Abstract
Apelin is the endogenous ligand for the APJ receptor and both apelin and APJ are expressed in the gastrointestinal (GI) tract. The aim of this study was to define ontogeny of apelin and APJ in the developing rodent GI tract by measuring expression levels and characterizing abundance and cellular localization at an embryonic stage (E18.5 or E21), two postnatal stages (P4, P16) and in the adult. Apelin and APJ mRNA levels were measured by real time RT-PCR, apelin and APJ-containing cells were identified by immunohistochemical (IHC) staining. Gastric, duodenal and colonic apelin and APJ mRNA levels were highest at birth and declined postnatally. In the postnatal rat stomach, few apelin peptide-containing cells were identified, the density of gastric apelin-containing cells increased progressively after weaning and into adulthood. A robust APJ immunostaining was observed postnatally in the epithelium, intestinal goblet cells and in smooth muscle cells. In the adult rat, APJ immunostaining in the surface epithelium and goblet cells decreased markedly. During the early postnatal period, in an apelin-deficient mouse, APJ expression and immunostaining in the gut were reduced suggesting that apelin regulates APJ. Together, our data support a role for the apelin-APJ system in regulation of smooth muscle, epithelial and goblet cell function in the GI tract.
Keywords: expression, immunohistochemistry, localization
INTRODUCTION
Apelin is the endogenous ligand for the APJ receptor [1]. The APJ receptor is a member of the G-protein-coupled receptor (GPCR) family [2] and is structurally related to the angiotensin and CXC chemokine receptors [3, 4]. Apelin was discovered by screening tissue extracts for their effects on extracellular acidification and inhibition of cAMP formation in a Chinese hamster ovary cell line transfected with the APJ cDNA [1]. Rat, mouse, cow and human apelin cDNAs have been characterized [1, 5] and encode a 77-amino-acid precursor peptide. A 36-amino-acid variant of apelin is the apparent parent peptide.
Apelin and APJ have a widespread distribution in the body [6–8]. Apelin and APJ are expressed in the brain, kidney, adipose tissue, heart, lung, retina, mammary gland and gastrointestinal tract (GI) [5, 9–17]. Apelin exerts a broad range of physiological actions including effects on heart contractility, blood pressure, blood vessel growth, appetite and drinking behavior, pituitary hormone secretion and the hypothalamic-pituitary-adrenal axis [17–24]. In the GI tract and pancreas, apelin has been shown to influence gastric acid secretion as well as intestinal and pancreatic hormone secretion [17, 25, 26].
During pregnancy and lactation, breast apelin expression increases ~7- to 20-fold [5, 27] and significant amounts of apelin are ingested by neonates. A putative target of ingested apelin is the GI tract, however, the extent to which APJ is expressed postnatally in the GI tract, and more importantly, where APJ is localized in the GI tract are not known.
The purpose of the present study, therefore, was to characterize APJ and apelin expression (mRNA levels) profiles as well as localization and abundance of APJ protein and apelin peptide in the developing mouse and rat GI tract. Additionally, the influence of apelin gene knockout on APJ mRNA and immunostaining intensity was investigated.
MATERIALS AND METHODS
Animals
All animal experiments were done in accordance with mandated standards of humane care and were approved by the Institutional Animal Care and Use Committees at the University of Texas Medical Branch and Stanford University. C57/BL6 mice (Figure 1 and Figure 3), 129SV mice (Figure 6 and Figure 7) and Sprague-Dawley rats (Figure 1–Figure 5) were maintained in air-conditioned and light-regulated rooms (lights on, 0600–1800 h) and given access to food and water ad libitum. All tissues were harvested from animals in the ad lib-fed condition. For generation of embryonic and postnatal tissues, mice and rats were mated in house. Rat and mouse litters were born at approximately 21 and 19.5–20 d gestation and kept with their mothers until 21 d postpartum. As indicated in figure legends, apelin and APJ expression levels were examined at one embryonic (E18.5, mouse; E21, rat) two postnatal (P4, P16) stages, and in the adult (1–3 months of age). For measurement of APJ expression levels or IHC localization of APJ protein in apelin gene knockout and control 129SV mice, GI tissues were harvested at E18.5, P7, P18, and P38. GI tissues were harvested from mice and rats of both sexes and either placed in a reagent called RNA later (Ambion, Austin, TX) or immediately extracted for total cellular RNA. Samples in RNA later were extracted at a later date. In all dissections, care was taken not to include the pancreas. For collection of pup and adult mouse and rat stomachs, the stomach body was separated from the rumen and the full-thickness stomach was extracted for total cellular RNA. The entire fetal stomach was extracted for total cellular RNA. Tissues were also placed in fixative (4% paraformaldehyde) for immunohistochemical staining.
