Abstract
Prolactin has been implicated in promoting paternal care behaviors but little evidence of causality has been found to date except for birds and fish. This study was designed to examine the possible causal relationships between prolactin and male parenting behaviors, reproductive hormones, and physical changes in cooperatively breeding common marmosets, Callithrix jacchus. Fifteen parentally experienced fathers were studied over three consecutive infant care periods during two weeks prior and three weeks following their mates' parturition under three treatment conditions: normal control pregnancy, decreased prolactin and elevated prolactin. The treatments significantly altered the serum prolactin levels in the fathers. Using three methods of determining a father's level of parental care: infant carrying, family effort and responsiveness to infant stimulus tests, we found that only the male response to infant stimuli was altered by the hormone treatments. Lowering prolactin significantly reduced male responsiveness to infant stimuli but elevating prolactin showed the same effect. Hormonal sampling indicated that testosterone levels showed an inverse relationship to prolactin levels during a normal peripartum period and prolactin treatment reduced this relationship. Prepartum estradiol levels were significantly elevated during the lowered prolactin treatment and estradiol was significantly lowered postpartum with the elevated prolactin treatment. Father's weight decreased significantly by the third week of infant care during the normal treatment. Males in the elevated prolactin treatment lost little or no weight from prepartum while in the lowered prolactin treatment showed the most weight loss. The present findings did not distinguish a direct causal relationship of prolactin on behavior in experienced fathers but did find an interaction with other hormones and weight gain.
Keywords: prolactin, paternal care, infant responsiveness, testosterone, estradiol, common marmoset, weight gain
Introduction
The pituitary hormone prolactin is involved in regulating many important systems in the body. It's most notable actions are found in the reproductive, metabolic, osmoregulatory and immunoregulatory systems (Ben-Jonathan et al., 2006). Within the reproductive system, prolactin is known for its effect on lactation and its involvement in the onset of rodent maternal behavior (McCarthy et al., 1994; Bridges et al., 1985). Prolactin is required for ovarian hormones to be effective in stimulating maternal behavior (Bridges and Ronsheim, 1990). Key target areas of the brain for maternal behavior have prolactin receptors (Pi and Grattan, 1998). Neurogenesis in the forebrain during pregnancy is stimulated by prolactin (Shingo et al., 2003). Nest building and pup retrieval are regulated by prolactin in the medial preoptic area of the rat brain (Bridges et al., 1997). Therefore, prolactin has a role in promoting maternal behaviors. In the rat, hormones regulate maternal behavior onset but maintenance of maternal behavior may be free from hormonal control where experienced rats don't rely on hormonal input to be maternal (Rosenblatt and Siegel, 1981; reviewed in Fleming et al., 2009).
Knowledge about prolactin's influence on paternal behavior in biparental species is limited except for birds and fish (Buntin, 1996; Páll et al., 2004). However, prolactin levels are elevated in the periparturitional period in fathers, both first-time and experienced fathers. Males do not undergo pregnancy; yet there are indications that males undergo hormonal and other physiological changes during their mate's gestational period (Wynne-Edwards and Reburn, 2000). Prolactin levels are elevated in males at the end of their mate's pregnancy in the common marmoset, (Callithrix jacchus), cotton-top tamarin (Saguinus oedipus), Goeldi's (Callimico goeldii) monkeys and some biparental rodents (Brown et al., 1995; Gubernick and Nelson, 1989; Reburn and Wynne-Edwards, 1999; Schradin et al., 2003; Ziegler and Snowdon, 2000; Ziegler et al., 2004; Schradin, 2008). Prolactin is elevated during the chick-rearing period in ring doves (Buntin, 1996) and an up-regulation and expression of the prolactin receptor occurs in parental fish with paternal behaviors (Khong et al., 2009). Additionally, direct evidence for both prolactin and steroid hormone involvement in paternal behavior has been shown in ring doves (Buntin and Tesch, 1985; Buntin, 1996) and fish (Blüm and Fiedler, 1965; Ruiter et al., 1986; Kindler et al., 1991; Páll et al., 2004).
Males from a number of biparental mammalian species show elevated prolactin while they are actively participating in infant care. Biparental rodent males, such as the Mongolian gerbil, California mouse and dwarf hamsters show elevated prolactin postpartum (Brown et al., 1995; Gubernick and Nelson, 1989; Reburn and Wynne-Edwards, 1999). The naturally biparental hamster, Phodopus campbelli, shows higher mRNA for prolactin receptors in the choroid plexus of the brain than nonpaternal males of Phodopus sungorus (Ma et al., 2005). In primates, cotton-top tamarins have elevated prolactin both pre- and postpartum (Ziegler et al., 2000). The common marmoset has elevated levels of prolactin postpartum specifically in fathers carrying infants (Dixson and George, 1982; Torii et al., 1998; Mota and Sousa, 2000) or when other group members carry infants (Roberts et al., 2001a). Human fathers also have been reported to exhibit increased prolactin levels before and after infant birth (Storey et al., 2000) and fathers are more responsive to infant cries when they have higher prolactin levels (Fleming et al., 2002).
Prolactin's role in promoting paternal care behaviors is unclear since pharmacological experiments do not support a causal role of prolactin on onset or maintenance of paternal care behaviors. Several studies have examined the reduction of prolactin through dopamine receptor agonists. In a study reported by Roberts et al. (2001b) who used mainly females, parentally inexperienced young marmosets reduced their retrieval of infants when their prolactin was suppressed with bromocryptine. However, another study using the D2 receptor agonist, Cabergoline, showed no changes in experienced marmoset father's infant care behaviors during observations made within the family context (Almond et al., 2006). In the biparental Djungarian hamster, (Phodopus cambelli), Brooks et al. (2005) found no reduction in pup retrieval or retrieval latency after suppression of prolactin pre and postpartum in first-time fathers using either Cabergoline or Bromocryptine. A lack of clear evidence for prolactin's effect on paternal care behaviors has been debated recently (Wynne-Edwards and Timonin, 2007; Schradin, 2007).
While a causal relationship between prolactin and paternal care behaviors has not been proven, the evidence for a correlational relationship between prolactin and paternal care is evident in many biparental species. Changes in prolactin levels in biparental males definitely occur. The role of prolactin before and during infant care periods for experienced fathers may have less to do with direct effect on behaviors as it does for other prolactin functions such as the metabolic roles of prolactin and its stimulation of other reproductive hormones.
Infant care requires energetic demands (Clutton-Brock, 1991). Species where infants need to be carried may present a greater energetic demand (Kirkwood and Underwood, 1984; Goldizen, 1987). However, the energetic demands of parenting may be shared by the mate in bi-parental species (Tardif, 1994) and by other group members in cooperatively care systems. Species of the neotropical primate family, Callithricidae (marmosets and tamarins), have been reported to have a high energetic cost to infant care (Tardif, 1994; Schradin and Anzenberger, 2001). These species develop large multiple infants (up to 20% of their body weight). Marmoset and tamarin infants are carried almost continuously for the first month after birth (Tardif et al., 1986). One cost associated with infant carrying is the inability of the carrier to leap as far as a noninfant carrier (Schradin and Anzenberger 2001). Another cost is the loss of body weight. Cotton-top tamarin fathers have been reported by two different laboratories to lose weight during the infant care period (Sanchez et al., 1999; Achenbach and Snowdon, 2001). Males varied in the amount of weight they lost but in both studies some males lost nearly 11% of their body weight. Males, without offspring to serve as helpers, lost the most weight (Achenbach and Snowdon, 2001). We have previously reported that expectant common marmoset and cotton-top tamarin fathers gain weight while their mate is pregnant (Ziegler et al., 2006).
