Abstract
Seasonal changes in the length of the daily photoperiod induce significant changes in social behavior. Hamsters housed in winter-like short photoperiods (SP) can express significantly higher levels of aggression than hamsters housed in long photoperiods (LP) that mimic summer. The mechanisms responsible for increasing aggressiveness in SP-exposed female hamsters are not well understood but may involve seasonal changes in the endocrine system. In experiment 1, the effects of SP exposure on the circulating levels of three adrenal hormones were determined. Short photoperiod exposure was found to significantly depress the circulating levels of cortisol and the adrenal androgen dehydropiandrosterone (DHEA) but significantly increased the circulating levels of the sulfated form of DHEA, DHEAS. Experiment 2 examined the effects of gonadal hormones on several different measures of aggression including its intensity in females housed in both long and short photoperiod. Exposure to SP resulted in high levels of aggression regardless of the endocrine state of the animal or the measure used to quantify aggression. In contrast, administration of estradiol to hamsters housed in LP significantly reduced aggression. The data of the present study support the hypothesis that SP-housed females are more aggressive than LP-housed females because SP exposure renders females insensitive to the aggression-reducing effects of ovarian hormones.
Keywords: dehydroepiandrosterone, cortisol, estrogen, seasonal, photoperiod, agonistic behavior, social behavior
Introduction
Seasonal changes in the duration of the daily photoperiod induce profound seasonal changes in the physiology and behavior of many species. A variety of avian and rodent species display seasonal alterations in a wide range of variables including reproduction, metabolism, immunity, body weight, and pelage color (for reviews see Bartness et al., 1993 and Leska and Dusza, 2007). For example, Syrian hamsters (Mesocricetus auratus) exposed to long, summer-like photoperiods (LP) containing more than12.5 h of light/day maintain their ability to reproduce. However, exposure to short photoperiods (SP) containing less than12.5 h of light/day for 6-8 weeks inhibits their capacity to reproduce by inducing a state of reproductive quiescence like that seen during winter conditions (Seegal and Goldman, 1975; Turek et al., 1975). In males, SP induces a dramatic regression in the size of the testes and significant reductions in circulating testosterone (T) (Turek et al., 1975) while in females, SP results in significant reductions in ovarian mass and in the circulating concentrations of estradiol (Jorgenson and Schwartz, 1985; for review: Nelson et al., 1990).
It is not surprising that exposure to short photoperiods can also produce substantial alterations in the social behavior of seasonal breeding species. In association with the decline in reproductive capacity following SP-exposure, the expression of aggression is dramatically heightened in SP-exposed male rodents despite the significant reductions in the circulating levels of gonadal hormones. Studies in both male Syrian hamsters (Jasnow et al., 2002; Garrett and Campbell, 1980) and Siberian hamsters (Phodopus sungorus) (Jasnow et al., 2000) have reported substantial increases in aggression in response to SP exposure. The mechanisms responsible for the elevated levels of aggression that occur in short photoperiod are not known. However, it seems unlikely that gonadal androgens play a significant role in mediating the seasonal changes in aggression since SP exposure substantially reduces testes function and circulating levels of testosterone.
In female Syrian hamsters, the effect of photoperiod on aggressiveness is less clear than in males (for a review: Albers et al., 2002). In LP-housed females exhibiting spontaneous estrous cycles, the duration of offensive aggression was found to be similar to that of SP-housed anestrous females regardless of whether the opponents were SP-or LP-housed females (Fleming et al., 1988). However, SP-housed females displayed significantly shorter proportions of time exhibiting defensive behavior than LP-housed females. In another study, no differences were found between LP- and SP-housed ovariectomized (OVX) females in the percentage of animals that attacked male or female LP-housed opponents (Elliott & Nunez, 1992). However, when LP- and SP-housed (OVX) females were administered various combinations of estradiol and progesterone, the percentage of SP-housed females that attacked their opponents was significantly greater than the percentage of LP-housed females administered the same hormone treatments. In LP, OVX hamsters display high levels of aggressive behavior and there is a substantial body of evidence that aggression levels vary over the estrous cycle suggesting that ovarian hormones can significantly alter aggressiveness (Ciaccio et al., 1979; Floody & Pfaff, 1977; Fraile et al., 1987; Meisel et al., 1988; Payne & Swanson, 1972; Takahashi & Lisk, 1983; Vandenbergh, 1971). In addition, administration of estradiol to OVX females housed in LP has been found to either reduce aggression (Carter et al., 1973; Ciaccio et al., 1979; Lisk & Nachtigall, 1988) or have no effect on aggression (Fraile et al., 1987; Meisel et al., 1988; Payne & Swanson, 1972; Vandenbergh, 1971), while estradiol combined with progesterone or progesterone alone has been consistently found to reduce aggression (Ciaccio et al., 1979; Floody and Pfaff, 1977; Meisel et al., 1988; Meisel & Sterner, 1990; Elliott & Nunez, 1992). Taken together, these data suggest the possibility that SP exposure increases aggression by altering levels of ovarian hormones (e.g., reducing estradiol) or by reducing the ability of ovarian hormones to reduce aggression. If so, then gonadally intact SP-housed, OVX SP-housed and OVX LP-housed hamsters should display significantly higher levels of aggression than intact LP-housed hamsters displaying estrous cyclicity. However, in a study examining this question the results were equivocal (Fleming, et al, 1988). Although intact LP-housed females displayed significantly shorter durations of aggression when tested with LP-housed opponents than intact SP-housed females tested with SP-housed opponents, no differences in the duration of aggression were observed between intact and OVX LP-or SP-housed females. Thus, it remains unclear whether increased aggression in SP-housed females is simply due to the lack of circulating ovarian hormones in Syrian hamsters.
In female Siberian hamsters, SP exposure significantly increases aggression in gonadally intact hamsters, and surprisingly, also significantly increases circulating levels of estradiol (Scotti et al., 2007). However, a trend toward reduced aggression was observed when OVX hamsters housed in LP or SP were implanted with Silastic capsules containing estradiol for four weeks. Thus, in Siberian hamsters, estradiol appears to reduce aggression whether hamsters are housed in LP or SP, while in Syrian hamsters housed in SP administration of ovarian hormones appears to have no effect on aggression. One purpose of the present study was to re-examine whether SP exposure increases aggression in Syrian hamsters by altering levels of ovarian hormones (e.g., reducing estradiol) or by reducing the ability of ovarian hormones to reduce several different measures of aggression including its intensity.