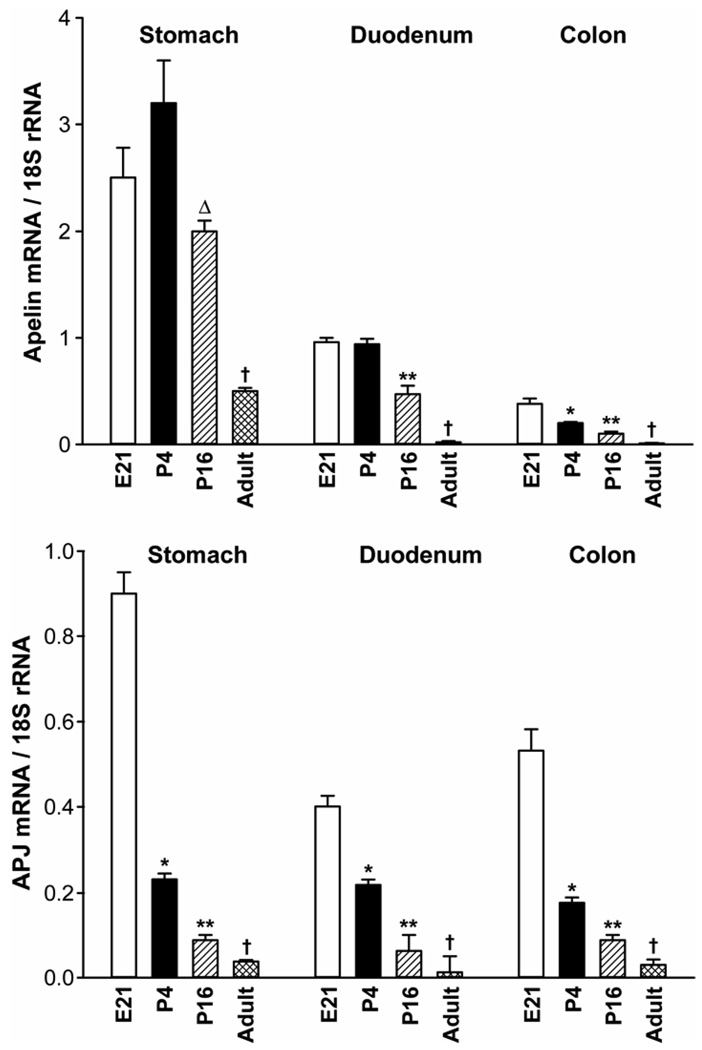
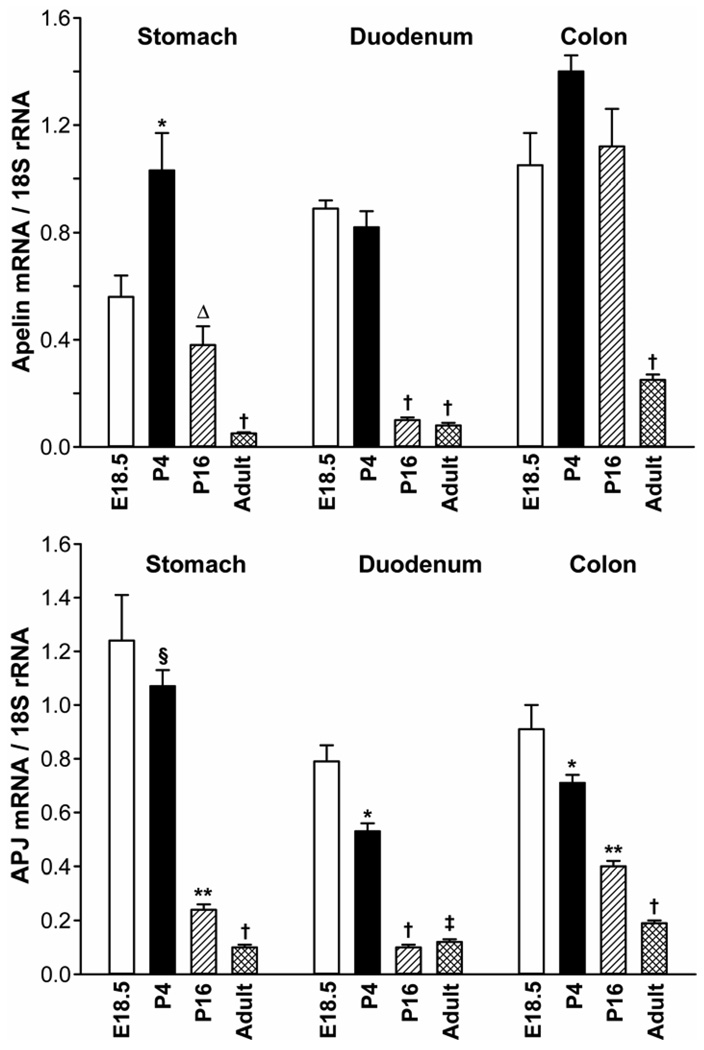
Figure 1.
Ontogeny of apelin and APJ expression (mRNA levels) in the rat stomach, duodenum and colon. N= 5 rats/group. *P < 0.05 vs E21, P16, adult values; **P < 0.05 vs E21, P4, adult values; ΔP < 0.05 vs P4, adult values; †P < 0.05 vs younger ages. E21 = embryonic day 21; P4 = postnatal day 4. The Ct values for apelin mRNA in the duodenum at E21 and APJ mRNA in the stomach at E21 are 27.6 and 26.2, respectively.
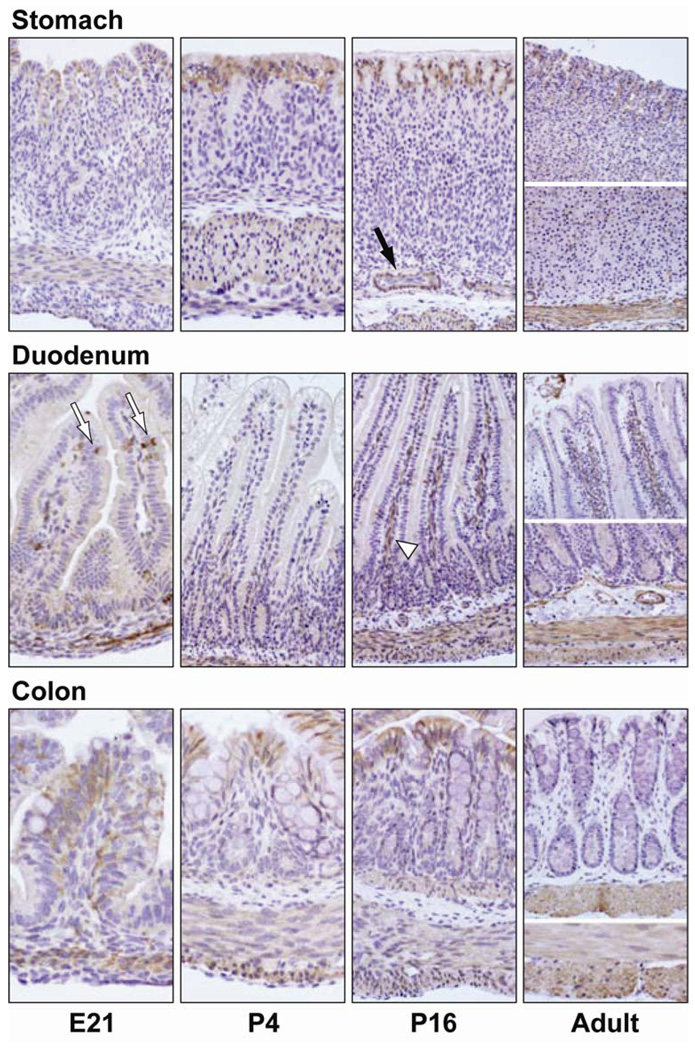
Figure 3.