Prolactin may play a role in the energetics of parenting. The hormone may work to change the metabolic process of energy absorption of food or increase the rate of food intake. Increased food consumption (hyperphagia) and corresponding elevations of prolactin have been found in biparental ring doves (Streptopelia risoria) (Buntin and Tesch, 1985). Peripherally or centrally administered prolactin in nonbreeding ring doves has been shown to induce marked hyperphagia (Buntin, 1989; Buntin and Figge, 1988; Buntin and Tesch, 1985). The feeding response to prolactin is sexually dimorphic in ring doves with males showing twice the increase of females (Buntin and Tesch, 1985). In humans when prolactin levels are elevated by hyperprolactinemia, patients gain weight and lowering prolactin in these patients also reduces weight gain suggesting a causative role for prolactin in weight gain (Greenman et al., 1998).
In this study, we attempt to determine the effects of prolactin on parental care behaviors, reproductive hormones, and weight gain in parentally experienced male marmosets. While prolactin's role on paternal behavior may be more like the maternal rat where prolactin is most effective during the onset of maternal behavior, we wanted to begin with the paternally experienced male who shows pronounced paternal behaviors and pronounced levels of prolactin during the perinatal period. We tested each male under 3 treatment conditions: lowered prolactin, elevated prolactin and normal control during the periparturitional period.
Methods
Animals
Common marmosets were socially housed at the Wisconsin National Primate Research Center. All males were parentally experienced (n = 15) and had fathered two or three previous litters and were between the ages of 2.5-9 years of age (5.27± 0.38) at the onset of testing (Mean ± SEM). To test their difference by treatment, males underwent three consecutive births and infant care periods under the following treatments: “lowered” prolactin by a D2 agonist (Cabergoline), “increased” prolactin by human recombinant prolactin (hPrl), and a “control”, normal condition where no manipulation of prolactin occurred. All males were in all treatment conditions and were assigned to one of the three treatments in a randomized order. Prolactin manipulations started 14 days prior to the estimated due date of their mate and continued for 28 days postpartum. Marmoset families were housed in cages that measured either 122 × 61 × 183 cm or 61 × 91 × 183 cm. Diets and husbandry details have been reported previously for this colony [Saltzman et al., 1997]. No changes in diet quantity or quality occurred during the testing period. In the morning, marmosets were fed a protein snack and a primary feed occurred near noontime that included a fruit or a vegetable portion. Water was provided ad libitum. Lighting was regulated on a 12:12 hour light/dark cycle and the humidity was maintained at approximately 40%. Housing conditions and behavioral testing met the guidelines for nonhuman primates and were approved by the Animal Care and Use Committee (IACUC) at the University of Wisconsin.
Behavioral testing
Behavioral tests and inter-male variability in responsiveness to infant stimuli have been described for these subjects in detail (Zahed et al., 2008). We collected three types of behavioral measures on parentally experienced fathers. Fathers were observed in their family groups for both (1) infant directed behaviors: touch, sniff, groom, face lick, anogenital lick, attempt to retrieve, successful retrieve, (2) infant carrying during the first two weeks following birth: percent time father carried, and on the third week postpartum for their (3) responsiveness to an infant stimuli outside the family group (infant's cries presented as a recording on digital voice recorder served as the stimulus): attempt to retrieve stimulus, search for stimulus, look at stimulus, manipulate stimulus, enter stimulus cage. Measuring a male's response to the infant vocalizations proved to be the most effective method to measure a male's motivation and interest in infant care (Zahed et al., 2008). Each father's frequency of caretaking behavior was summed for analysis. Trained observers used a hand held Tungsten C Palm Pilot (2005) and the “Hand Obs” observation program (Dr. Kim Wallen, Emory University). The testing that occurred outside of the family cage consisted of a specially designed infant response cage where the male could cross a bridge to enter the cage with the infant stimulus. Males were habituated to the testing cage for three consecutive days and had alternating stimulus and non-stimulus (control) testing days. We additionally tested a vocal control (vowel E sound) presented to seven experienced fathers and found the behaviors: look at stimulus and search for stimulus were significantly different between the stimulus vocal, the vocal control and the non vocal control, (Look at stimulus: F = 6.46, p = 0.027, Search for stimulus: F = 11.22, p = 0.003, where the stimulus vocal was significantly different, p < 0.05).
Parentally experienced fathers were examined for their weight loss from their average weight for the last two weeks of their mate's pregnancy with weekly weights for the three weeks following birth. Males were weighed each week on the same day between 0900-1000. To collect the weight, males were ushered into their nest boxes and then weighed on a scale (Sartorius, Model BP12000-S, d=0.1 g). The weight of the box was torn leaving the weight of the marmoset. The male was weighed five times in a row and the average weight was recorded for the week. Blood (0.2 ml) was collected prior to weighing via femoral puncture and stored at -20°C until analyses.
Prolactin manipulation
Cabergoline (Dostinex©), a dopamine agonist, was used to lower prolactin beginning 14 days prior to the female's estimated due date through to 28 days postpartum. Cabergoline powder, at 0.1 mg/kg, was mixed with a dropper of Ensure® (Ross Nutrition, Abbott Labs, Columbus, OH), a milk-like treat and given orally every 72 hours to the male while he was in his home cage. Males readily consumed the treat from a dropper. All males received the Ensure treat in all treatment conditions but only the lowered prolactin condition received the cabergoline in the Ensure. Cabergoline was given every three days between 1400 to 1500 hours, and was given in enough time to suppress the prolactin before the blood sample was taken the following morning as indicated by prolactin analysis. All males were blood sampled on the same schedule while cabergoline was given consistently every three days.
Prolactin was raised above normal prolactin levels by using human recombinant prolactin (hPRL) provided by Dr. AF Parlow (AFP795). For pilot males, we attempted to elevate the prolactin by providing a 1.54 mg dose by time-release pellets (21-day release, Innovative Research of America, Sarasota, FL). However, these pellets did not raise the level of prolactin in the blood over the level of our control parentally experienced fathers (mean ± S.E. = 4.17±0.83 ng/ml). Therefore, we chose to use the osmotic minipump (Alzet, CA) for our test males. The same dose, 1.54 mg of hPRL, was administered in a 28-day osmotic minipump (0.25 μl per hour). The hPRL was dissolved in sterile buffer (0.1 M NaHC03 + 0.15 M NaCL + 1.6% glycerol) and inserted into the pump a day prior to insertion into the male marmoset. Pumps were inserted subcutaneously into the mid dorsal area beginning 14 days prior to the female's due date, and a second pump was inserted 21-24 days after the first pump at approximately 8-10 days after the birth of infants. The position of the pumps on the lower area of a male's back precluded any interference with infant carrying since infant carrying occurs nearer the neck. In a preliminary study we tested a saline control osmotic pump over a one-month period and found that there was no difference in the male's weight due to carrying the pump. Due to the limited size of the marmoset cages, urine collection for hormonal analysis was not feasible. Blood was collected on the same schedule for all three periparturition periods. Males were bled once weekly between 0900 to 1000 beginning 14 days prior to the female's estimated due date. After infants were born, blood sampling occurred postpartum on day 3, one sample between days 4-7, 2 samples were taken between days 8-14 postpartum and three additional samples on days 20, 23, and 27 postpartum. Males were restrained and 0.2 ml of blood was taken from their femoral vein, followed by weighing. Blood was then separated and stored at -20° C until analyses.
