Abstract
Sialylated forms of the Fc fragment of immunoglobulin G, produced by the human α2–6 sialyltransferase ST6Gal-I, were identified as potent anti-inflammatory mediators in a mouse model of rheumatoid arthritis and are potentially the active components in IVIG therapies. The activities and specificities of hST6Gal-I are, however, poorly characterized. Here MS and NMR methodology demonstrates glycan modification occurs in a branch-specific manner with the α1–3Man branch of the complex, biantennary Fc glycan preferentially sialylated. Interestingly, this substrate preference is preserved when using a released glycan, suggesting that the apparent occlusion of glycan termini in Fc crystal structures does not dominate specificity.
The existence of specific glycans on glycoproteins is known to play an important role in the regulation of their activities in vivo (1). The synthesis of these glycans occurs through a complex process involving a host of glycosidases and glycosyltransferases; the activity and specificity of which leads to specific glycan structures. Understanding their specificity can be an important step toward understanding glycoprotein function and the design of enzymes that can produce altered enzymes for therapeutic purposes. Recently an interesting link between a specific glycan modification of the Fc fragment of immunoglobulin G (IgG) and rheumatoid arthritis (RA) has been uncovered (2). Using a mouse RA model, the glycoform of the Fc fragment, with α2–6 linked N-acetylneuraminic acid N-glycan termini (sialylated IgG-Fc), was shown to be an active component of intravenous IgG anti-inflammatory therapy (IVIG) (3). IVIG is an effective treatment for various autoimmune disorders in which massive quantities of IgG (1–2 g/kg) are administered at a high cost with potentially dangerous complications (4). The sialylated form is a minor component produced by the action of the sialyltransferase, hST6Gal-I. Preparation of this specific form for therapeutic application may well reduce risks in IVIG therapy. Here we investigate the specificity of hST6Gal-I in sialylating the IgG-Fc fragment.
IgG is a primary effector of the secondary phase of the adaptive immune system as a blood-borne scavenger of foreign particles. The ~55kDa Fc domain is a stable dimer and separable from the Fab domains of the IgG molecule following proteolysis. Each Fc monomer contains one N-linked glycan at Asn297, which in healthy human patients is characterized by a mixture of mostly complex-type, biantennary structures (Figure 1A) with 4–11% sialylation (5) on the α1–3Man branch of the glycan (6). In a crystal structure of an IgG molecule a large portion of the glycans were observed sandwiched between the two polypeptide monomers (7), suggesting that unlike many N-glycans (8–11) the Fc glycan is conformationally restricted. This suggests that any inherent enzyme specificity for branch sialylation may be strongly influenced by accessibility in the protein structure.
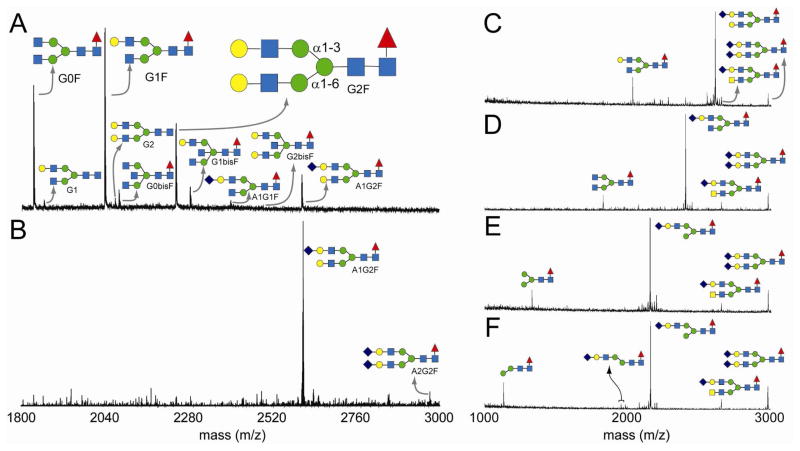
Figure 1.