Another endocrine gland that may be involved in regulating seasonal changes in aggression is the adrenal. Studies in birds and hamsters suggest adrenal hormones, specifically hormones secreted by the adrenal cortex, may play a critical role in regulating seasonal changes in aggression. In male birds, there is evidence that the aromatization of the adrenal androgen dehydroepiandrosterone (DHEA) into estrogen is involved in the seasonal changes in aggression (Soma and Wingfield, 2001; Soma et al., 2000). In male Syrian and Siberian hamsters, melatonin secretion during short photoperiod exposure has been shown to increase aggression (Demas et al., 2004; Jasnow et al., 2002). There is evidence that melatonin-induced aggression is mediated by hormones secreted from the adrenal cortex since complete adrenalectomy, but not removal of the adrenal medulla, significantly reduced melatonin-induced aggression (Demas et al., 2004). There is also evidence that DHEAS, the sulfated form of DHEA, is associated with the facilitation of aggression in male mice (Nicolas et al., 2001). Taken together, these data suggest that hormones originating from the adrenal cortex may be involved in regulating the seasonal variations in aggression.
Little is known about the factors that regulate the circulating levels of adrenocortical hormones such as DHEA and DHEAS in mammals. In male Syrian hamsters, exposure to SP significantly increases the circulating concentrations of DHEA when compared to hamsters housed in LP (Caldwell et al., 2008). In the present study, we evaluated whether there are seasonal changes in the circulating levels of DHEA, DHEA-S as well as cortisol in female Syrian hamsters and the circulating levels of these hormones following aggressive interactions.
Materials and Methods
Animals and housing conditions
Adult female Syrian hamsters (Charles River Laboratories: Wilmington, MA) were maintained in reverse light cycles to mimic “summer-like” long photoperiod (LP) (14:10, L:D) or “winter-like” short photoperiod (SP) (8:16, L:D). Animals weighed 120-140 g at the start of the experiment and were housed individually in polycarbonate cages (40 × 20 × 20 cm) with corncob and cotton bedding materials and wire mesh tops. Food (Purina Rodent Chow) and water were provided ad libitum. All experimental protocols were approved by the Georgia State University Institutional Animal Care and Use Committee.
Experiment 1: Photoperiod-dependent changes in adrenal hormones
Female hamsters were individually housed in LP (n=35) or SP (n=40) conditions for 10 weeks. Throughout the 10-week period, estrous cycles were monitored by the examination of post-ovulatory secretions. In SP, females began to show acyclicity at week 6 with all photoperiod responders acyclic by week 8. We continued to monitor SP-housed females through week 10 so that estrous cycles had ceased for at least two weeks prior to sacrifice. Females were euthanized in groups at hourly intervals from three hours prior to the onset of the dark phase until four hours into the dark phase. At the time of sacrifice, females were injected with a lethal dose of sodium pentobarbital and blood was collected from the inferior vena cava after the hamsters were deeply anesthetized (i.e., less than two minutes following injection of anesthesia). In LP-housed females the stage of the estrous cycle on the day of sacrifice was recorded.
Experiment 2: Effects of treatment and photoperiod on agonistic behavior
Fifty adult female hamsters were individually housed in LP or SP as described above. Estrous cycles were monitored throughout the experiment by examination of postovulatory discharge. At week six, 50 female intruders were individually housed in LP conditions. Two weeks after intruders had acclimated to housing conditions, and after eight weeks of photoperiod exposure experimental animals were further subdivided into the following groups. One-third of experimental females in both photoperiod conditions were bilaterally ovariectomized (OVX) and 62 subcutaneously implanted in the scapular region with two Silastic capsules filled with estradiol benzoate (OVX + EB) (Sigma, St. Louis, MO). Another one-third were OVX and implanted with two empty Silastic capsules (OVX). The remaining experimental animals received an incision in the muscle tissue that was subsequently sutured, but OVX and implantation of capsules were not performed (sham group). To avoid estrous cycles but to maintain levels of estradiol within the physiological range intruder females (n=50) were OVX and implanted with one capsule of EB. Four weeks following surgery, animals were tested using the resident-intruder paradigm. Immediately following the behavioral test, animals were sacrificed and blood drawn from the inferior vena cava.
Ovariectomies and hormonal manipulations
All females were deeply anesthetized with sodium pentobarbital (50 mg/kg). Prior to surgery, animals were given a subcutaneous injection of the analgesic, ketaprophen (5 mg/kg). Previous studies in our laboratory have shown that implantation of one 10 mm EB-filled Silastic capsule (0.062 in i.d. × 0.125 in o.d.) produces an average of 179.06±17.07 pg/ml of circulating estradiol (Guzman, Karom, and Albers, unpublished observations). Each 10 mm Silastic capsule was filled with 5 mm of EB, and sealed with Factor II 6382 RTV Silicone and Elastomer as previously described (Faruzzi et al., 2005). Using this approach we targeted this amount of circulating hormone in our intruder females to approximate the amount of estradiol in a diestrous female. The experimental females were implanted with 2 EB-filled capsules in order to double this concentration. Following surgery, skin incisions were repaired using tissue adhesive and animals received 1 ml of physiological saline to prevent surgery-induced dehydration.
Behavioral Testing
After five weeks of recovery, experimental females were tested for aggression using the resident-intruder paradigm. The intruders were approximately the same weight as the experimental hamsters (intruders: 145.9±1.9g; experimental: 142.6±2.6g). The cages of experimental females were not changed for two weeks prior to testing. Five minute tests occurred between two and three hours after the onset of the dark phase under red light. Sham-OVX hamsters were tested on diestrus 1. All tests were videotaped, and the behavior was scored by a scorer blind to the treatment of the experimental animal. The latency to attack and the number of attacks were quantified for each test period. In addition, the total duration of social, non-social, submissive and defensive, or aggressive behavior was recorded for each test. Because the intensity of aggression appeared to differ in some of the experimental groups we further categorized aggressive behavior as either low intensity or high intensity (Table 1). Several behavioral tests (n=5) were terminated prior to five minutes because of the intensity of the aggression was likely to lead to injury. As a result, we also analyzed the duration of high and low intensity aggression as a percentage of the duration of the test period, i.e., the duration of time from the beginning of the test until the test was terminated because of the intensity of aggression or until five minutes of testing was completed. Defensive behavior was quantified using the criteria of Fleming et al., 1988. Experimental animals were euthanized immediately following the behavioral test.
Table 1.