Prolactin (PRL) concentration was determined and validated for an I125 radioimmunoassay. This assay uses NHPP recombinant cynomologus monkey PRL (AFP1059, National Hormone and Peptide Program) as the standard reference preparation, and anti-cynomologus monkey PRL for the antibody (AFP291891). We used 50 μl of marmoset serum with 100 μl antibody and 100 μl PRL trace (10,000 cpm, in house iodination of AFP1059, IODOGEN, Pierce Chemical Co.). After incubation overnight at room temperature, a second antibody was added at 500μl (3% goat, anti-rabbit gamma globulin in assay buffer) and then samples were incubated again overnight at room temperature. Samples were centrifuged for 30 minutes at 2780 Gs, 7° C and the fluid was aspirated, leaving the pellet intact. Samples were counted in a gamma counter for 10 minutes. Serial dilutions of marmoset serum were parallel to the standard curve, t = 1.19, p > 0.05 and accuracy was 96.77 ± 1.29% n = 8. Pooled marmoset serum was used to determine the intra and inter-assay coefficient of variation for the monkey prolactin assay: n=7, intra CV = 4.57, inter CV = 13.09.
After the prolactin assay, samples for each male where ample serum was available were combined to provide two conditions: prepartum and postpartum for measurement of testosterone and estradiol for each male. To determine the levels of testosterone (T) and estradiol (E2), samples of 200 μl of marmoset serum plus 300 μl of distilled water were extracted with 5 ml of diethyl ether. After five minutes of vortexing and centrifugation (3 minutes at 1500 rpm), the aqueous portion of the sample was flash-frozen and the solvent phase was decanted and dried under air in a water bath. Samples were reconstituted into 1 ml 30% methanol and purified with solid phase extraction (StrataX, 30 mg, phenomenx) as per directions with the addition of 20% methanol for the rinse and eluting the sample in 2 ml of methanol. After drying, the sample was reconstituted in 25 μl ACN: H2O (50:50) and injected onto a high-performance liquid chromatography (HPLC, Beckman-Coulter, Fullerton, CA) system to separate the steroids following the methods reported in Ziegler et al. (2004a). Each sample was run for 15 minutes and fractions for E2 and T were collected on a Gilson fractionator (Gilson, Middleton, WI). Samples were dried and reconstituted back to 200 μl in ethanol and the individual fractions were assayed for either T or E2. The T was validated for marmoset serum and assayed by EIA according to the methods described in Ziegler et al. (2005) at a volume of 15 μl. Since all samples for T and E2 were assayed in three or less assay, we report the lab values: Intra and interassay coefficients of variation for the T EIA were 3.2 and 12.0%, n=11. E2 was validated and assayed with a radioimmunoassay as described in Saltzman et al. (1998) using 200 μl amounts. Intra and interassay coefficients of variation for the E2 were 3.8 and 15.7%, n=11
Statistical analysis
The data from the parentally experienced males were compared across prolactin treatments (lowered, normal, elevated) as repeated measures within subjects design. Differences by treatment for prolactin levels were determined by repeated measures ANOVA with pair-wise comparisons by Tukey. Male responsiveness to infant cues for the three behavioral tests were log transformed and compared across the three treatments as repeated measures ANOVA with Tukey posthoc. T-tests were used to determine significant differences for steroid levels both as pre- and postpartum in each treatment.
Significant differences in weight across weeks for the males in the normal control condition were assessed by within subjects repeated measures ANOVA with Tukey post hoc. To compare males between treatments, the mean weight lost during the infant care period from the prepartum amount was determined for all males in all conditions. An average weight loss was determined by comparing the most amount of weight lost during the three-week parenting period with the mean weight of the last two weeks prior to birth for each male in each condition. Repeated measures ANOVA was used to determine a difference in weight loss between treatments with Bonferroni's Multiple Comparison Test between treatments. A Spearman Rank Correlation was used to compare the mean weight loss with their mean prolactin levels during the infant care period for all males during their normal infant care period.
Results
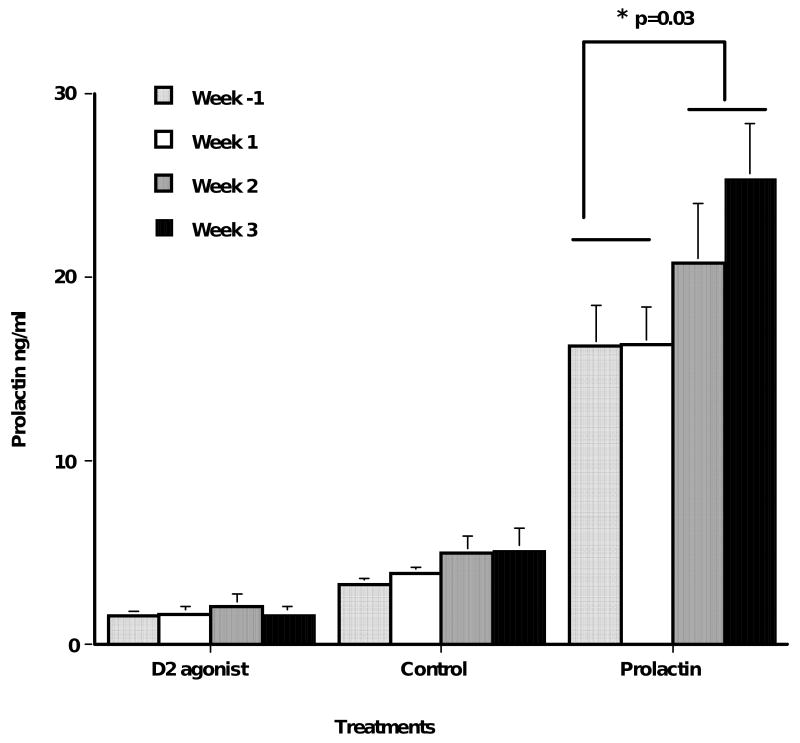
Fourteen of our 15 parentally experienced males went through all three-treatment conditions. Mean serum prolactin levels over the four weeks of treatment were significantly different by treatment (F = 46.6, p = 0.0001; treatments: lowered prolactin: 1.4±0.4 ng/ml; normal: 4.2±0.8 ng/ml; elevated prolactin: 19.3±2.3 ng/ml). Mean increased prolactin treatment was significantly higher than the other treatments (p = 0.0001). Figure 1 shows the mean prolactin levels for the different treatments by week. Prolactin concentrations were also different by week. Males in the normal control treatment, or non hormonal manipulation, showed higher levels of prolactin in the second and third week postpartum than in the week before and after birth (t= 2.0, p=0.07, N=15) and males in the elevated prolactin treatment showed significantly higher levels in the second and third weeks postpartum (t = 2.48, p = 0.03, N = 11).
Figure 1.
Mean serum prolactin levels in experienced male marmoset fathers during the prepartum (last two weeks prior to birth) and weeks 1, 2 & 3 following birth. All males under went three succesive treatments during their peripartum periods: lowering prolactin with a D2 agonist, normal control, and increased prolactin in random order. All males were actively involved in infant care during this time. Prolactin levels were significanly different by treatment (p=0.0001) and by week (p=0.03) in the increased condition.
Parenting behaviors were significantly different by treatment only when tested outside the family group during the infant stimulus response tests, Figure 2. There were no significant differences in frequencies of infant directed behaviors by treatment for the paternal behavior within the family context or infant carrying time. Males, however, showed significant differences by treatment in their responses to infant stimulus (F = 5.05, p = 0.02, N = 13). The lowered prolactin treatment was significantly lower than the normal treatment (p < 0.05) and the elevated treatment was also significantly lower than the normal treatment (p < 0.05).
Figure 2.