Mass spectrometry based determination of glycan structures. (Panel A) N-glycans isolated from the immunoglobulin G Fc fragment with the G2F glycoform illustrating the α1–3Man and α1–6Man branches of the glycan. (Panel B) Galactosylation followed by sialylation of the Fc fragment results in primarily digalactosylated, monosialylated glycan. (Panels C–F) Enzymatic determination of the branch containing an N-acetylneuraminic acid residue: (Panel C) monosialylated glycan treated with β-galactosidase (Panel D) and N-acetylglucosaminidase (Panel E) resulted in the removal of those residues on the non-reducing end not protected by a terminal sialic acid. (Panel F) The sialylated, β-galactosidase- and N-acetylglucosaminidase-treated glycan was not affected by an α1–2,3 mannosidase, suggesting the terminal mannose residue is α1–6 linked. Residues are denoted by symbols: N-acetylneuraminic acid (purple diamond), galactose (yellow circle), N-acetylgalactosamine (yellow square), N-acetylglucosamine (blue square), mannose (green circle) and fucose (red triangle).
The glycans of the Fc fragment, as purified from human serum, are heterogeneous with a majority of the termini lacking both sialic acid and galactose residues (as shown in Figure 1A). However, they may be remodeled to near homogeneity by fully galactosylating the N-glycan (3, 12). This step will greatly assist in providing substrates for testing enzyme specificity. The substrates can also be sialylated with isotopically labeled sugars to aid the eventual interpretation of structural and dynamic measurements performed on the glycoprotein and glycoprotein – receptor complexes using solution NMR spectroscopy. Sialylation is achieved in vitro using the human CMP-N-acetylneuraminic acid:galactose(β1–4)-N-acetylglucosamine-R (α2–6)sialyltransferase, ST6Gal-I. This enzyme is ubiquitously expressed in human tissues and is primarily responsible for generating α2–6 linked sialic acid at N-glycan termini in situ (13, 14). Complete galactosylation of native IgG-Fc was readily achieved with UDP-galactose and a bovine galactosyltransferase (see Experimental Procedures, Supporting Information); however sialylation with CMP-13C-[1,2,3]-N-acetylneuraminic acid and ST6Gal-I occurred on a much slower timescale and resulted in primarily monosialylated glycan (Figure 1B), even after four days with daily replenishment of the sugar nucleotide donor and ST6Gal-I. The amount of disialylated material increased, albeit slightly, upon repeating the sialylation procedure using monosialylated Fc fragment, and the disialylated material displayed a molecular weight consistent with the incorporation of two 13C-labeled N-acetylneuraminic acids that could be used for NMR investigations (data not shown).
To determine whether ST6Gal-I placed N-acetylneuraminic acid specifically on one branch or randomly on both branches of the monosialylated, glycan was characterized using mass spectrometry and NMR. Following enzymatic liberation and purification, the glycan was incubated with obligate exoglycosidases to sequentially remove terminal β1–4-linked galactose, β1–2-linked N-acetylglucosamine and α1–3-linked mannose residues. Mass spectra of the glycan mixture following these digestions, shown in Figure 1C–F, demonstrated that galactose and N-acetylglucosamine residues were removed from the branch of the glycan not affected by an α 1–2,3 mannosidase; suggesting these residues were on the α1–6Man branch and the N-acetylneuraminic acid residue is on the α1–3Man branch of the complex biantennary glycan. A dramatically less intense group of peaks corresponding to a sialylated glycan that had lost a mannose residue was observed (Figure 1F), suggesting the majority (>95%) of the N-acetylneuraminic acid was on the α1–3Man branch (see Figure 1A for branch definition). This starting glycan mixture contained a small amount of monogalactosylated material, which following galactosidase and N-acetylglucosaminidase treatment was digested by the α1–2,3 mannosidase, verifying the enzyme is capable of fully digesting a branched substrate.