Ontogeny of APJ protein in the stomach, duodenum and colon of the developing rat. APJ containing cells were identified by immunohistochemical staining with a rabbit antibody generated against the amino terminal 28 residues of rat APJ (brown color). APJ immunostaining is observed in the surface epithelium, goblet cells (white arrows) and smooth muscle layer of the GI tract. APJ staining intensity in the surface epithelium decreased during development. APJ immunostaining in duodenal goblet cells was most intense at E21, P4 and P16 stages, duodenal and colonic goblet cells did not immunostain for APJ in the adult rat. APJ immunostaining in the smooth muscle layer was robust at all ages. Blood vessels (black arrow) and intestinal venules (white arrow head) immunostained for APJ. Omission of the APJ antibody or use of a control rabbit serum in place of the rabbit APJ antibody failed to detect APJ-positive cells (data not shown). Photos were captured with a 20× objective.
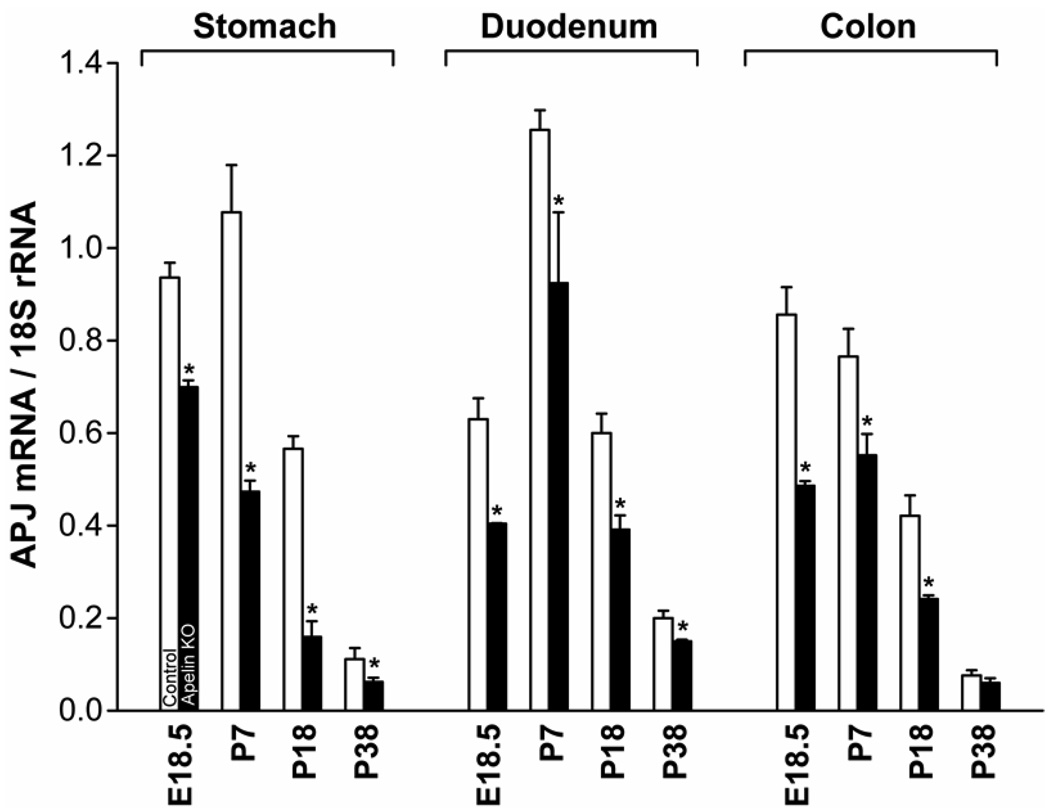
Figure 6.
Influence of apelin gene knockout on APJ expression levels in the developing mouse GI tract. Apelin gene knockout resulted in a significant decrease in APJ expression levels in the developing GI tract. * P< 0.05 vs control mice of same age. N= 3 mice/group. The Ct values for APJ mRNA in the stomach at E18.5 is 24.8.
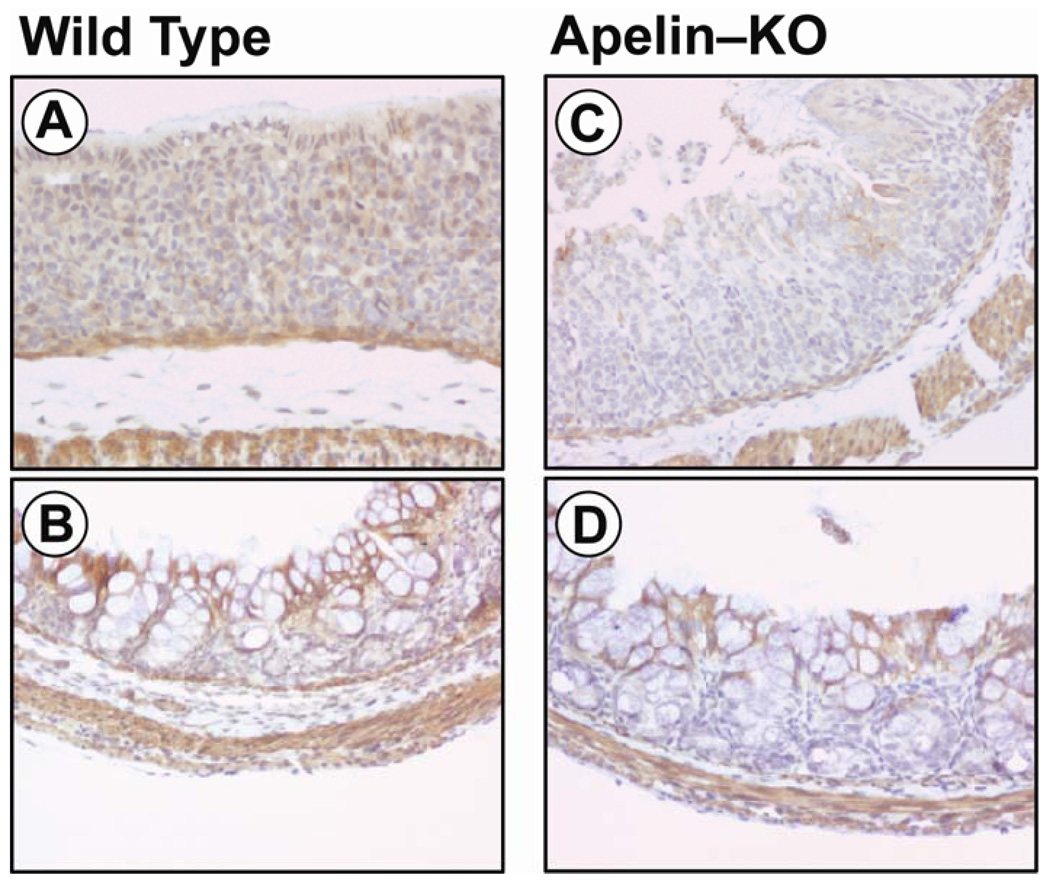
Figure 7.