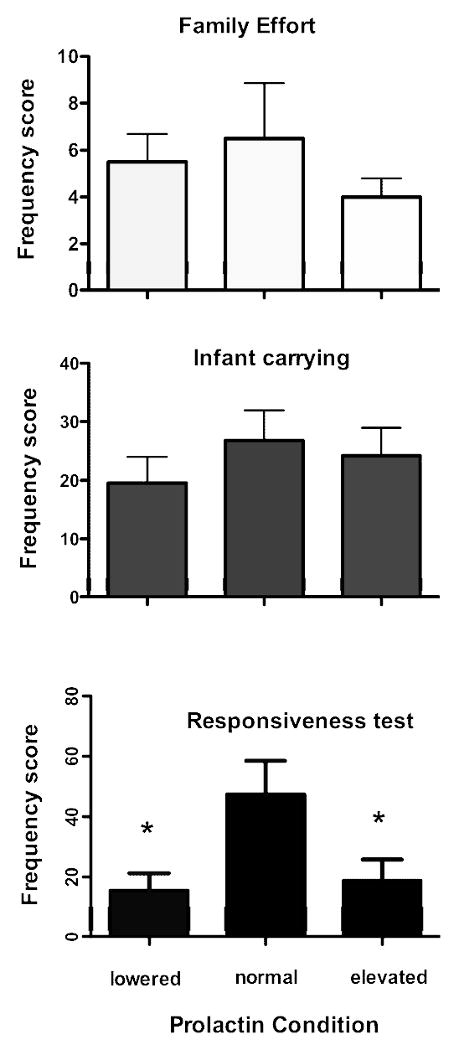
Mean frequency scores per observation in parentally experienced fathers for their infant directed behaviors under three treatments: lowered prolactin (D2 agonist), nontreated control, elevated prolactin (human recombinant). Males showed no differences in behavioral frequencies between treatments for their family effort behaviors or percent carrying effort in the family. Males did show significant differences between treatments for their infant responsivenss behaviors, * = p<0.05.
We compared the specific paternal behavior in the responsiveness to infant stimulus. Significant differences between the three treatments occurred for the individual behaviors: searching for infant (Friedman test = 6.65, p = 0.036), look at stimulus (F = 7.51, p = 0.02), and nearly significant for manipulate stimulus (F = 5.77, p = 0.055). The normal condition was significantly higher than the other treatments, p < 0.05.
Differences between mean concentrations of estradiol and testosterone pre and postpartum were examined under the three treatments, Figure 3. Estradiol levels were not different pre and postpartum in fathers during the normal condition. However, significant differences occurred in estradiol levels under the lowered and elevated prolactin conditions. Prepartum levels of estradiol were significantly higher than the postpartum levels when prolactin levels were lowered (t = 2.4, df = 6, p = 0.03). During the elevated prolactin treatment mean levels of estradiol were significantly lower during the postpartum period than the prepartum (t = 5.75, df = 4, p = 0.002). Mean testosterone levels were significantly different pre to postpartum in males during the normal condition (t = 1.89, df = 13, p = 0.04) but no changes in testosterone levels occurred due to lowering or elevating prolactin.
Figure 3.
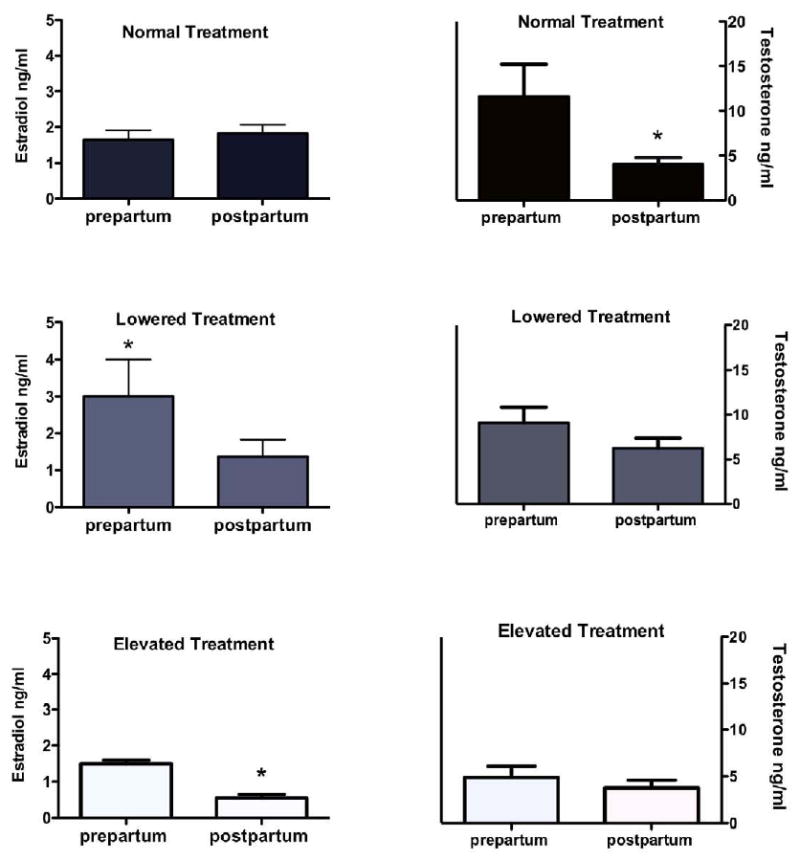
Mean level of estradiol (left panel) and testosterone (right panel) pre and post partum during three treatments: normal, lowered and elevated. Lowering prolactin significantly increased estradiol pre partum while elevating prolactin decreased estradiol. Testosterone was significantly reduced under normal treatment postpartum.
Analysis of weight data indicated that males lost weight during the postpartum period when they were caring for infants. Males who had gone through a non-treated, normal period of expectant fatherhood and the following three weeks after birth, showed a significant decrease in weight following birth (F = 4.5, p = 0.008, N = 15; Figure 4). Prepartum weights were significantly higher than week 3 (Bonferroni's Multiple Comparison Test, p < 0.05). Males had lost the most weight by the third week following birth during the infant care period in the normal condition.
Figure 4.
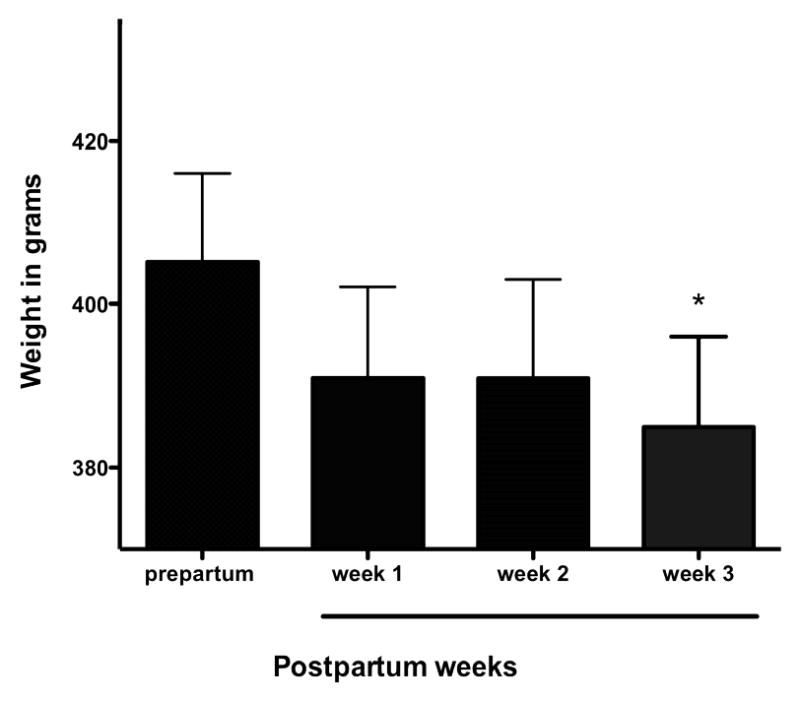
Mean weight (in grams), for 15 marmoset fathers during a normal peripartum period (normal treatment). Father's weight was significantly lower during the third infant carrying week than prepartum (P < 0.05).