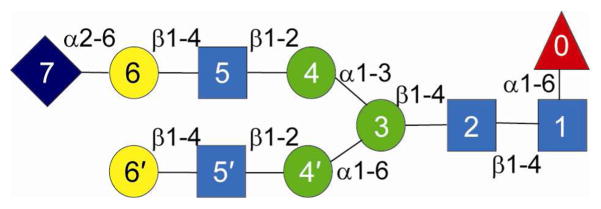
High-resolution NMR spectroscopy was performed to confirm the configuration of the monosialylated glycan. Residue types were assigned based on COSY and TOCSY spectra, and the branch-specific assignment was achieved using a NOESY spectrum (Figure S1A) that showed interresidue connectivities traced from the branched mannose to the N-acetylglucosamine residues. A branch-specific assignment of the galactose residues was made through an NOE between the anomeric proton of one of the two identified galactose residues to the H4 of the N-acetylglucosamine residue on the α1–3Man branch of the glycan (Figure S1A). This galactosyl residue has H1 and H5 shifts of 4.442 and 3.829 ppm, respectively, whereas the galactosyl residue on the α1–6Man branch of the glycan has H1 and H5 shifts of 4.470 and 3.728 ppm, respectively. Values for H1 and H5 proton shifts of the galactosyl residue in an N-acetylneuraminate-α(2–6)-galactose-β(1–4) N-acetylglucosamine-β(1–2)-R moiety of a complex-type biantennary glycan were previously determined to be 4.440 and 3.818 ppm, respectively, and 4.470 and 3.732, respectively, for a terminal galactosyl residue in a galactose-β(1–4)-N-acetylglucosamine-β(1–2)-R moiety (15, 16). Based on this chemical shift analysis, the galactosyl residue on the α1–3Man likely harbors the α2–6-linked N-acetylneuraminic acid modification as shown in Figure 2. These results are consistent with the mass spectrometry-based configuration analysis presented above.
Figure 2.
The proposed structure of the monosialylated glycan as determined by enzymatic digests and NMR spectroscopy is shown with the linkages indicated. The residue symbols are the same as those in Figure 1.
In crystal structures the glycans on the Fc fragment reside in the cavity between dimer subunits, and it is easy to imagine that preferential occlusion contributes to specificity. Therefore, the branch specificity of human ST6Gal-I towards a released biantennary glycan was tested by following the time course of sialylation using the released digalactosyl, biantennary glycans from IgG-Fc. This shows the rapid conversion to a monosialylated form, followed by the slow conversion to a disialylated form (Figure S1B). At 16h a primarily monosialylated form was observed (Figure S2A, S3). This material was further analyzed using the enzymatic analysis coupled with mass spectrometric analysis demonstrated above and determined that like the Fc glycan, the single N-acetylneuraminic acid on the released glycan was attached to the α1–3Man branch (Figure S2). The polypeptide component of the Fc fragment does, however, appear to inhibit the sialylation reaction, in that the rate of released glycan sialylation is at least 5-fold greater than Fc-conjugated glycan sialylation (Figure S3). This effect may well be due to restricted access to the glycan.in the Fc dimer. However, this inhibitory effect on the sialylation reaction does not alter branch specificity. The terminal galactosyl residue on the α1–3Man branch of the complex-type, biantennary, Fc-conjugated glycan is sialylated more efficiently than the galactosyl residue on the α1–6Man branch even in the absence of attachment to the protein.
An inherent branch specificity of sialyltransferases is not without support from previous studies. Preference of the ST6Gal-I from bovine colustrum towards the terminal α1–3Man-linked galactose had been observed in vitro using isolated glycans as substrates (17–19). Additional studies by Conradt and coworkers also demonstrated that other glycoproteins coexpressed with human ST6Gal-I contained a higher percentage of α2–6-linked N-acetylneuraminic acid on the α1–3Man branch, suggesting some independence of protein substrate (20).
The preservation of branch specificity towards either an Fc-conjugated or released glycan suggests a large portion of the glycan is recognized by ST6Gal-I in one of two manners. The enzyme either recognizes one entire terminal galactose-N-acetlylglucosamine-mannose-mannose tetrasaccharide moiety and prefers a substrate with the α1–3Man linkage over an α1–6Man linkage; or the enzyme simultaneously recognizes both branch termini and selectively sialylates the α1–3Man-linked galactosyl residue, discriminating glycan termini based on spatial or conformational arrangement. In either scenario the scope of acceptor substrate recognition is likely substantial, and suggests a large portion of the Fc glycan is accessible to the enzyme in conformations unlike those seen in the available crystal structures. Measurements of glycan dynamics and conformation in solution will be helpful in characterizing the more extended structures. Investigations of glycans in complex with hST6Gal-I may uncover the origin of branch specificity and allow engineering of sialyltransferases with less specificity.
The anti-inflammatory properties of sialyl-Fc have been attributed to a disialylated form, and despite the data presented here showing monosialylated Fc-conjugated glycan (Figure 1B), however, a more indepth comparison of anti-inflammatory activity of monosialylated Fc with disiaylated Fc preparation may be illuminating. It should be possible to obtain a homogenous disialylated preparation using either a greater concentration of enzyme or a more active preparation of ST6Gal-I than used here. However, production would certainly be more efficient with a hST6Gal-I engineered to have di-siaylating activity or a transferase from another source that inherently has this activity.