Criteria used to score aggressive behavior & intensity
| Behavior | Definition |
|---|---|
| Latency to initial attack | Attack defined as biting/pushing conspecific onto side to initiate roll fight |
| Number of attacks | Attack defined as biting/pushing conspecific onto side |
| Duration of high intensity aggression | Attack biting/roll fighting |
| Duration of low intensity aggression | Offensive “push” behavior, chase behavior, pinning the subordinate |
| Average duration per attack | Duration of high intensity aggression divided by the number of attacks |
| Duration of social behavior | Scored when animals are in contact with each other; includes nose-to-nose investigation or non-aggressive “following” behavior/olfactory investigation, grooming flanks |
| Duration of non-social behavior | Scored when animals are investigating the cage, sitting, “escape” behavior (trying to climb out of the cage, but not out of fear or submission), grooming of face and head |
| Duration of defensive behavior | Tail-up posture, pinned on the ground, defensive posturing (hands up, protecting self), flee |
Radioimmunoassay
Serum was separated following centrifugation and stored at -20 °C until the time of processing. Assay kits were purchased from Beckman Coulter Diagnostic Systems Laboratories (Webster, TX) for E2, DHEA, and DHEAS. Following the determination of E2, DHEA and DHEAS in experiment 1, we measured cortisol with the remaining serum samples (n=31). In Experiment 2, E2, DHEA, and DHEAS, and cortisol concentrations were determined in samples from all animals.
Serum E2 concentrations were assessed using an RIA kit (DSL 43100). The linearity of dilution was used to validate the kit and yielded 94% recovery. The interassay coefficient of variation was 6%. The intraassay coefficient of variation was 2%. The sensitivity of the assay was 3.19-1500 pg/ml. The cross-reactivity with other related compounds was less than 1%.
Serum DHEA concentrations were assessed using an RIA kit (DSL 9000). The linearity of dilution was 87% recovery. The interassay coefficient of variation was 5% and intraassay coefficient of variation, 4%. The sensitivity of the assay was 0.55 - 29.93 nmol/L. . The cross-reactivity was less than 1% or non-detectable for other related compounds.
An RIA kit was also used to quantify serum DHEAS concentrations (DSL 3500). The linearity of dilution was 94%. The inter- and intra-assay coefficients of variation were 4% and 2%, respectively. The sensitivity of this assay was 10.84 - 1667.19 nmol/L. The cross-reactivity with DHEA was 41%. Due to the fact that DHEAS concentrations in hamster serum are about 100-fold higher than DHEA, this cross-reactivity did not significantly reduce the accuracy of the measurements of DHEAS immunoreactivity. For other related compounds, the cross-reactivity was less than 7.3% or non-detectable.
Finally, to determine circulating cortisol concentrations, we used an RIA kit (DSL 2100). The linearity of dilution was 93%. Interassay coefficient of variation was 2% and the intraassay coefficient of variation was 3%. The sensitivity was 2.17 - 2736.83 nmol/L. The cross-reactivity with prednisolone was 33.3% and less than 10% with any other related compounds.
Statistical analyses
All statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS) version 11.0. Females that did not respond to SP conditions (n=2) were dropped from the study. Non-responders were defined as those females that continued to display estrous cycles despite housing in SP conditions. Differences in E2, DHEA, and DHEAS across the estrous cycle were examined using a one-way ANOVA. Tukey’s HSD test was used for post hoc analysis when a significant difference was detected as a result of estrous cycle stage. Mean E2 concentrations for each photoperiod were further analyzed for differences using an independent-samples t test. Additionally, we analyzed whether differences occurred in circulating cortisol, DHEA, and DHEAS using time and photoperiod as independent variables. These data were analyzed using a two-factor, independent-samples analysis of variance (ANOVA). In Experiment 2, a two-factor ANOVA was used to determine whether differences in aggressive behavior existed as a result of treatment or photoperiod, or an interaction of the two. Where significance was indicated, the data were analyzed post hoc with Tukey’s HSD test. P ≤ 0.05 was considered significant for all analyses. Defensive behavior was analyzed with a Chi-Square analysis because of the lack of homogeneity of variance according to the Levene’s test.
Results
Experiment 1: Photoperiod-dependent changes in adrenal hormones
Females housed in SP had circulating levels of estradiol of 131.0±3.5 pg/ml while hamsters housed in LP had average circulating levels of estradiol of 369.6±33.6 pg/ml over the estrous cycle. These differences were statistically significant (t(71)= -7.36, p≤0.001). Significant differences were also found in estradiol when LP females were compared across the stages of the estrous cycle (F3, 31=5.77, p≤0.01) (Table 2). The highest estradiol concentrations were found in proestrus females compared to females on either day of diestrus (p≤0.05). On diestrus 1 females also had significantly lower estradiol concentrations compared to females on estrus (p≤0.05). There was a trend for differences in DHEA concentrations across the stages of the estrous cycle, however this trend did not reach statistical significance (p=0.08). In contrast, significant differences in DHEAS concentrations were observed across the stages of the estrous cycle (F3, 31=3.48, p≤0.05). Specifically, DHEAS concentrations were higher on the day of estrus compared to diestrus 1 (p≤0.05). Trends were detected between concentrations of DHEAS on estrus and the remaining days of the estrous cycle, diestrus 2 and proestrus (p=0.13 and p=0.09, respectively).
Table 2.
Concentrations of estradiol, DHEA, and DHEAS during the estrous cycle
| Estrous Cycle Stage | Estradiol (pg/ml) | DHEA (nmol/L) | DHEAS (nmol/L) |
|---|---|---|---|
| Diestrus 1 | 241.82 ± 13.06a | 1.22 ± 0.04 | 72.41 ± 9.01b |
| Diestrus 2 | 261.68 ± 26.79a,b | 1.23 ± 0.05 | 81.61 ± 10.20a,b |
| Proestrus | 499.62 ± 72.09c | 1.29 ± 0.04 | 81.65 ± 7.31a,b |
| Estrus | 461.39 ± 78.9b,c | 1.54 ± 0.18 | 120.06 ± 17.53a |
a, b, or c indicate statistical differences of p≤0.05
There were main effects of photoperiod on both DHEA (F1, 57=17.80, p≤0.001) (Fig 1A) and DHEAS (F1, 57=12.30, p≤0.001) (Fig 1B). DHEA concentrations were significantly higher in LP-housed females, while circulating DHEAS levels were higher in the SP-housed group. No effect of time was found, nor was any interaction of time and photoperiod indicated (p>0.05). There was no main effect of photoperiod on cortisol concentrations (p>0.05), but there was a main effect of time (F3, 23=3.43, p≤0.05) (Fig 1C). There was also an interaction between photoperiod and time (F3, 23=3.03, p≤0.05). LP-housed females had higher concentrations of cortisol compared to their SP-housed counterparts at the time points representing 2 and 3 hours after the onset of the dark phase. SP-housed females did not show the same fluctuation in cortisol as LP-housed females.