The amount of weight males lost from prebirth levels was significantly different due to treatment (F = 3.5, p = 0.047, N = 13). Figure 5 shows the mean weight loss postpartum from the mean prepartum levels for the males by treatment condition. Males with lowered prolactin levels lost significantly more weight than males receiving increased prolactin treatments (Z = 5.61, p = 0.01), but not more than the non-manipulated normal fathers. Males with elevated prolactin levels lost the least or no weight.
Figure 5.
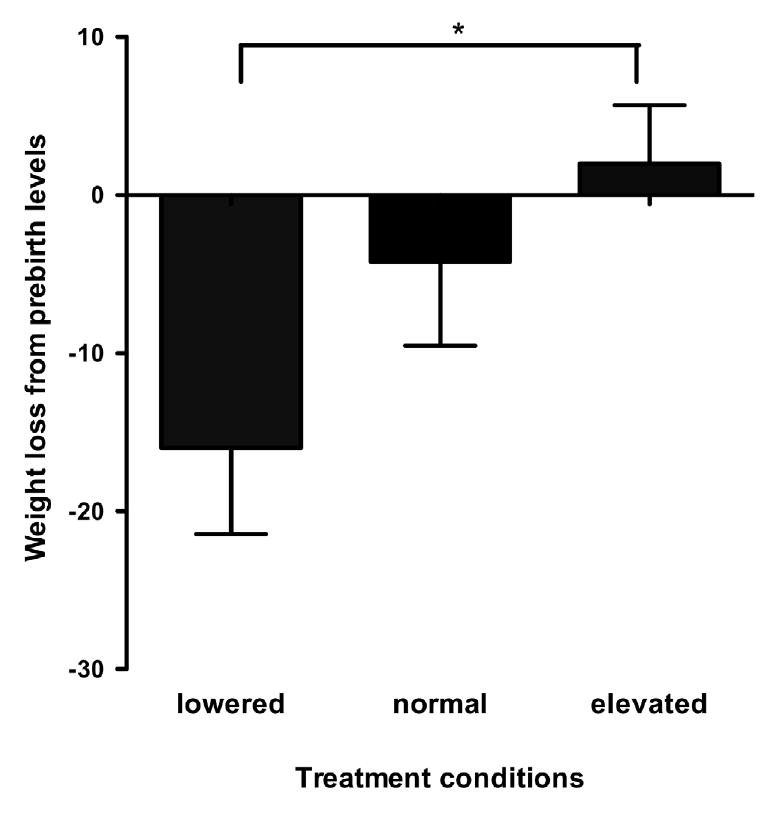
Mean weight loss for parentally experienced fathers during the three week postpartum period from the prepartum period for 13 fathers by treatment. Fathers lost significantly more weight in the lowered prolactin condition than in the increased prolactin condition (p=0.01).
A significant negative correlation per male was found between the mean amount of weight lost during a normal infant care period and mean prolactin levels (rs=-0.63, p = 0.04). The males, who lost the least amount of weight, or none, had the highest prolactin levels postpartum.
Discussion
This study identifies an important role for prolactin in paternally experienced marmosets. However, rather than a direct effect on the expression of paternal care, prolactin influences a male's weight during the infant care period. Elevated prolactin postpartum, when males are actively caring for infants, may work to prevent excessive weight loss during their added energetic demands. Hormonal responses to the prolactin changes indicate that changes in both testosterone and estradiol are involved in paternal changes with parenting. However, we did not find a direct causal relationship of prolactin to behavior because elevated prolactin levels did not enhance paternal behavior. We did not manipulate prolactin in first-time and non fathers, and therefore do not know if prolactin changes would have affected the onset of paternal behaviors as occurs in the maternal rat (Rosenblatt and Siegel, 1981). This would be an important next step in understanding the role of prolactin in biparental primate paternal behavior.
The reduction of prolactin by use of the D2 agonist, Cabergoline, appeared to act specifically on a male's motivation to respond to infant cries rather to general parenting participation, per se. As has been reported in an earlier study on parentally experienced male marmosets, this D2 agonist does not appear to influence the time a parentally experienced male spends with his infants within the family (Almond et al., 2006). Yet our results were also consistent with the findings of Roberts et al. (2001b) in that another D2 agonist, Bromocriptine, also lowered serum prolactin and disrupted responsiveness to infants in inexperienced common marmosets, although most of these subjects were female. Activation of the dopamine D2 receptor appears to mediate dopaminergic action on maternal behavior (Bridges, 1996). Dopamine suppresses prolactin release and thereby would reduce prolactin's effect on parenting responses to infant stimuli. Exploration of the effects of manipulating hormone levels in parentally inexperienced male marmosets may provide a key difference in results from those obtained from the experienced fathers. It is clear the inexperienced males already behave differently in response to infant stimuli than experienced fathers (Zahed et al., 2008) and the difference might potentially be reduced or eliminated with elevated prolactin levels.
If prolactin was responsible for the experienced males' infant responsiveness, then males should have shown an increase in infant responsiveness with the prolactin implants. However, elevating prolactin did not increase infant directed behaviors or response to infant stimuli in our study. Elevated prolactin surprisingly decreased infant responsiveness behaviors to levels below normal as occurred with the D2 agonist. One explanation might be that there is a threshold level for the efficacy of prolactin with effective prolactin stimulation being more in the normal range of prolactin during the periparturitional periods. Our elevated prolactin was higher than the normal condition. Once a male is experienced in infant care, there may be less of a need for additional prolactin with prolactin's behavioral role as onset of paternal responsiveness as it is for the maternal rat (Rosenblatt and Siegle, 1981).
Testosterone did show an inverse relationship with prolactin. In the normal condition (no prolactin manipulation) we found that testosterone levels were significantly lower during the infant care period than before infant birth. Prepartum levels of testosterone were around normal levels found for male marmosets from other studies (Ziegler et al., 2005) while testosterone levels were significantly lower during the postpartum period. The relationship with prolactin was not altered by prolactin manipulation but was altered by the normal increase in prolactin with infant care. The weeks following infant birth are a time when prolactin levels are elevated during infant care. The association of prolactin with the postpartum period and infant contact has been reported previously (Dixson and George, 1982; Mota and Sousa, 2000, Schradin et al., 2003). However, the lowered prolactin treatment did reduce the difference in the pre to postpartum levels with testosterone levels not as suppressed as under the normal condition postpartum. When prolactin levels were elevated, we found that both pre and postpartum levels of testosterone were as low as postpartum in the normal condition. Prolactin appears to be suppressing testosterone, especially under parenting conditions.
In biparental primates, such as marmosets and tamarins, a reduction in testosterone would not be expected since their mate has a postpartum ovulation within two to three weeks of birth. Cotton-top tamarin males show a sustained elevation in testosterone and DHT during the ten days around their mate's postpartum ovulation while they are actively engaged in infant care and in sexual activity (Ziegler et al., 2004). However, fathers in the Weid's marmoset (Callithrix kuhli) have decreased testosterone levels with increasing infant-carrying rates (Nunes et al., 2001). Apparently, the testosterone to prolactin relationship in biparental primates can adjust to the simultaneous parenting and breeding relationship by lowering testosterone for parenting and increasing testosterone during the female's fertile period. The flexible changes in testosterone are most likely due to testosterone's response to fertility and parenting signals. Due to the long generation time of sperm production in primates (Amann, 2008), testosterone would be facilitating behavioral responses in the male rather than increasing fertility by sperm production.