Influence of apelin gene knockout on APJ immunostaining intensity in the early postnatal mouse stomach and colon. A reduced APJ immunostaining is observed in the stomach (panel C) and colon (panel D) of apelin-deficient mice when compared to wild-type mice (panels A, B).
Figure 5.
Ontogeny of apelin and APJ expression in the mouse stomach, duodenum and colon. N= 5 mice/group. *P < 0.05 vs E18.5, P16, adult values; **P < 0.05 vs E18.5, P4, adult values; ΔP < 0.05 vs P4, adult values; †P < 0.05 vs younger ages; §P<0.05 vs P16, adult values. E18.5 = embryonic day 18.5; P4 = postnatal day 4. The Ct values for apelin and APJ mRNA in the colon at E18.5 are 28.2 and 26.2, respectively.
The apelin gene knockout mouse was generated in the laboratory of T. Quertermous [28]. In brief, the translation start site (ATG) in the first exon of the apelin gene was disrupted by knocking in bacterial β-galactosidase (lacZ gene) followed by neomycin resistance gene. The construct was electroporated into mouse ES cells (derived from agouti 129SV mouse). After homologous recombination, the targeted, inactivated apelin bearing ES cells were selected with G418. Selected ES cells were injected to mouse blastocysts to generate a chimeric apelin mouse. Chimeric mice were then bred with 129SV mice to get the germline chimeric mouse. The germline offspring were then backcrossed for ten generations to generate knockout mice used in the present experiments.
Real time RT-PCR measurements of apelin and APJ expression
For measurements of apelin and APJ expression, total cellular RNA was extracted and purified as described previously [17]. RNA was quantified by spectrophotometry. Before real time PCR analyses, total RNA was used as a template to synthesize first strand cDNA by the random priming method using the AdvantageTM RT-for-PCR kit (BD Biosciences-Clontech, Palo Alto, CA). Briefly, 1 µg of total RNA was incubated with 1 µl random primer at 70°C for 2 min, chilled on ice, and mixed with a solution containing 4 µl reaction buffer (5x), 1 µl deoxynucleotide (dNTP) (10 mM), 0.5 µl recombinant RNase inhibitor (40 U/µl) and 1.0 µl of MMLV reverse transcriptase (200 U/µl). The mixture (20 µl) was incubated for 1 h at 42°C and the reverse transcriptases were denatured at 94°C for 5 min. Apelin and APJ mRNA levels were then measured by real time RT-PCR assays as described [17]. Assays were done using an Applied Biosystems 7000 sequence detection system (Foster City, CA). Applied Biosystems Assays-By-Design containing a 20x assay mix of primers and TaqMan MGB probes (6-FAM dye-labeled probe) were used for the target genes: mouse apelin (accession no. NM_013912); rat apelin (accession no. AF179679); mouse APJ (accession no. NM_011784); rat APJ (accession no. NM_031349); and a pre-developed mouse and rat 18S rRNAs (VIC dye-labeled probe). TaqMan assay reagent (P/N 4319413E) was used for the internal control. Primers were designed to span exon-exon junctions. Probe sequences were searched against the NCBI database.
Immunohistochemical (IHC) Staining
For IHC localization of apelin peptide and APJ protein, mouse and rat tissue specimens were fixed in buffered formalin or 4% paraformaldehyde and then processed for identification of apelin- or APJ-containing cells [17].
Fixed, paraffin-embedded tissue sections (5 µm) were deparaffinized and rehydrated. After deparaffinization, slides were treated with 1% H2O2 in PBS for 15 min, followed by microwave antigen retrieval at 100°C for 10 min in Dako target retrieval solution (Dako Corp., Carpinteria, CA) in an H2800 microwave processor (Energy Beam Sciences, Inc., Agawam, MA) or water bath. After sequential 15-min incubations with 0.1% avidin and 0.01% biotin (Vector Laboratories, Inc., Burlingame, CA), slides were then incubated in 0.05% casein (Sigma) in 0.05% Tween 20 (Dako) in PBS for 30 min to block nonspecific protein binding. Either a primary rabbit polyclonal antibody generated against apelin-36 (Phoenix Laboratories, Belmont, CA) at a dilution of 1:300, or a rabbit antibody generated against the 29-amino terminal residues of rat APJ at a dilution of 1:750–1:1000 (gift of D. Kolson, Univ. of Pennsylvania) were applied to sections for 60 min. The apelin antibody recognizes large and small forms of apelin (Apelin-36, Apelin-13, etc.). For negative controls, either rabbit serum Ready-to-Use (InnoGenex, San Ramon, CA) was applied or the primary antibody was omitted, immunoreactive apelin and APJ staining were not observed in these cases. The apelin antibody does not recognize structurally unrelated peptides. Biotinylated F(ab')2 fragment of swine anti rabbit immunoglobulins (Dako) served as the secondary antibody and was detected by streptavidin-horseradish peroxidase and colorized by diaminobenzidine (Dako). Slides were counterstained with Mayer’s modified hematoxylin before mounting and viewed under a Nikon Eclipse E600 microscope. Images were captured with a Nikon DXM1200 digital camera and ACT-1 (version 2.00) program.
Statistical analyses
Results are expressed as the mean ± SEM. Data were analyzed by t-test or one-way ANOVA and subsequently with Newman-Keuls test when appropriate. P<0.05 was considered significant.
RESULTS
Ontogeny of apelin and APJ expression in the rat GI tract
The aim of this study was to characterize apelin and APJ expression (mRNA levels) in the developing rat stomach, duodenum and colon. We measured apelin and APJ expression at a single embryonic stage (E21), two postnatal stages (P4, P16), and in the adult rat (~1–3 months old).