Figure 1. Effects of photoperiod on circulating DHEA, DHEAS, and cortisol concentrations across time.
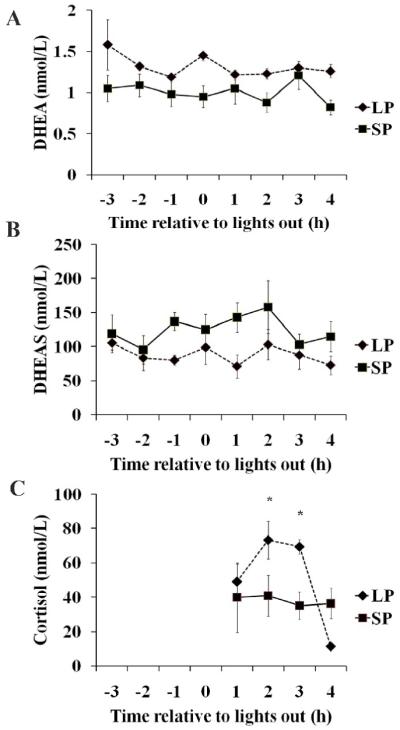
Time is expressed as hours (h) prior to and following the onset of the dark phase. A.) A main effect of photoperiod was found on DHEA concentrations (p≤0.001), but neither a main effect of time (p>0.05) nor an interaction of time and photoperiod (p>0.05) were detected. B.) There was a main effect of photoperiod on DHEAS concentrations (p≤0.001), but no main effect of time (p>0.05) nor an interaction of photoperiod and time (p>0.05). C.) Cortisol concentrations were not significantly different as a result of photoperiod (p>0.05), but a significant effect of time was detected (p≤0.05) as well as an interaction of photoperiod and time (p≤0.05). Post-hoc comparisons show significantly higher cortisol concentrations in LP females compared to SP females sacrificed two and three hours after the onset of the dark phase. * indicates significance
Experiment 2: Effects of treatment and photoperiod on agonistic behavior
The circulating concentrations of estradiol in each group can be seen in Table 3. As expected, there was a main effect of treatment on estradiol concentrations (F2, 40=87.75, p≤0.001), but there was no main effect of photoperiod on estradiol concentrations (F1, 40=0.44, p>0.05). A significant interaction was detected between photoperiod and treatment (F2, 40=5.85, p≤0.01). Females implanted with EB-filled or empty capsules had no differences in estradiol as a function of photoperiod (p>0.05), but LP-housed sham females on diestrous 1 had significantly higher concentrations of estradiol compared to SP-housed sham females (p≤0.05). Intruder females that had been OVX and implanted with one EB-filled capsule had mean estradiol concentrations of 199.52 ± 9.36 (mean ± s.e.m.), which is similar to the concentrations of estradiol seen in the sham, diestrous females (p>0.05)
Table 3.
Estradiol concentrations of LP and SP treatment groups (Mean ± S.E.M.)
| Photoperiod | Treatment | Estradiol (pg/ml) |
|---|---|---|
| Long Photoperiod | Sham | 219.12 ± 11.75a |
| EB | 319.93 ± 20.40b | |
| Empty | 139.43 ± 5.13c | |
| Short Photoperiod | Sham | 149.19 ± 9.90c |
| EB | 358.84 ± 25.07b | |
| Empty | 144.45 ± 12.17c |
a, b, or c indicate statistical differences of p≤0.05
An analysis of non-aggressive social behaviors (Fig 2A), i.e. contact time between resident and intruder, revealed a main effect of treatment (F2, 40=5.22, p≤0.01) but no main effect of photoperiod (F1, 40=0.42, p>0.05). There was also no interaction between photoperiod and treatment (F2, 40=1.72, p>0.05). Surprisingly, SP-housed sham females had greater durations of non-aggressive social behavior compared to SP-housed OVX females (p≤0.05). The duration of defensive behavior was also quantified and no significant differences (χ2(2)=3.11,p>0.05) were observed across groups. In the LP group, the durations of defensive behavior for the sham, OVX and OVX + EB groups were 8.7±7.1 sec (mean ± SEM), 48.8±31.1 and 63.5±33.7, respectively. In the SP group, the durations of defensive behavior for the sham, OVX and OVX + EB groups were 14.4±10.7, 38.8±21.8 and 0±0, respectively.
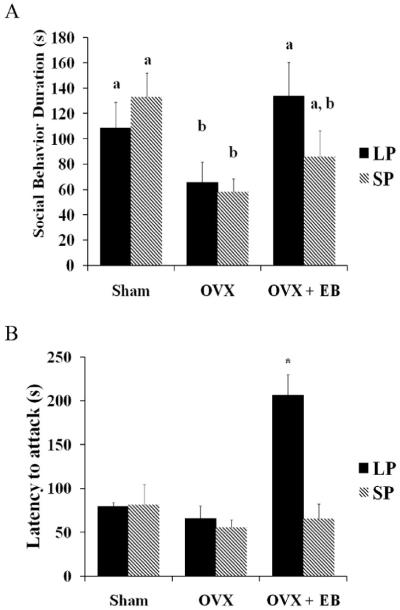
Figure 2. Effects of photoperiod and treatment on the duration of social behavior and the latency to attack.
A.) A main effect of treatment revealed that OVX females exhibited significantly lower durations of social behavior than sham females (p≤0.01), but there was no main effect of photoperiod (p>0.05). B.) In LP, females that were OVX and given EB treatment displayed significantly longer latencies to attack compared to all other groups reflecting differences due to photoperiod, time, and an interaction of the two (p≤0.001 for each). a, b, or * indicate significance
There was a significant main effect of treatment (F2, 39=11.04, p≤0.001) and photoperiod (F1, 39=12.53, p≤0.001) on latency to attack, as well as a significant interaction between the two (F2,39=10.93, p≤0.001) (Fig 2B). LP-housed, EB-treated OVX females had a significantly higher latency to attack than any other group (p≤0.001 in all cases). There was a main effect of photoperiod (F1, 40=6.26, p≤0.05) and treatment (F2, 40=3.44, p≤0.05) on the total duration of aggression (Fig 3A). An interaction between photoperiod and treatment approached, but did not reach, statistical significance (F2, 40=2.95, p=0.06). OVX hamsters administered EB and housed in LP had significantly shorter durations of aggression than OVX hamsters housed in LP but not given EB (p=0.05). LP-housed sham females did not have significantly longer durations of aggression than EB-treated OVX females (p>0.05). Overall, SP-housed females had longer durations of aggressive behavior. This effect is particularly evident in comparing LP-housed, EB-treated OVX females to the SP-housed OVX females given empty capsules, and the SP-housed, EB-treated OVX females (p≤0.05 for both comparisons). Interestingly, post hoc analysis did not detect a statistical difference in the total duration of aggression between LP-housed, EB-treated OVX females and the SP sham group (p>0.05).