Prolactin manipulations affected estradiol levels differently before and after infant birth. Estradiol levels were significantly higher prepartum with the lowered prolactin treatment and significantly lower postpartum during the elevated prolactin treatment. These inverse relationships most likely indicate the different roles of estrogen before infant birth than after. Estradiol does not appear to be required for paternal care in dwarf hamsters, Phodopus campbelli, (Hume and Wynne-Edwards, 2006) but it is well known that estradiol is a key hormone in the induction of maternal behavior (Bridges, 1996). All other maternal hormones such as progesterone, prolactin and oxytocin require some exposure to estradiol to produce their stimulatory effects. Estradiol has a permissive action that allows prolactin to stimulate the neural processes (Moltz et al., 1970; Zarrow et al., 1971).
While more data is needed on the role of estradiol and testosterone on infant care behaviors, the present results suggest that there is an interaction of prolactin with estradiol and testosterone during the periparturitional period for fathers. Prolactin by itself does not initiate maternal behavior, but decreases the latency to the onset of maternal behavior in rats (Bowen et al., 1996; Freeman et al., 2000). Whether the interaction of the steroid hormones with prolactin acts the same in paternal males as it does in maternal females has yet to be determined. While males do not have the same pregnancy induced changes in estradiol to promote prolactin changes, it does appear that estradiol is a stimulus in males as well. The increase in prolactin due to infant contact appears to be the same in both sexes. Even non-paternal male rats will show the same induction of brain prolactin receptors of the long form mRNA expression as occurs in female rats (Sakaguchi et al., 1996). Exposure to estrogens increases the expression of prolactin receptors in the brain and elevates serum prolactin levels. Estrogens stimulate prolactin both in the hypothalamus and the pituitary (Lieberman et al., 1982). There appears to be a feedback loop where estrogen stimulates prolactin, which then negatively feeds back on estrogen. This relationship may change depending on whether or not infants are present.
Our results indicate that there is an energetic cost to caring for infants for common marmoset fathers. Fathers experience a significant weight loss while carrying infants during a normal three-week postpartum period. They also show increased prolactin levels during this time. This suggests that prolactin plays a role in buffering father marmosets from losing too much weight. Manipulating prolactin levels resulted in changes in the amount of weight a male lost, providing evidence of a causative role for prolactin. Males with high levels of prolactin show no or limited weight loss, while males who had lowered or low prolactin showed a decrease in body weight.
Each male marmoset in this study went through all three treatments in a randomized order that allowed us to follow each male's physiological changes under the three conditions. These males showed a significant physiological response to the prolactin. This provided for a more powerful test of their weight response to prolactin and eliminated a source for between-groups differences.
Recently, prolactin's actions as a metabolic hormone have been reviewed extensively (Ben-Jonathan et al., 2006). In rats, chronic elevations of prolactin are associated with increased food intake and body weight. This has been found by use of dopamine antagonists, daily injections of prolactin and with ectopic pituitaries (Bayatt et al., 1993; Moore et al., 1986; Baptisda et al., 2004). The use of a dopamine agonist, bromocryptine, shows the reverse affect and lowers weight gain (Knudtzan et al., 1986). Bromocriptine reduction is most effective in rats that are lactating, suggesting their enhanced sensitivity (Bayatt et al., 1993). Humans also show weight gain with prolactinomas (Doknic et al., 2002; Greenman et al., 1998) and also with antipsychotic drugs (Baptista et al., 2000). Treatment to lower prolactin in these cases will lower body weight and occurs in both men and women. Interestingly, not all patients show the clear response to prolactin and therefore, it is hypothesized that there may be variations in the expression of the prolactin receptor or signaling process (Ben-Jonathan et al., 2006).
While the above studies elucidate the relationship of prolactin to weight, they do not address the normal condition of increased energetic load due to parental care and its affect on prolactin. Our study examines a unique condition where males gain weight prior to their mate's pregnancy and then lose weight during their extensive infant care period. While weight gains occur during pregnancy in concert with increasing prolactin in male marmosets and tamarins (Ziegler et al., 2006; Ziegler and Snowdon, 2000; Ziegler et al., un published data), we saw a loss of weight following infant birth while prolactin was increasing. Our studies on prolactin manipulations suggest that weight in the experienced male common marmoset is responsive to prolactin levels. Therefore, the data suggest that males may have lost even more weight while carrying infants if a normal increase in prolactin, associated with infant care, had not occurred.
Infant carrying has been associated with increased prolactin in the common marmoset. Fathers carrying infants have elevated prolactin after infant contact (Dixson and George, 1982; Torii et al., 1998; Mota and Sousa, 2000). Additionally, other helpers in the group or even unfamiliar subadult marmosets have an increase in prolactin with infant carrying (Roberts, et al., 2001; Mota and Sousa, 2000). These data and ours suggest that prolactin rises in response to infant contact and that this assists in preventing severe weight loss in fathers.
Carrying infants is costly and is considered to be the most costly form of parental care after lactation (Altmann and Samuels, 1992; Kramer, 1998). Within a cooperative breeding system of the marmosets and tamarins, males are a necessary component in ensuring the survival of their offspring (Snowdon, 1996). The cost of paternal care is less when there are other offspring in the family to share the carrying burden of the newborn infants. Physiological changes in metabolic hormones, such as prolactin, may protect males during the stressful event of carrying multiple infants and ensure a higher reproductive advantage in these species.
While these studies have examined the role of prolatin on the maintenance of paternal care they have not addressed the role of prolactin in the onset of infant care. The present results suggest that prolactin levels may be more important in the energetics of paternal care in parentally experienced fathers rather than in promoting specific infant care behaviors. To fully understand the role of prolactin on facilitating paternal care in the common marmoset, the next process would be to manipulate prolactin in first-time fathers to determine its role in the onset of infant care where prolactin may have a stronger behavioral role.