Supplementary Material
Acknowledgments
We wish to thank Prof. Kelley Moremen for suggestions regarding ST6Gal-I enzymology, Drs. Roberto Sonon and Parastoo Azadi for help with permethylation-based glycan analysis, and Dr. John Glushka for assistance with NMR spectroscopy. This research was funded by grants R01GM033225 and P41RR005351 from the NIH.
Footnotes
SUPPORTING INFORMATION PARAGRAPH
Details of the experimental procedures including glycan remodeling, glycan analysis, exoglycosidase treatments and NMR analysis as well as three supplementary figures (S1–S3) and a table of the chemical shift assignments are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Gabius HJ. Crit Rev Immunol. 2006;26:43–79. doi: 10.1615/critrevimmunol.v26.i1.30. [DOI] [PubMed] [Google Scholar]
- 2.Kaneko Y, Nimmerjahn F, Ravetch JV. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 3.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dwyer JM. New Eng J of Med. 1992;326:107–116. doi: 10.1056/NEJM199201093260206. [DOI] [PubMed] [Google Scholar]
- 5.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. Anl Rev Imm. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 6.Wormald MR, Rudd PM, Harvey DJ, Chang SC, Scragg IG, Dwek RA. Biochemistry. 1997;36:1370–1380. doi: 10.1021/bi9621472. [DOI] [PubMed] [Google Scholar]
- 7.Guddat LW, Herron JN, Edmundson AB. P Natl Acad Sci USA. 1993;90:4271–4275. doi: 10.1073/pnas.90.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyss DF, Choi JS, Li J, Knoppers MH, Willis KJ, Arulanandam AR, Smolyar A, Reinherz EL, Wagner G. Science. 1995;269:1273–1278. doi: 10.1126/science.7544493. [DOI] [PubMed] [Google Scholar]
- 9.Wyss DF, Choi JS, Wagner G. Biochemistry. 1995;34:1622–1634. doi: 10.1021/bi00005a019. [DOI] [PubMed] [Google Scholar]
- 10.DeBeer T, VanZuylen CWEM, Leeflang BR, Hard K, Boelens R, Kaptein R, Kamerling JP, Vliegenthart JFG. Eur J of Biochem. 1996;241:229–242. doi: 10.1111/j.1432-1033.1996.0229t.x. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher CM, Harrison RA, Lachmann PJ, Neuhaus D. Protein Sci. 1993;2:2015–2027. doi: 10.1002/pro.5560021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raju TS, Briggs JB, Chamow SM, Winkler ME, Jones AJ. Biochemistry. 2001;40:8868–8876. doi: 10.1021/bi010475i. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein J, Lee EU, Mcentee K, Lai PH, Paulson JC. J Biol Chem. 1987;262:17735–17743. [PubMed] [Google Scholar]
- 14.Harduin-Lepers A, Recchi MA, Delannoy P. Glycobiology. 1995;5:741–758. doi: 10.1093/glycob/5.8.741. [DOI] [PubMed] [Google Scholar]
- 15.Wieruszeski JM, Michalski JC, Montreuil J, Strecker G. Glycoconjugate J. 1989;6:183–194. doi: 10.1007/BF01050647. [DOI] [PubMed] [Google Scholar]
- 16.Vliegenthart JFG, Dorland L, van Halbeek H. Adv Carbohydr Chem Biochem. 1983;41:209–374. [Google Scholar]
- 17.Paulson JC, Prieels JP, Glasgow LR, Hill RL. J Biol Chem. 1978;253:5617–5624. [PubMed] [Google Scholar]
- 18.Joziasse DH, Schiphorst WE, van den Eijnden DH, van Kuik JA, van Halbeek H, Vliegenthart JF. J Biol Chem. 1985;260:714–719. [PubMed] [Google Scholar]
- 19.Joziasse DH, Schiphorst WE, Van den Eijnden DH, Van Kuik JA, Van Halbeek H, Vliegenthart JF. J Biol Chem. 1987;262:2025–2033. [PubMed] [Google Scholar]
- 20.Grabenhorst E, Hoffmann A, Nimtz M, Zettlmeissl G, Conradt HS. Eur J Biochem. 1995;232:718–725. doi: 10.1111/j.1432-1033.1995.718zz.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.