In the rat stomach, apelin expression levels were maximal at the E21 and P4 stages, and then declined progressively into adulthood. Apelin expression levels in the rat stomach were significantly lower at the P16 and the adult stages when compared to apelin expression levels at the E21 and P4 stages.
In the duodenum, apelin expression levels were highest at the E21 and P4 stages, apelin expression levels in the duodenum then decreased progressively at the P16 and adult stages.
In the rat colon, apelin expression levels were highest at the E21 stage and then declined progressively at the P4, P16 and adult stages. In the colon and duodenum of the adult rat, apelin mRNA levels were marginal when compared to earlier ages and to the adult rat stomach.
In general, apelin expression levels in the rat stomach were higher when compared to apelin expression levels in the duodenum and colon.
In the rat stomach, duodenum and colon, APJ expression levels were maximal at the E21 stage (Figure 1). APJ expression levels then declined postnatally and into adulthood in a dramatic fashion.
Ontogeny of apelin peptide in the rat stomach
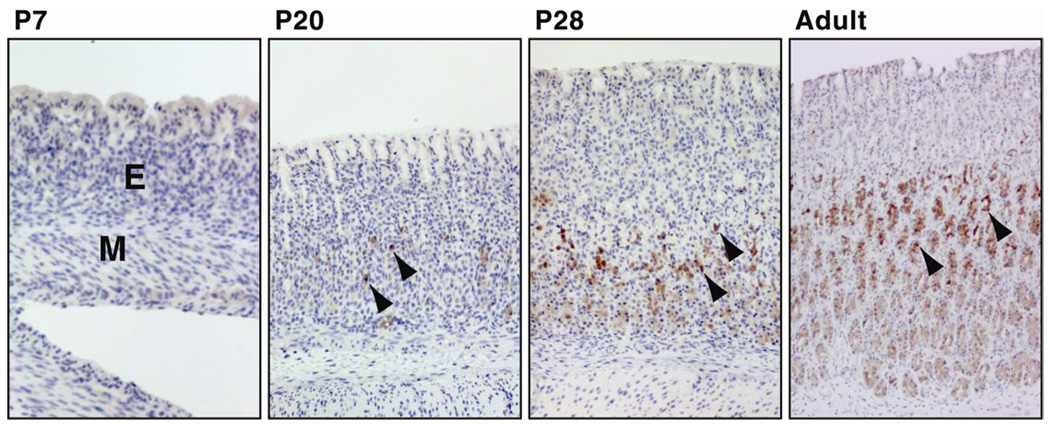
Ontogeny, localization and abundance of apelin peptide-containing cells were characterized in the rat stomach at P7, P13, P20, P28 and in the adult by IHC staining. At the P2, P7 and P13 stages, apelin-positive cells were not identified in the rat stomach (Figure 2, P2 and P13 not shown). The density of apelin-positive cells increased at the P20 and P28 stages and was maximal in the adult stomach. Apelin-positive cells were observed primarily in the middle and lower mucosal regions of gastric glands. Our laboratory and others [29, 30] have previously reported that apelin immunostaining occurs in gastric mucous neck, parietal, chief and endocrine cells.
Figure 2.
Ontogeny of apelin immunoreactivity in the oxyntic mucosa of the developing rat stomach. Apelin-containing cells (brown color) were not detected at P2 (not shown), P7 and P13 (not shown) and were observed initially in the rat stomach at 20 days of age (P20) by IHC. Apelin-containing cells are localized primarily in the middle and basal region of gastric glands (arrow heads). The density of apelin-containing cells increased progressively with age. Omission of apelin antibody or use of control rabbit serum in place of the rabbit apelin antibody failed to detect apelin-positive cells (not shown). Photos were captured with a 10× objective, M = muscle layer of stomach; E = gastric mucosal epithelium; P7 = postnatal day 7.
Ontogeny of APJ protein in the rat GI tract
An earlier report indicates APJ immunostaining in the rat stomach [25]. In the present study, a diffuse APJ immunostaining was observed in the surface epithelium of the developing rat stomach and colon (Figure 3) and, in the developing rat duodenum, APJ immunostaining was observed in goblet cells (Figure 4, panel C), non-goblet epithelial cells (Figure 4, Panel D) and in the crypt region (Figure 3).
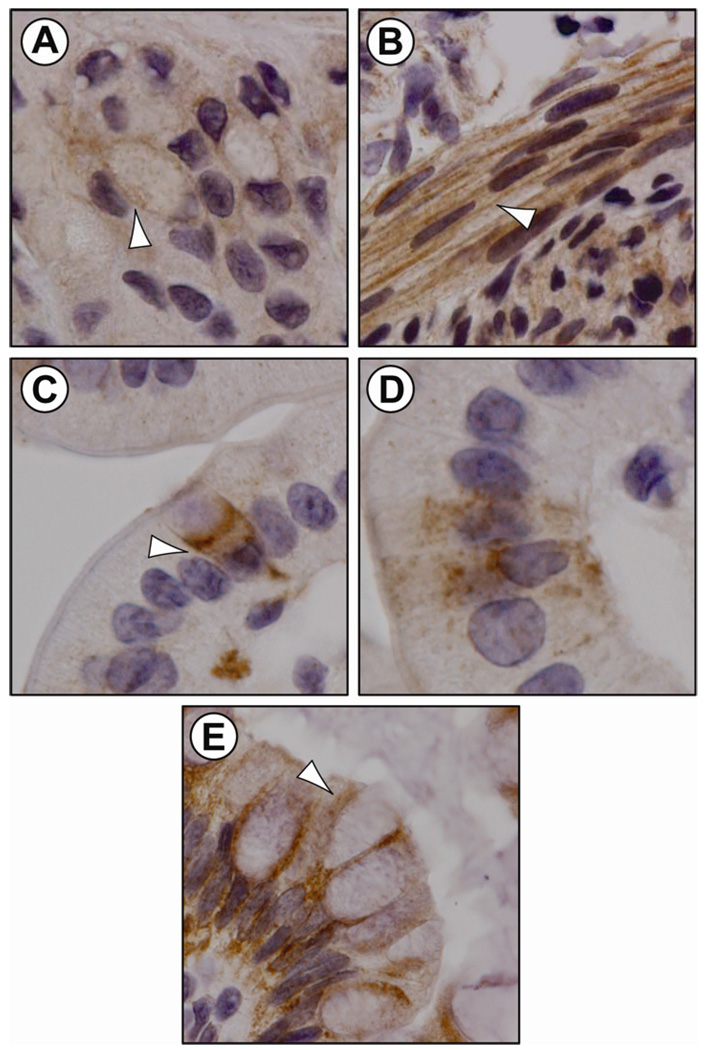
Figure 4.
