656. [DOI] [PubMed] [Google Scholar]
- Kime DE. ‘Classical’ and ‘non-classical’ reproductive steroids in fish. Rev Fish Biol Fish. 1993;3:160–180. [Google Scholar]
- Landys MM, Ramenofsky M, Wingfield JC. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol. 2006;148:132–49. doi: 10.1016/j.ygcen.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Markham MR, Allee SJ, Goldina A, Stoddard PK. Melanocortins regulate the electric waveforms of gymnotiform electric fish. Horm Behav. 2009;55:306–313. doi: 10.1016/j.yhbeh.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham MR, Stoddard PK. Adrenocorticotropic hormone enhances the masculinity of an electric communication signal by modulating the waveform and timing of action potentials within individual cells. J Neurosci. 2005;25:8746–54. doi: 10.1523/JNEUROSCI.2809-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J. Evolution and the Theory of Games. Cambridge University Press; Cambridge: 1982. [Google Scholar]
- Mills A, Zakon HH. Chronic androgen treatment increases action potential duration in the electric organ of Sternopygus. J Neurosci. 1991;11:2349–61. doi: 10.1523/JNEUROSCI.11-08-02349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A, Zakon HH, Marchaterre MA, Bass AH. Electric organ morphology of Sternopygus macrurus, a wave-type, weakly electric fish with a sexually dimorphic EOD. J Neurobiol. 1992;23:920–32. doi: 10.1002/neu.480230712. [DOI] [PubMed] [Google Scholar]
- Miranda M, Silva AC, Stoddard PK. Use of space as an indicator of social behavior and breeding systems in the gymnotiform electric fish Brachyhypopomus pinnicaudatus. Environ Biol Fish. 2008;83:379–389. [Google Scholar]
- Neat FC, Taylor AC, Huntingford FA. Proximate costs of fighting in male cichlid fish: the role of injuries and energy metabolism. Anim Behav. 1998;55:875–82. doi: 10.1006/anbe.1997.0668. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AV. Social modulation of androgen levels in male teleost fish. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:203–15. doi: 10.1016/s1096-4959(01)00523-1. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Lopes M, Carneiro LA, Canario AV. Watching fights raises fish hormone levels. Nature. 2001;409:475. doi: 10.1038/35054128. [DOI] [PubMed] [Google Scholar]
- Overli O, Harris CA, Winberg S. Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav Evol. 1999;54:263–75. doi: 10.1159/000006627. [DOI] [PubMed] [Google Scholar]
- Pankhurst NW, Barnett CW. Relationship of population density, territorial interaction and plasma levels of gonadal steroids in spawning male demoiselles Chromis dispilus (Pisces: Pomacentridae) Gen Comp Endocrinol. 1993;90:168–76. doi: 10.1006/gcen.1993.1071. [DOI] [PubMed] [Google Scholar]
- Parker GA. Assessment strategy and the evolution of fighting behaviour. J Theor Biol. 1974;47:223–43. doi: 10.1016/0022-5193(74)90111-8. [DOI] [PubMed] [Google Scholar]
- Pottinger TG, Moran TA. Differences in plasma cortisol and cortisone dynamics during stress in two strains of rainbow trout (Onchorhynchus mykiss) J Fish Biol. 1993;43:121–130. [Google Scholar]
- Prestwich KN, Brugger KE, Topping M. Energy and communication in three species of hylid frogs: power input, power output and efficiency. J exp Biol. 1989;144:53–80. [Google Scholar]
- Prestwich KN, Walker TJ. Energetics of singing in crickets: effects of temperature in three trilling species (Orthoptera: Gryllidae) J Comp Physiol B. 1981;143:199–212. [Google Scholar]
- Remage-Healey L, Bass AH. Rapid elevations in both steroid hormones and vocal signaling during playback challenge: a field experiment in Gulf toadfish. Horm Behav. 2005;47:297–305. doi: 10.1016/j.yhbeh.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Romero LM. Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol. 2004;19:249–55. doi: 10.1016/j.tree.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Salazar VL, Stoddard PK. Sex differences in energetic costs explain sexual dimorphism in the circadian rhythm modulation of the electrocommunication signal of the gymnotiform fish Brachyhypopomus pinnicaudatus. J exp Biol. 2008;211:1012–20. doi: 10.1242/jeb.014795. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. Why Zebras Don't Get Ulcers. W.H. Freeman and Company; New York: 1994. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Silva A, Quintana L, Ardanaz JL, Macadar O. Environmental and hormonal influences upon EOD waveform in gymnotiform pulse fish. J Physiol Paris. 2002;96:473–84. doi: 10.1016/S0928-4257(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Smith JM. The logic of animal conflict. Nature. 1973;246:15–18. [Google Scholar]
- Stoddard PK, Allee SJ, Markham MR. The 8th Congress of the International Society of Neuroethology. Vancouver, B.C., Canada: 2007a. Synergy between androgens and melanocortins in regulation of gymnotiform electric waveforms. [Google Scholar]
- Stoddard PK, Markham MR, Salazar VL. Serotonin modulates the electric waveform of the gymnotiform electric fish Brachyhypopomus pinnicaudatus. J exp Biol. 2003;206:1353–62. doi: 10.1242/jeb.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard PK, Markham MR, Salazar VL, Allee S. Circadian rhythms in electric waveform structure and rate in the electric fish Brachyhypopomus pinnicaudatus. Physiol Behav. 2007b;90:11–20. doi: 10.1016/j.physbeh.2006.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard PK, Zakon HH, Markham MR, McAnelly L. Regulation and modulation of electric waveforms in gymnotiform electric fish. J Comp Physiol A. 2006;192:613–24. doi: 10.1007/s00359-006-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CH, Winberg S. Interactions between the neural regulation of stress and aggression. J exp Biol. 2006;209:4581–9. doi: 10.1242/jeb.02565. [DOI] [PubMed] [Google Scholar]
- Taigen TL, Wells KD. Energetics of vocalization by an anuran amphibian (Hyla versicolor) J Comp Physiol B. 1985;155:163–170. [Google Scholar]
- Wingfield JC, Ball GF, Dufty AM, Hegner RE, Ramenofsky M. Testosterone and aggression in birds. Am Scient. 1987;75:602–608. [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF. The Challenge Hypothesis - Theoretical Implications For Patterns Of Testosterone Secretion, Mating Systems, And Breeding Strategies. Am Nat. 1990;136:829–846. [Google Scholar]
- Wingfield JC, Kitaysky AS. Endocrine responses to unpredictable environmental events: Stress or anti-stress hormones? Integr Comp Biol. 2002;42:600–609. doi: 10.1093/icb/42.3.600. [DOI] [PubMed] [Google Scholar]
- Zahavi A. Mate selection - a selection for a handicap. J theor Biol. 1975;53:205–14. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]
- Zakon H, McAnelly L, Smith GT, Dunlap K, Lopreato G, Oestreich J, Few WP. Plasticity of the electric organ discharge: implications for the regulation of ionic currents. J exp Biol. 1999;202:1409–16. doi: 10.1242/jeb.202.10.1409. [DOI] [PubMed] [Google Scholar]
- Zakon HH. The effects of steroid hormones on electrical activity of excitable cells. Trends Neurosci. 1998;21:202–7. doi: 10.1016/s0166-2236(97)01209-5. [DOI] [PubMed] [Google Scholar]
- Zakon HH, Thomas P, Yan HY. Electric organ discharge frequency and plasma sex steroid levels during gonadal recrudescence in a natural population of the weakly electric fish Sternopygus macrurus. J Comp Physiol A. 1991;169:493–9. doi: 10.1007/BF00197661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