Figure 3. Effects of photoperiod and treatment on the duration of aggression and the duration of high and low intensity aggression.
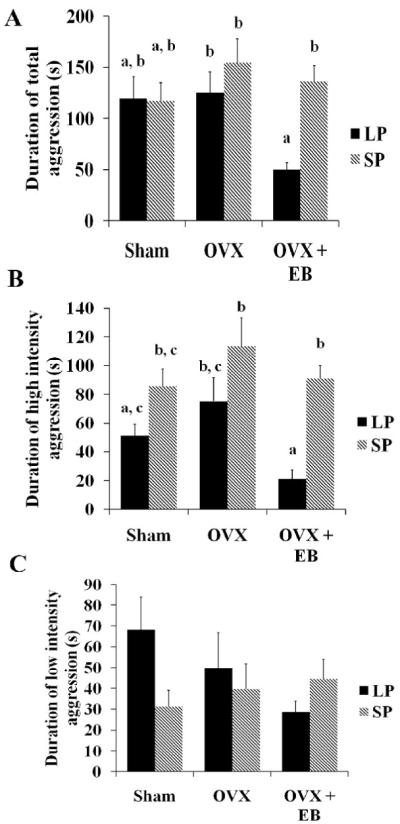
A.) Sham and OVX females did not display photoperiod-dependent differences in total duration of aggression while EB significantly reduced this duration only in LP-housed females (p≤0.05). B.) Main effects of treatment and photoperiod were detected (p≤0.05 for both) on the duration of high intensity aggression, but the duration of high intensity aggression was only attenuated by EB treatment in LP-housed females. C.) No significant differences in the duration of low intensity aggression were detected due to photoperiod, treatment, or an interaction between photoperiod and treatment (p>0.05). a, b, or c indicate significance
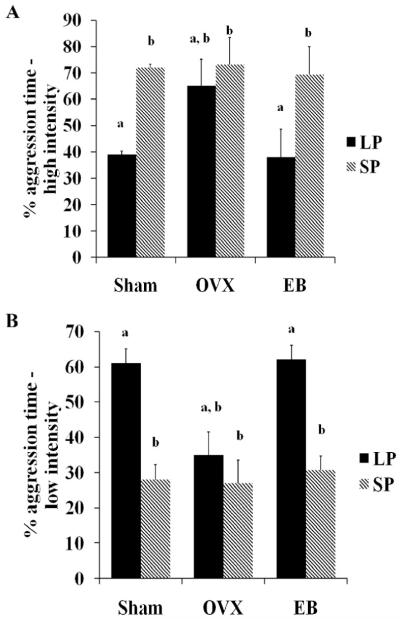
Because the intensity of aggression appeared to be particularly high in SP-housed females we also examined the duration of high intensity and low intensity aggression (see Table 1). SP-housed females had greater durations of high intensity aggression (Fig 3B) compared to LP-housed females, as evidenced by a significant main effect of photoperiod (F1, 40=19.99, p≤0.001). In addition, a main effect of treatment was detected (F2, 40=4.69, p≤0.05), but there was no interaction (F2, 40=1.13, p>0.05). In LP-housed OVX females, administration of EB resulted in significantly shorter duration of high intensity aggression compared to OVX LP-housed females implanted with empty capsules (p≤0.05). However, the duration of high intensity aggression in LP-housed sham females was not significantly different from either of the LP-housed OVX groups (p>0.05). The duration of high intensity aggression in LP-housed EB-treated OVX females was also significantly shorter than in all SP-housed groups (p≤0.05 for all cases). Conversely, there was no main effect of photoperiod (F1, 40=1.11, p>0.05) or treatment (F2, 40=0.60, p>0.05) on the duration of low intensity aggression (Fig 3C). The interaction of photoperiod and treatment was not statistically significant, but a trend was detected (F2, 40=2.38, p=0.11). Five of the behavioral tests had to be terminated prior to their completion because it seemed likely that the hamsters would be injured. As a result, we also calculated high intensity aggression and low intensity aggression as a percentage of the total duration of aggression (Fig 4A & B). Using this approach, there was a significant main effect of photoperiod on the percentage of high intensity aggression (F1, 39=17.86, p≤0.001). No main effect of treatment (F2,39=2.4, p=0.10) or interaction of photoperiod and treatment (F2,39=2.26, p=0.10) were detected. However, SP-housed EB-treated OVX females spent a significantly greater portion of their duration of aggression in a high intensity encounter compared to LP-housed, EB-treated OVX females (p≤0.05). Furthermore, LP-housed EB-treated OVX animals had significantly less of their total aggression time in a high intensity interaction compared to all SP-housed groups (p≤0.05 for all cases). SP-housed sham females also had greater percentages of high intensity aggression compared to LP-housed sham females (p≤0.05). There was a main effect of photoperiod on the percentage of low intensity aggression (F1, 40=14.27, p≤0.001), but no main effect of treatment (F2, 40=2.11, p>0.05) or interaction (F2, 40=1.60, p>0.05). LP-housed EB-treated OVX females had significantly higher percentages of low intensity aggression compared to SP-housed OVX (p≤0.05) and sham females (p≤0.01).
Figure 4. Effects of photoperiod and treatment on the percentages of high and low intensity aggression.