APJ localization by IHC staining in the developing rat GI tract (embryonic, early postnatal). APJ-containing cells (white arrow) in the rat stomach epithelium (panel A: stomach, P16) and smooth muscle layer (panel B: stomach, E21). Duodenal goblet cell (panel C: duodenum, E21) and non-goblet, flask-shaped cells (panel D: duodenum, E21) immunostained for APJ. Colonic goblet cells immunostained for APJ (panel E: colon, P16). APJ immunoreactivity was detected predominantly at the plasma membrane of enteric cells.
In the surface epithelium of the stomach, APJ immunostaining was most intense at the P4 and P16 stages, APJ staining intensity in the surface epithelium decreased in the adult stomach (Figure 3). In the stomach epithelium, APJ immunostaining was associated with the cell membrane and cytoplasm (Figure 4, panel A). In the developing rat duodenum, APJ immunostaining in goblet cells was most intense at E21, P4 and P16 stages. In the adult rat, APJ immunostaining in duodenal goblet cells was less intense when compared to earlier ages. In the developing rat colon, APJ immunostaining in the surface epithelium was most intense at the E21, P4 and P16 stages. APJ immunostaining decreased in the adult colon. In the rat colon, APJ immunostaining was associated with the cell membrane of goblet and absorptive epithelial cells (Figure 4, panel E). A strong APJ immunostaining was also present in the smooth muscle layer of the rat stomach, duodenum and colon (Figure 3). In contrast to the surface epithelium, APJ immunostaining in the smooth muscle layer was robust at all ages examined, APJ immunostaining was associated primarily with the cell membrane and some APJ immunostaining was observed in the cytoplasm (Figure 4, panel B). APJ immunostaining was also observed in venules of intestinal villi and in endothelial cells (Figure 3).
Ontogeny of apelin and APJ expression in the mouse GI tract
The aim of this study was to characterize apelin expression levels in the developing mouse stomach, duodenum and colon. Apelin expression was measured at a single embryonic stage (E18.5), two postnatal stages (P4, P16), and in the adult mouse (~1–3 months old).
In the mouse stomach, maximal apelin expression levels were measured at P4, stomach apelin expression levels then declined progressively at the P16 and adult stages (Figure 5). Apelin expression levels were significantly lower at E18.5 when compared to levels at P4.
In the duodenum, apelin expression levels were highest at the E18.5 and P4 stages, apelin expression levels in the duodenum then decreased dramatically at the P16 and adult stages.
In the colon, apelin expression levels were maximal at the E18.5, P4 and P16 stages, apelin expression levels in the colon were dramatically lower in the adult.
In the mouse, apelin expression levels were of similar magnitudes in the stomach, duodenum and colon.
In the mouse duodenum and colon, APJ expression levels were maximal at the E18.5 stage (Figure 5). In the mouse stomach, maximal APJ expression levels were measured at the E18.5 and P4 stages. In the stomach, duodenum and colon, APJ expression levels then declined postnatally and into adulthood in a dramatic fashion.
Apelin gene knockout decreases APJ expression levels and immunostaining in the GI tract
The aim of this experiment was to examine the influence of an apelin-deficient condition on APJ mRNA levels and immunostaining intensity in the developing GI tract. In the apelin-deficient mouse, APJ mRNA levels decreased significantly in the developing stomach, duodenum and colon (Figure 6). In the developing stomach, APJ expression levels in the apelin gene knockout mouse are reduced 20–70%, in the developing duodenum APJ mRNA levels are reduced approximately 30% and in the developing colon APJ mRNA levels are reduced 30–40%. APJ expression levels in the GI tract of apelin-deficient mice at P38 are either reduced marginally or unchanged when compared to wild-type mice. In the developing apelin gene knockout mouse, APJ immunostaining in the surface epithelium of the stomach (Figure 7, panel C) and colon (panel D) was less intense when compared to the intensity of APJ immunostaining of WT mice (panels A and B). APJ immunostaining in the apelin-deficient mouse is also less intense at E18.5 and P18 (data not shown). At P38, APJ immunostaining in the GI tract in apelin-deficient mice did not differ when compared to WT mice (data not shown).
DISCUSSION
The aim of the present study was to characterize the ontogeny of apelin and APJ expression as well as ontogeny, localization and abundance of apelin peptide and APJ protein in the developing rodent GI tract. Furthermore, the influence of apelin gene knockout on APJ expression and immunostaining intensity in the developing mouse GI tract was examined. In this study, we demonstrate that expression levels of apelin and APJ are regulated developmentally and in a parallel fashion. In the rat and mouse stomach, duodenum and colon, apelin and APJ expression levels are at maximal or near maximal levels at birth, expression levels then decline precipitously postnatally. Interestingly, the profile of apelin peptide production (ie, immunohistochemical staining) does not parallel apelin expression levels and differs, in part, when compared to the profile of APJ protein production in the postnatal GI tract. For instance, in the rat stomach, apelin-containing cells are not detectable in neonates and appear near the time of weaning (P20). The density of gastric apelin-staining cells continues to increase progressively after weaning and a maximal density coincides with low gastric apelin expression levels. In contrast, APJ immunostaining intensity in the surface epithelium, goblet cells and smooth muscle layer of the GI tract are strong and coincide with high APJ expression levels in neonates. In the surface epithelium and goblet cells, APJ staining intensity continues to parallel APJ expression levels since both decrease as rat pups mature. In contrast to the pattern of APJ immunostaining in the surface epithelium, an intense APJ immunostaining is observed in the smooth muscle layer of the GI tract during the entire lifespan of the rat and does not parallel the decline in enteric APJ expression levels. These results confirm and extend an earlier study from our laboratory [17] and others [4, 31], and suggest that APJ localized in the smooth muscle layer and apelin are regulated at the level of translation and not at the level of their mRNA abundance whereas APJ in the surface epithelium is regulated at the transcriptional level.