Acknowledgments
We acknowledge the expertise of the animal care staff for their special care of the marmosets, especially Megan Sosa, and undergraduate assistance from Jennifer Gorst, Marcia Ramaker and Colin Devlin. We thank Dan Wittwer for his assistance with the steroid assays. This work was funded by the NIH: MH070423 to T.E. Ziegler and in part by support from the Wisconsin National Primate Research Center, RR000167, a component of the National Institutes of Health. This facility was constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach GG, Snowdon CT. Costs of caregiving: weight loss in captive adult male cotton-top tamarins (Saguinus oedipus) following the birth of infants. Internat J Primatol. 2001;23:179–189. doi: 10.1023/A:1013210226793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond EA, Ziegler TE, Snowdon CT. Suppression of prolactin does not reduce infant care by parentally experienced male common marmosets (Callithrix jacchus) Amer J Primatol. 2006;70:560–565. doi: 10.1016/j.yhbeh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Altmann J, Samuels A. Costs of maternal care: Infant-carrying in baboons. Behav Ecol & Sociobiol. 1992;29:391–398. doi: 10.1002/(SICI)1096-8644(199809)107:1<71. [DOI] [Google Scholar]
- Amann RP. The cycle of the seminiferous epithelium in humans: A need to revisit? J Androl. 2008;29:469–487. doi: 10.2164/jandrol.107.004655. [DOI] [PubMed] [Google Scholar]
- Baptista T, de Baptista EA, Lalonde J, Plamondon J, Kin NM, Beaulier S, Joober R, Richard D. Comparative effects of the antipsychotics sulpiride and risperidone in female rats on energy balance, body composition, fat morphology and macronutrient selection. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1305–1311. doi: 10.1016/j.pnpbp.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Baptista T, Lacruz A, De Mendoza D, Medoza JM, Silvera R, Angeles F, Mendoza MT, Hernandez L. Body weight gain after administration of antipsychotic drugs: correlation with leptin, insulin and reproductive hormones. Pharmacopsychiat. 2000;33:81–88. doi: 10.1055/s-2000-8451. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR. Focus on prolactin as a metabolic hormone. Trends Endocrinol & Metab. 2006;17:110–116. doi: 10.1016/j.tem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Blüm V, Fiedler K. Hormonal control of reproductive behavior in some cichlid fish. Gen Comp Endocrinol. 1965;5:186–196. doi: 10.1016/0016-6480(65)90113-9. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Keyes PL, Warren JS, Townson DH. Prolactin-induced regression of the rat corpus luteum: expression of monocyte chemoattractant protein-1 and invasion of macrophages. Biol Reprod. 1996;54:1120–1127. doi: 10.1095/biolreprod54.5.1120. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Dibiase R, Loundes DD. Prolactin stimulation of maternal behavior in female rats. Science. 1985;227:782–784. doi: 10.1126/science.3969568. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Ronsheim PM. Prolactin (PRL) regulation of maternal behavior in rats: bromocriptine treatment delays and PRL promotes the rapid onset of behavior. Endocrinol. 1990;126:837–848. doi: 10.1210/endo-126-2-837. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Ronsheim PM. Prolactin (PRL) regulation of maternal behavior in rats: bromocriptine treatment delays and PrL promotes the raid onset of behavior. Endocrinol. 1990;126:837–848. doi: 10.1210/endo-126-2-837. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Robertson MC, Shiu RPC, Sturgis JD, Henriquez BM, Mann PE. Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity, and behavioral potencies of rat PRL and rat placental lactogen I. Endocrinol. 1997;13:8, 756–763. doi: 10.1210/endo.138.2.4921. [DOI] [PubMed] [Google Scholar]
- Brooks PL, Vella ET, Wynne-Edwards KE. Dopamine agonist treatment before and after the birth reduces prolactin concentration but does not impair paternal responsiveness in Djungarian Hamsters, Phodopus campbelli. Horm Behav. 2005;47:358–366. doi: 10.1016/j.yhbeh.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brown RE, Murdoch T, Murphy PR, Moger WH. Hormonal responses of male gerbils to stimuli from their mate and pups. Horm Behav. 1995;29:474–491. doi: 10.1006/hbeh.1995.1275. [DOI] [PubMed] [Google Scholar]
- Buntin JD. Time course and response specificity of prolactin-induced hyperphagia in ring doves. Physiol & Behav. 1989;45:903–909. doi: 10.1016/0031-9384(89)90213-8. [DOI] [PubMed] [Google Scholar]
- Buntin JD, Tesch D. Effects of intracranial prolactin administration on maintenance of incubation readiness, ingestive behavior and gonadal condition in ringdoves. Horm Behav. 1985;19:188–203. doi: 10.1002/jez.1402320330. [DOI] [PubMed] [Google Scholar]
- Buntin JD, Figge GR. Prolactin and growth hormone stimulate food intake in ring doves. Pharmacol, Biochem & Behav. 1988;31:533–540. doi: 10.1003/gcen.1998.7239. [DOI] [PubMed] [Google Scholar]
- Buntin JD. Neural and hormonal control of parental behavior in birds. In: Slater PJB, Rosenblatt JS, Snowdon CT, Milinski M, editors. Parental Care: Evolution, Mechanisms, and Adaptive Significance. Academic Press; San Diego: 1996. pp. 161–213. [Google Scholar]
- Byatt JC, Staten NR, Salsgiver WJ, Kostelc JG, Collier RJ. Stimulation of food intake and weight gain in mature female rats by bovine prolactin and bovine growth hormone. Amer J Physiol Endocrinol Metab. 1993;264:E986–E992. doi: 10.1152/ajpendo.1993.264.6.E986. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH. The costs of breeding. In: Krebs JR, Clutton-Brock T, editors. The evolution of parental care. Princeton, New Jersey: Princeton University Press; 1991. pp. 31–47. [Google Scholar]
- Dixson AF, George L. Prolactin and parental behaviour in a male New World primate. Nature. 1982;299:551–553. doi: 10.1038/299551a0. [DOI] [PubMed] [Google Scholar]
- Doknic M, Pekic S, Zarkovic M, Bedic-Stojanoska M, Dieguez C, Casanueva F, Popovic V. Dopaminergic tone and obesity: an insight from prolactinomas treated with bromocriptine. Eur J Endocrinol. 2002;147:77–84. doi: 10.1530/eje.0.1470077. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steriner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm Behav. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Numan M, Bridges RS. Father of mothering: Jay S. Rosenblatt. Horm Behav. 2009;55:484–487. doi: 10.1016/j.yhbeh.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1524–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Goldizen AW. Facultative polyandry and the role of infant-carrying in wild saddleback tamarins (Saguinus fuscicollis) Behav Ecol Sociobiol. 1987;20:99–109. [Google Scholar]
- Greenman Y, Tordjman K, Stern N. Increased body weight associated with prolactin secreting pituitary adenomas: weight loss with normalization of prolactin levels. Clin Endocrinol. 1998;48:547–553. doi: 10.1046/j.1365-2265.1998.00403.x. [DOI] [PubMed] [Google Scholar]
- Guberneck DJ, Nelson RJ. Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus. Horm Behav. 1989;23:203–210. doi: 10.1016/0018-506x(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Hume JM, Wynne-Edwards KE. Paternal responsiveness in biparental dwarf hamsters (Phodopus campbelli) does not require estradiol. Horm Behav. 2006;49:538–544. doi: 10.1016/j.yhbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Khong HK, Kuah MK, Jaya-Ram A, Shu-Chien AC. Prolactin receptor mRNA is upregulated in discus fish (Symphysodon aequifasciata) skin during parental phase. Comp Biochem Physiol B Biochem Mol Biol. 2009;152:18–28. doi: 10.1016/j.cbpb.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Kindler PM, Bahr JM, Gross MR, Philipp DP. Hormonal regulation of parental care behavior in nesting male bluegills: do the effects of bromocriptine suggest a role for prolactin? Physiol Zool. 1991;64:310–322. [Google Scholar]
- Kirkwood JK, Underwood SJ. Energy requirements of captive cotton-top tamarin (Saguinus oedipus oedipus) Folia Primatol (Basel) 1984;42:180–187. doi: 10.1159/000156160. [DOI] [PubMed] [Google Scholar]
- Knudtzon J, Johansen PW, Haug E, Gautivik K. Effects of hypersecretion of growth hormone and prolactin on plasma levels of glucagon and insulin in GH3-cell-tumor-bearing rats, and the influence of bromocriptine treatment. Life Science. 1986;39:617–621. doi: 10.1016/0024-3205(86)90042-1. [DOI] [PubMed] [Google Scholar]