A.) There was a significant main effect of photoperiod on the percentage of total aggression time spent in high intensity aggression (p≤0.001). EB treatment did not attenuate this effect in either photoperiod. B.) There was a statistically significant main effect of photoperiod on the percentage of total aggression time spent in low intensity aggression (p≤0.001). LP-housed females spent a greater portion of time spent in an aggressive interaction at a low intensity, however no differences were detected due to treatment or an interaction of photoperiod and treatment (p>0.05). a, b indicate significance
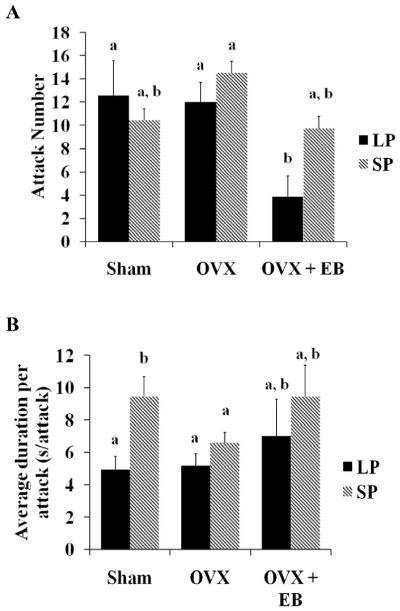
There was no main effect of photoperiod on attack number (F1, 40=1.41, p>0.05) nor was a significant interaction detected (F2, 40=1.72, p>0.05) (Fig 5A). There was, however, a main effect of treatment (F2, 40=5.03, p≤0.01). OVX females housed in LP and treated with EB attacked significantly less than OVX females in either LP or SP given empty capsules (p≤0.05) and sham (p≤0.05) hamsters housed in LP. Surprisingly, of the SP-housed females, only SP-housed OVX females given empty capsules had significantly more attacks compared to LP-housed EB-treated OVX females. We calculated duration of high intensity aggression/attack number and found only a main effect of photoperiod (F1, 38=5.43, p≤0.05). Overall, SP-housed females had greater durations per attack than LP-housed animals (Fig 5B). Specifically, SP-housed sham females had significantly higher attack durations compared to LP-housed sham and LP-housed OVX females (p≤0.01 for both).
Figure 5. Effects of photoperiod and treatment on attack number and duration per attack.
A.) A main effect of treatment, but not of photoperiod, was found on attack number (p≤0.01). EB treatment in LP-housed females significantly reduced the number of attacks compared to the other LP-housed female groups. B.) Although there was not a significant photoperiod-dependent difference in the attack number of the sham groups, SP was associated with significantly longer durations per attack (p≤0.05). SP-housed sham females exhibited significantly longer durations per attack compared to their LP-housed counterparts (p≤0.01). These durations were also significantly higher in SP-housed sham females compared to OVX females in both photoperiods. a, b indicate significance
Following the aggressive encounter, there was no main effect of photoperiod (F1, 40=0.152, p>0.05), treatment (F2, 40=0.028, p>0.05), nor an interaction between photoperiod and treatment (F2, 40=0.362, p>0.05) in the circulating concentrations of DHEA (Fig 6A). There was however, a main effect of photoperiod (F1, 40=7.11, p≤0.01) and treatment on DHEAS concentrations (F2, 40=6.00, p≤0.01). The interaction between photoperiod and treatment approached, but did not reach, statistical significance (F2, 40=2.75, p=0.08). SP-housed sham females had significantly higher DHEAS concentrations following an aggressive encounter than all other groups (p≤0.05 for all comparisons) (Fig 6B). There were also main effects of photoperiod (F1, 39=4.79, p≤0.05) and treatment (F2, 39=3.587, p≤0.05) on cortisol concentrations, but not an interaction between photoperiod and treatment (F2, 39=0.50, p>0.05). LP-housed EB-treated OVX animals had significantly higher circulating cortisol concentrations compared to all groups (p≤0.05) except LP-housed OVX females given empty capsules (p>0.05) (Fig 6C).
Figure 6. Adrenal hormone concentrations following an aggressive encounter.
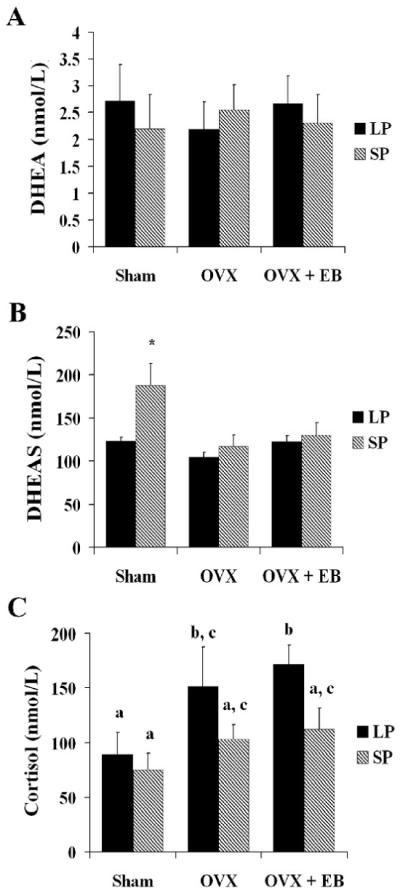
A.) There were no significant differences in DHEA concentrations between treatment groups or photoperiod groups after an aggressive encounter. B.) Main effects of photoperiod and treatment were detected on circulating DHEAS concentrations (p≤0.01 for both), but an interaction of the two was not detected (p>0.05). The DHEAS concentrations of SP-housed sham females were significantly higher compared to all other groups. C.) Sham females showed significant differences in cortisol concentrations as a function of photoperiod and treatment (p≤0.05). In the case of LP-housed sham females, these concentrations were significantly lower than the other LP-housed groups. a, b, c or * indicate significance
Discussion
In the present study, female Syrian hamsters singly housed in SP conditions for 10 weeks were found to have significantly lower levels of circulating DHEA and significantly higher levels of DHEAS than females housed in LP. In contrast, previous studies in male Syrian hamsters found that SP exposure significantly increased DHEA levels compared to LP-exposed animals (Caldwell, et al., 2008). In females, it seems unlikely that the SP-induced reduction in DHEA concentrations is the result of the concomitant reduction in gonadal hormone concentrations since gonadectomy has little or no effect on circulating levels of DHEA (Pieper and Lobocki, 2000). The present study found no statistically significant changes in circulating levels of DHEA over the estrous cycle. However, DHEAS levels were significantly modulated over the estrous cycle with the greatest concentrations occurring on estrus. Previous studies in Syrian hamsters have found DHEA and DHEAS concentrations to peak in the hours preceding the onset of the dark phase (Pieper and Lobocki, 2000), however in the present study no significant differences were observed in either DHEA or DHEAS levels during the three hours prior to lights-off. In summary, DHEA and DHEAS concentrations appear to be regulated by photoperiodic mechanisms in Syrian hamsters; however the existing data suggest that the changes in DHEA and DHEAS levels are not the result of photoperiodic induced changes in circulating gonadal hormones.
Previous studies in male Syrian hamsters have found that SP exposure significantly reduces circulating levels of cortisol and that SP exposure additionally dampens the 24 hr rhythm in cortisol (Ottenweller et al, 1987; Ronchi et al., 1998). The present study provides the first data on the effects of SP exposure on the circulating levels of cortisol in female Syrian hamsters. Although, there was no main effect of photoperiod on cortisol concentrations there was a significant interaction between photoperiod and time suggesting that SP exposure reduces cortisol concentrations and dampens the rhythm of cortisol. Differences in basal concentrations of circulating adrenal hormones may indicate a change in HPA activity due to SP exposure.