The developmental patterns of apelin mRNA and peptide levels differ when compared to other GI peptides [32, 33]. For instance, mRNA and peptide levels of gastrin, a stomach peptide, and of peptide YY (PYY), an ileo-colonic peptide, are marginal at birth and increase either during the suckling period or after weaning. The high apelin mRNA levels at birth suggest a role for apelin in the development of the GI tract postnatally, however, the absence of apelin immunostained cells in the early postnatal period argues against this idea. It should be pointed out that endogenously produced apelin peptide in the GI tract may be redundant during the early postnatal period since breast milk contains high levels of apelin peptide [5, 17]. The elevation in the density of gastric apelin peptide-containing cells after weaning suggests that apelin synthesis, like PYY [32], is triggered by the switch from breast milk to solid food and a reduced dependence upon maternally derived nutrition.
Localization of APJ in the surface epithelium, goblet cells and crypt cell region of the GI tract supports a role for milk-borne apelin in activating the mucosal APJ receptor during the suckling period. Additionally, APJ localization in intestinal goblet cells implies that the apelin-APJ system has a role in regulation of mucin secretion. One function of goblet cells is secretion of mucus for maintenance of the epithelial barrier [34, 35]. Interestingly, APJ localization is not detected in intestinal Brunner’s glands, Brunner’s glands also secrete mucus [36]. Furthermore, our results show that the intensity of APJ immunostaining in the surface epithelium and the density of APJ positive duodenal and colonic goblet cells decrease in the adult rat. Together, these observations suggest that the epithelial and goblet cell APJ receptor is activated by luminal apelin derived from milk. APJ immunostaining is also evident in the crypt cell region of the small intestine implying that apelin has a role on epithelial proliferation. An earlier report from our laboratory shows that apelin can stimulate intestinal epithelial proliferation [37].
Our IHC studies demonstrate that APJ is localized in the cell membrane and cytoplasm of enteric cells. APJ localization is expected on the cell membrane; however, it is not uncommon to localize G-protein coupled receptors in the cytoplasm [38]. The APJ receptor localized on enteric smooth muscle cells may be activated either by luminal apelin that is absorbed into the systemic circulation from the gut lumen or from other apelin sources in the body. In the smooth muscle layer of the GI tract, a strong APJ immunostaining is seen at all ages suggesting that the apelin-APJ system maintains an important role in regulation of smooth muscle function throughout the entire lifespan.
APJ is also localized in enteric blood vessels. APJ has been described in blood vessels previously and is thought to be involved in blood vessel growth [13, 14, 19].
Another objective of our study was to examine the influence of apelin gene knockout on APJ expression and protein levels in the developing mouse GI tract. In the apelin-deficient mouse, APJ mRNA and protein levels are reduced at E18.5, P7 and P18 implying that apelin influences APJ synthesis in the GI tract. The source of apelin that regulates APJ production is most likely milk-borne apelin. At P38, enteric APJ mRNA levels and APJ immunostaining intensity did not differ in apelin-deficient and wild-type mice implying that the effect of apelin in regulating APJ production decreased.
Consideration of the present findings with earlier reports suggests that the enteric apelin-APJ system may have multiple roles in the GI tract. Luminal apelin may be involved in regulation of intestinal hormone secretion [17], gastric acid secretion [25], regulation of APJ receptor expression and production and in mucus secretion.
ACKNOWLEDGMENTS
This work is supported by grants from the National Institutes of Health (P01 DK35608), Broad Medical Research Program (IBD-0118), and Crohn’s and Colitis Foundation of America (Ref. #1821).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 2.O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 3.Devic E, Paquereau L, Vernier P, Knibiehler B, Audigier Y. Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech Dev. 1996;59:129–140. doi: 10.1016/0925-4773(96)00585-0. [DOI] [PubMed] [Google Scholar]
- 4.Devic E, Rizzoti K, Bodin S, Knibiehler B, Audigier Y. Amino acid sequence and embryonic expression of msr/apj, the mouse homolog of Xenopus X-msr and human APJ. Mech Dev. 1999;84:199–203. doi: 10.1016/s0925-4773(99)00081-7. [DOI] [PubMed] [Google Scholar]
- 5.Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, Onda H, Tatemoto K, Fujino M. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta. 1999;1452:25–35. doi: 10.1016/s0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 6.Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107:198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Lee DK, George SR, O'Dowd BF. Unravelling the roles of the apelin system: prospective therapeutic applications in heart failure and obesity. Trends Pharmacol Sci. 2006;27:190–194. doi: 10.1016/j.tips.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Masri B, Knibiehler B, Audigier Y. Apelin signalling: a promising pathway from cloning to pharmacology. Cell Signal. 2005;17:415–426. doi: 10.1016/j.cellsig.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpene C, Audigier Y, Saulnier-Blache JS, Valet P. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–1771. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 10.Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, Sugaya T, Matsuzaki H, Yamamoto R, Shiota N, Okunishi H, Kihara M, Umemura S, Sugiyama F, Yagami K, Kasuya Y, Mochizuki N, Fukamizu A. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279:26274–26279. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 11.Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, Hinuma S, Baba A. Apelin is a novel angiogenic factor in retinal endothelial cells. Biochem Biophys Res Commun. 2004;325:395–400. doi: 10.1016/j.bbrc.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 12.Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, Nishizawa N, Kitada C, Onda H, Nishimura O, Fujino M. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta. 2001;1538:162–171. doi: 10.1016/s0167-4889(00)00143-9. [DOI] [PubMed] [Google Scholar]