- Kramer PA. The costs of human locomotion: maternal investment in child transport. Amer J Phys Anthropol. 1998;107:71–85. doi: 10.1002/(SICI)1096-8644(199809)107:1<71::AID-AJPA6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Lieberman ME, Maurer RA, Claude P, Gorski J. Prolactin synthesis in primary cultures of pituitary cells; regulation by estradiol. Molecular Cellular Endocrinol. 1982;25:277. doi: 10.1016/0303-7207(82)90084-3. [DOI] [PubMed] [Google Scholar]
- Ma E, Lau J, Grattan DR, Lovejoy DA, Wynne-Edwards KE. Male and female prolactin receptor mRNA expression in the brain of a biparental and a uniparental hamster, Phodopus, before and after the birth of a litter. J Neuroendocrinol. 2005;17:81–90. doi: 10.1111/j.1365-2826.2005.01278.x. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Curran GH, Siegel HI. Evidence for the involvement of prolactin in the maternal behavior of the hamster. Physiol Behav. 1994;55:181–184. doi: 10.1016/0031-9384(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Moltz H, Lubin M, Leon M, Numan M. Hormonal induction of maternal behavior in the ovariectomized nulliparous rat. Physiol Behav. 1970;12:1373–1377. doi: 10.1016/0031-9384(70)90122-8. [DOI] [PubMed] [Google Scholar]
- Moore BJ, Gerardo-Gettens T, Horwitz BA, Stern JS. Hyperprolactinemia stimulates food intake in the female rat. Brain Res Bull. 1986;17:220–225. doi: 10.1016/0361-9230(86)90226-1. [DOI] [PubMed] [Google Scholar]
- Mota MT, Sousa MBC. Prolactin levels of fathers and helps related to alloparental care in common marmosets, Callithrix jacchus. Folia Primatol. 2000;71:22–26. doi: 10.1159/000021727. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Martin RD. Energy intake during reproduction in captive common marmosets (Callithrix jacchus) Physiol & Behav. 1999;65:849–854. doi: 10.1016/s0031-9384(98)00249-2. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. Interactions among paternal behavior, steroid hormones, and parental experience in the male marmosets (Callithrix kuhli) Horm and Behav. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Páll MK, Liljander M, Borg B. Prolactin diminishes courtship behaviour and stimulates fanning in nestling male three-spined sticklebacks, Gasterousteus aculeatus. Behaviour. 2004;141:1511–1519. [Google Scholar]
- Pi XJ, Grattan DR. Differential expression of the two forms of prolactin receptor mRNA within microdissected hypothalamic nuclei of the rat. Mol Brain Res. 1998;59:1–12. doi: 10.1016/s0169-328x(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Reburn CJ, Wynne-Edwards KE. Hormonal change in males of a naturally biparental and a uniparental mammal. Horm Behav. 1999;35:163–176. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Lawler TL, Wegner FH, Norcross JL, Newman JD. Prolactin levels are increased after infant retrieval and carrying in parentally inexperienced common marmosets. Physiol Behav. 2001a;72:713–720. doi: 10.1016/s0031-9384(01)00430-9. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Jenkins KT, Lawler T, Jr, Wegner FH, Newman JD. Bromocriptine administration lowers serum prolactin and disrupts parental responsiveness in common marmosets (Callithrix j. jacchus) Horm Behav. 2001b;39:106–112. doi: 10.1006/hbeh.2000.1639. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Siegel HI. Factors governing the onset and maintenance of maternal behavior among non-primate mammals: The role of hormonal and non-hormonal factors. In: Gubernick DJ, Klopfer PH, editors. Parental Care in Mammals. Plenum Press; New York: 1981. pp. 13–76. [Google Scholar]
- Ruiter AJHD, Bonga SEW, Slijkhuis B, Baggerman B. The effect of prolactin on fanning behavior in the male three-spined stickleback, Gasterosteus aculeatus L. Gen Comp Endocrinol. 1986;64:273–283. doi: 10.1016/0016-6480(86)90014-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi K, Tanaka M, Ohkubo T, Doh-Ura K, Fujikawa T, Sudo S, Nakashima K. Induction of brain prolactin receptor long-form mRNA expression and maternal behavior in pup-contacted male rats: promotion by prolactin administration and suppression by female contact. Neuroendocrinol. 1996;63:559–568. doi: 10.1159/000127085. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Schultz-Darken NJ, Abbott DH. Familial influences on ovulatory function in common marmosets (Callithrix jacchus) Am J Primatol. 1997;41:159–177. doi: 10.1002/(SICI)1098-2345(1997)41:3<159::AID-AJP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Sánchez S, Peláez R, Gil-Bürman C, Kaumanns W. Costs of infant-carrying in the cotton-top tamarin (Saguinus oedipus) Am J Primatol. 1999;48:99–111. doi: 10.1002/(SICI)1098-2345(1999)48:2<99::AID-AJP2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Schradin C. Comments to K.E. Wynne-Edwards and M.E. Timonin, 2007. Paternal care in rodents: Weakening support of hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm & Behav. 2007;52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]; Horm Behav. 52:557–559. doi: 10.1016/j.yhbeh.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Schradin C, Anzenberger G. Costs of infant carrying in common marmosets, Callithrix jacchus: an experimental analysis. Anim Behav. 2001;62:289–295. [Google Scholar]
- Schradin C, Reeder DM, Mendoza SP, Anzenberger G. Prolactin and paternal care: comparison of three species of monogamous New World monkeys. J Comp Psychol. 2003;117:166–175. doi: 10.1037/0735-7036.117.2.166. [DOI] [PubMed] [Google Scholar]
- Schradin C. Differences in prolactin levels between three alternative male reproductive tactics in striped mice (Rhabdomys pumilio) Proc R Soc B. 2008;275:1047–1052. doi: 10.1098/rspb.2008.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Massam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neruogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol Human Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- Tardif SD. Relative energetic cost of infant care in small-bodied neotropical primates and its relation to infant-care patterns. Amer J Primatol. 1994;34:133–143. doi: 10.1002/ajp.1350340205. [DOI] [PubMed] [Google Scholar]
- Torii R, Moro M, Abbott DH, Nigi H. Urine collection in the common marmoset, Callithrix jacchus, and its applicability to endocrinological studies. Primates. 1998;39:407–417. [Google Scholar]
- Wynne-Edwards KE, Reburn CJ. Behavioral endocrinology of mammalian fatherhood. TREE. 2000;15:464–468. doi: 10.1016/s0169-5347(00)01972-8. [DOI] [PubMed] [Google Scholar]
- Wynne-Edwards KE, Timonin ME. Paternal care in rodents: Weakening support of hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm & Behav. 2007;52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Zahed SR, Prudom SL, Snowdon CT, Ziegler TE. Male Parenting and Response to Infant Stimuli in the common marmoset (Callithrix jacchus) Amer J Primatol. 2007;69:1–15. doi: 10.1002/ajp.20460. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Gnadelman R, Denenberg VH. Prolactin: Is it an essential hormone for maternal behavior in the mammal? Horm Behav. 1971;2:353–354. [Google Scholar]
- Ziegler TE, Snowdon CT. Preparental hormone levels and parenting experience in male cotton-top tamarins, Saguinus oedipus. Horm Behav. 2000;38:159–167. doi: 10.1006/hbeh.2000.1617. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Washabaugh KF, Snowdon CT. Responsiveness of expectant male cotton-top tamarins, Saguinus oedipus, to mate's pregnancy. Horm Behav. 2004a;45:84–92. doi: 10.1016/j.yhbeh.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Jacoris S, Snowdon CT. Sexual communication between breeding male and female cotton-top tamarins (Saguinus oedipus) and its relationship to infant care. Amer J Primatol. 2004b;64:57–70. doi: 10.1002/ajp.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Wittwer DJ. Fecal steroid research in the field and laboratory: Improved methods for storage, transport, processing and analysis. Amer J Primatol. 2005;67:159–174. doi: 10.1002/ajp.20175. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Prudom SL, Schultz-Darken NJ, Kurian AV, Snowdon CT. Pregnancy weight gain: marmoset and tamarin dads show it too. Royal Soc Biol Let. 2006;2:181–183. doi: 10.1098/rsbl.2005.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]