In the present study, the effects of photoperiod and estradiol on aggressiveness was evaluated by examining multiple measures of aggression including the latency to attack, the number of attacks, the duration of aggression as well as the intensity of aggression. The intensity of aggression in SP-housed females could be quite high. In fact, several of the behavioral tests of SP-housed females had to be terminated due to their intensity and the concern for injury. As a result, comparison of the total duration of aggression did not always represent a true reflection of the observed intensity of aggression. To address this issue, we quantified the duration of high intensity and low intensity aggression (see Table 1) and reported it as an absolute value or as a percentage of the total duration of aggression. This approach revealed that SP-housed females displayed significantly more high intensity aggression such as biting and roll-fighting than hamsters housed in LP. One measure of aggression where no differences were found between LP- and SP-housed hamsters was the number of attacks. However, the absence of differences in the number of attacks between photoperiod groups may have been due to the early termination of some of the behavioral tests in SP-housed hamsters. As a result, we also calculated the intensity of these encounters by determining the duration of high intensity aggression per attack. The duration of roll fighting/attack was significantly longer in SP-housed females compared to those housed in LP. In summary, exposure to SP was found to result in the display of high levels of aggression regardless of the endocrine state of the animal or the measure used to quantify aggression.
In the present study, estradiol administration to OVX females housed in LP significantly increased the latency to attack, and reduced the duration of aggression, the duration of high intensity aggression, the percentage of high intensity aggression and the mean duration of high intensity aggression/attack compared to OVX females housed in SP and given estradiol. No significant differences were observed in any measure of aggression between the LP- or SP-housed OVX hamsters given empty Silastic capsules and the SP-housed OVX hamsters given estradiol for four weeks. In contrast, estradiol administered for four weeks was associated with a trend towards reduced aggression in OVX Siberian hamsters whether they were housed in LP or SP (Scotti et al., 2007). Interestingly, estradiol may have more potent effects on high intensity aggression than on low intensity aggression. Estradiol significantly reduced the duration of high intensity aggression when measured as an absolute value or when measured as a percentage of the total duration of aggression but had no significant effects on low intensity aggression. There was also the suggestion that lower levels of estradiol might also reduce at least some measures of aggression in LP. The amount of high intensity aggression measured as a percentage of the total duration of aggression and the duration of high intensity aggression/attack number was significantly reduced in intact sham OVX females who were tested on diestrus compared to females housed in SP. Taken together, these data suggest that SP exposure reduces or eliminates the ability of estradiol to reduce aggression.
The present data are also consistent with previous studies that demonstrated that SP exposure reduces the ability of progesterone to decrease aggression (Elliott and Nunez, 1992). In the present study, high levels of aggression were consistently observed in the SP-housed sham females even though progesterone levels have been reported to be substantially increased as a result of SP exposure (Bridges & Goldman, 1975; Jorgenson & Schwartz, 1985). Thus, aggression appears to occur consistently in SP even when Syrian hamsters are exposed to estradiol or progesterone, suggesting that SP turns off the ability of ovarian hormones to reduce aggression. Therefore, the data of the present study support the hypothesis that SP-housed females are more aggressive than LP-housed females because SP renders females insensitive to the aggression-reducing effects of ovarian hormones.
In previous studies the effects of estradiol on aggression in LP-housed females have been less consistent than observed in the present study. It seems likely that differences in methodology across these studies may have contributed to the inconsistency in the results. These differences include the form and duration of estradiol administration, how aggression was measured, the gender, size and hormonal state of the opponent and the testing apparatus (e.g. home cage versus neutral arena). Since one of the goals of the present study was to determine whether estradiol could reduce aggression in SP-housed females, we administered estradiol continuously in a Silastic capsule for an extended interval, i.e. four weeks. Many of the other studies examined the effects of estradiol on aggression in LP when it was administered for shorter durations. In addition, circulating levels of estradiol were measured in the present study. The amount of estradiol administered to OVX females mimicked the levels of estradiol seen during the peak of the estrous cycle. The sham OVX group housed in LP were tested on diestrus and found to have substantially less estradiol than the OVX animals administered estradiol and substantially more estradiol than the OVX group. Another substantial difference between the present study and previous work is the way that aggression was measured. Some of the previous studies used discrete measures of aggression (e.g., latency to attack), while others used composite measures (e.g. the duration of aggressive behaviors). The present study employed both discrete and composite measures and also measured the intensity of aggression. In general, these different measures of aggression tended to be similar across treatment groups. However, unlike the other measures of aggression, few between-group differences were observed in either measure of low intensity aggression.
A previous study by Fleming et al. (1988) found that one of the most prominent differences in agonistic behavior between females housed in LP versus SP was in the ratio of offensive behaviors to defensive behaviors. SP-housed females had significantly higher ratios of offensive behaviors to defensive behaviors. However, in the present study the amount of defensive behavior was quite variable and no significant differences were observed between groups. The differences in these results may be due to differences in experimental design. For example, the use of the resident-intruder testing paradigm in the present study might have reduced the duration of defensive behaviors compared to Fleming et al. (1988) that used a neutral testing area. One of the goals of the present study was to analyze the components of aggression utilizing a method to differentiate high and low intensity behaviors. Traditionally, a total duration of aggression is used to determine differences between groups. The differences in total testing times, however, prompted us to analyze percentages of observed behaviors as well as the average duration per attack. The calculation of these measures and the statistical differences between them more accurately reflected the interactions observed during the aggressive encounters.
Following the behavioral tests which occurred 2-3 hours after the onset of the dark phase, blood was sampled for measurements of DHEA, DHEAS and cortisol. DHEA concentrations did not differ significantly in any treatment group or as a result of photoperiod. However, DHEAS was significantly higher in SP-housed sham females compared to all other treatment groups. The absence of a significant effect of photoperiod on DHEA and DHEAS concentrations across groups was not surprising since the data from experiment 1 suggest that there are not dramatic differences between photoperiods at this time of day. There appears to be little relationship between the concentrations of DHEA and DHEAS and the various measures of aggression. As a result, these data provide little support for the possibility that aggressive behavior alters the levels of these hormones or that the hormones are involved in the activation or inhibition of aggression.
In experiment 1, cortisol concentrations were found to be significantly lower in SP-housed females compared to LP-housed females 2-3 hours after lights-off. A similar trend was observed in the cortisol concentrations following agonistic encounters. Again there was no clear relationship between the circulating levels of cortisol and the various measures of aggression, thus suggesting that aggressive behavior does not alter the levels of these hormones or that cortisol is responsible for the activation or inhibition of aggression.