- 13.Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept. 2004;118:119–125. doi: 10.1016/j.regpep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept. 2005;126:233–240. doi: 10.1016/j.regpep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G, Bolaky JE, Herrity NC, Murdock P, Darker JG. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem. 2003;84:1162–1172. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 16.O'Carroll AM, Selby TL, Palkovits M, Lolait SJ. Distribution of mRNA encoding B78/APJ, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim Biophys Acta. 2000;1492:72–80. doi: 10.1016/s0167-4781(00)00072-5. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Anini Y, Wei W, Qi X, O'Carroll AM, Mochizuki T, Wang HQ, Hellmich MR, Englander EW, Greeley GH., Jr Apelin, a new enteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology. 2004;145:1342–1348. doi: 10.1210/en.2003-1116. [DOI] [PubMed] [Google Scholar]
- 18.Ashley E, Chun HJ, Quertermous T. Opposing cardiovascular roles for the angiotensin and apelin signaling pathways. J Mol Cell Cardiol. 2006;41:778–781. doi: 10.1016/j.yjmcc.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Cox CM, D'Agostino SL, Miller MK, Heimark RL, Krieg PA. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol. 2006;296:177–189. doi: 10.1016/j.ydbio.2006.04.452. [DOI] [PubMed] [Google Scholar]
- 20.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O'Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 21.O'Shea M, Hansen MJ, Tatemoto K, Morris MJ. Inhibitory effect of apelin-12 on nocturnal food intake in the rat. Nutr Neurosci. 2003;6:163–167. doi: 10.1080/1028415031000111273. [DOI] [PubMed] [Google Scholar]
- 22.Reaux A, De Mota N, Skultetyova I, Lenkei Z, El Messari S, Gallatz K, Corvol P, Palkovits M, Llorens-Cortes C. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 2001;77:1085–1096. doi: 10.1046/j.1471-4159.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- 23.Sunter D, Hewson AK, Dickson SL. Intracerebroventricular injection of apelin-13 reduces food intake in the rat. Neurosci Lett. 2003;353:1–4. doi: 10.1016/s0304-3940(03)00351-3. [DOI] [PubMed] [Google Scholar]
- 24.Taheri S, Murphy K, Cohen M, Sujkovic E, Kennedy A, Dhillo W, Dakin C, Sajedi A, Ghatei M, Bloom S. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochem Biophys Res Commun. 2002;291:1208–1212. doi: 10.1006/bbrc.2002.6575. [DOI] [PubMed] [Google Scholar]
- 25.Lambrecht NW, Yakubov I, Zer C, Sachs G. Transcriptomes of purified gastric ECL and parietal cells: identification of a novel pathway regulating acid secretion. Physiol Genomics. 2006;25:153–165. doi: 10.1152/physiolgenomics.00271.2005. [DOI] [PubMed] [Google Scholar]
- 26.Sorhede Winzell M, Magnusson C, Ahren B. The APJ receptor is expressed in pancreatic islets and its ligand, apelin, inhibits insulin secretion in mice. Regul Pept. 2005;131:12–17. doi: 10.1016/j.regpep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Qi X, Wei W, Englander EW, Greeley GH., Jr Characterization of the 5'-regulatory regions of the rat and human apelin genes and regulation of breast apelin by upstream stimulatory factor. FASEB J. 2006;20:2639–2641. doi: 10.1096/fj.06-6315fje. [DOI] [PubMed] [Google Scholar]
- 28.Sheikh AY, Chun HJ, Glassford AJ, Kundu RK, Kutschka I, Ardigo D, Hendry SL, Wagner RA, Chen MM, Ali ZA, Yue P, Huynh DT, Connolly AJ, Pelletier MP, Tsao PS, Robbins RC, Quertermous T. In vivo genetic profiling and cellular localization of apelin reveals a hypoxia-sensitive, endothelial-centered pathway activated in ischemic heart failure. Am J Physiol Heart Circ Physiol. 2008;294:H88–H98. doi: 10.1152/ajpheart.00935.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambrecht NW, Yakubov I, Scott D, Sachs G. Identification of the K efflux channel coupled to the gastric H-K-ATPase during acid secretion. Physiol Genomics. 2005;21:81–91. doi: 10.1152/physiolgenomics.00212.2004. [DOI] [PubMed] [Google Scholar]
- 30.Susaki E, Wang G, Cao G, Wang HQ, Englander EW, Greeley GH., Jr Apelin cells in the rat stomach. Regul Pept. 2005;129:37–41. doi: 10.1016/j.regpep.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Saint-Geniez M, Masri B, Malecaze F, Knibiehler B, Audigier Y. Expression of the murine msr/apj receptor and its ligand apelin is upregulated during formation of the retinal vessels. Mech Dev. 2002;110:183–186. doi: 10.1016/s0925-4773(01)00558-5. [DOI] [PubMed] [Google Scholar]
- 32.Gomez G, Zhang T, Rajaraman S, Thakore KN, Yanaihara N, Townsend CM, Jr, Thompson JC, Greeley GH. Intestinal peptide YY: ontogeny of gene expression in rat bowel and trophic actions on rat and mouse bowel. Am J Physiol. 1995;268:G71–G81. doi: 10.1152/ajpgi.1995.268.1.G71. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi K, Peitsch W, Johnson LR. Mucosal gastrin receptor. V. Development in newborn rats. Am J Physiol. 1981;240:G163–G169. doi: 10.1152/ajpgi.1981.240.2.G163. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Vilar J, Hill RL. The structure and assembly of secreted mucins. J Biol Chem. 1999;274:31751–31754. doi: 10.1074/jbc.274.45.31751. [DOI] [PubMed] [Google Scholar]
- 35.Verburg M, Renes IB, Meijer HP, Taminiau JA, Buller HA, Einerhand AW, Dekker J. Selective sparing of goblet cells and paneth cells in the intestine of methotrexate-treated rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1037–G1047. doi: 10.1152/ajpgi.2000.279.5.G1037. [DOI] [PubMed] [Google Scholar]
- 36.Schumacher U, Duku M, Katoh M, Jorns J, Krause WJ. Histochemical similarities of mucins produced by Brunner's glands and pyloric glands: A comparative study. Anat Rec A Discov Mol Cell Evol Biol. 2004;278:540–550. doi: 10.1002/ar.a.20046. [DOI] [PubMed] [Google Scholar]
- 37.Han S, Wang G, Qiu S, de la Motte C, Wang HQ, Gomez G, Englander EW, Greeley GH., Jr Increased colonic apelin production in rodents with experimental colitis and in humans with IBD. Regul Pept. 2007;142:131–137. doi: 10.1016/j.regpep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Schulz S, Stumm R, Rocken C, Mawrin C, Schulz S. Immunolocalization of full-length NK1 tachykinin receptors in human tumors. J Histochem Cytochem. 2006;54:1015–1020. doi: 10.1369/jhc.6A6966.2006. [DOI] [PubMed] [Google Scholar]