In summary, the inability of estradiol to affect aggression in SP, supports the hypothesis that females housed in SP are more aggressive than females housed in LP because ovarian hormones reduce aggression in LP-housed females. The observation that DHEA concentrations are higher in LP-housed females compared to SP-housed females is in contrast to what has been shown in male Syrian hamsters (Caldwell et al., 2008). In addition, the alterations in concentrations of adrenal hormones in SP indicate that HPA activity may change overall as a function of photoperiod in female Syrian hamsters.
Acknowledgements
The authors would like to thank the Georgia State Department of Animal Resources, especially Dean Blake, Jesse Britt, Robert Bynes, Jonathan Edwards, Pat Hicks, Cindy Marshall, and DeAndre West for their excellent animal care. Also, Dr. Michael Hart and Matthew Davis contributed with their veterinary expertise and input on surgical techniques. This work was supported by NSF grants IBN 9876754 and IOS-0923301 as well as NIH grant MH62641 awarded to H.E.A. Portions of this work were presented at the 2008 meeting of the Society for Neuroscience (Washington, D.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers HE, Huhman KL, Meisel RL. Hormonal basis of social conflict and communication. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain, and Behavior. Vol. 1. Academic Press; San Diego: 2002. pp. 393–433. [Google Scholar]
- Bartness TJ, Bradley J, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: What has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J. Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Goldman BD. Diurnal rhythms in gonadotropins and progesterone in lactating and photoperiod induced acyclic hamsters. Biol. Reprod. 1975;13:617–622. doi: 10.1095/biolreprod13.5.617. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Smith DA, Albers HE. Photoperiodic mechanisms controlling scent marking: Interactions of vasopressin and gonadal steroids. Eur. J. Neurosci. 2008;27:1189–1196. doi: 10.1111/j.1460-9568.2008.06071.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Michael SJ, Morris AH. Hormonal induction of female sexual behavior in male and female hamsters. Horm. Behav. 1973;4:129–141. [Google Scholar]
- Ciaccio LA, Lisk RD, Reuter LA. Prelordotic behavior in the hamster: A hormonally modulated transition from aggression to sexual receptivity. J. Comp. Physiol. Psych. 1979;93:771–780. doi: 10.1037/h0077604. [DOI] [PubMed] [Google Scholar]
- Demas GE, Polacek KM, Durazzo A, Jasnow AM. Adrenal hormones mediate melatonin-induced increases in aggression in male Siberian hamsters (Phodopus sungorus) Horm. Behav. 2004;46:582–591. doi: 10.1016/j.yhbeh.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Elliott AS, Nunez AA. Photoperiod modulates the effects of steroids on sociosexual behaviors of hamsters. Physiol. Behav. 1992;51:1189–1193. doi: 10.1016/0031-9384(92)90307-n. [DOI] [PubMed] [Google Scholar]
- Faruzzi AN, Solomon MB, Demas GE, Huhman KL. Gonadal hormones modulate the display of submissive behavior in socially defeated female Syrian hamsters. Horm. Behav. 2005;47:569–575. doi: 10.1016/j.yhbeh.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Phillips A, Rydall A, Levesque L. Effects of photoperiod, the pineal gland, and the gonads on agonistic behavior in female golden hamsters (Mesocricetus auratus) Physiol. Behav. 1988;44:227–234. doi: 10.1016/0031-9384(88)90143-6. [DOI] [PubMed] [Google Scholar]
- Floody OR, Pfaff DW. Aggressive behavior in female hamsters: the hormonal basis for fluctuations in female aggressiveness correlated with estrous state. J. Comp. Physiol. Psychol. 1977;91:443–464. doi: 10.1037/h0077341. [DOI] [PubMed] [Google Scholar]
- Fraile IG, McEwen BS, Pfaff DW. Progesterone inhibition of aggressive behaviors in hamsters. Physiol. Behav. 1987;39:225–229. doi: 10.1016/0031-9384(87)90013-8. [DOI] [PubMed] [Google Scholar]
- Garrett JW, Campbell CS. Changes in social behavior of the male golden hamster accompanying photoperiodic changes in reproduction. Horm. Behav. 1980;14:303–319. doi: 10.1016/0018-506x(80)90020-3. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short days and exogenous melatonin increase aggression of male Syrian hamsters (Mesocricetus auratus) Horm. Behav. 2002;42:13–20. doi: 10.1006/hbeh.2002.1797. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short-day increases in aggression are inversely related to circulating testosterone concentrations in male Siberian hamsters (Phodopus sungorus) Horm. Behav. 2000;38:102–110. doi: 10.1006/hbeh.2000.1604. [DOI] [PubMed] [Google Scholar]
- Jorgenson KL, Schwartz NB. Shifts in gonadotropin and steroid levels that precede anestrus in female golden hamsters exposed to a short photoperiod. Biol. Reprod. 1985;32:611–618. doi: 10.1095/biolreprod32.3.611. [DOI] [PubMed] [Google Scholar]
- Leska A, Dusza L. Seasonal changes in the hypothalamo-pituitary-gonadal axis in birds. Reprod. Biol. 2007;7:99–126. [PubMed] [Google Scholar]
- Lisk RD, Nachtigall MJ. Estrogen regulation of agonistic and proceptive responses in the golden hamster. Horm. Behav. 1988;22:35–48. doi: 10.1016/0018-506x(88)90029-3. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sterner MR, Diekman MA. Differential hormonal control of aggression and sexual behavior in female Syrian hamsters. Horm. Behav. 1988;22:453–466. doi: 10.1016/0018-506x(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sterner MR. Progesterone inhibition of sexual behavior is accompanied by an activation of aggression in female Syrian hamsters. Physiol. Behav. 1990;47:415–417. doi: 10.1016/0031-9384(90)90102-a. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Badura LL, Goldman BD. Mechanisms of seasonal cycles of behavior. Ann. Rev. Psychol. 1990;41:81–108. doi: 10.1146/annurev.ps.41.020190.000501. [DOI] [PubMed] [Google Scholar]
- Nicolas LB, Pinoteau W, Papot S, Routier S, Guillaumet G, Mortaud S. Aggressive behavior induced by the steroid sulfatase inhibitor COUMATE and by DHEAS in CBA/H mice. Brain Res. 2001;922:216–222. doi: 10.1016/s0006-8993(01)03171-7. [DOI] [PubMed] [Google Scholar]
- Ottenweller JE, Tapp WN, Pitman DL, Natelson BH. Adrenal, thyroid, and testicular hormone rhythms in male golden hamsters on long and short days. Am. J. Physiol. 1987;253:R321–R328. doi: 10.1152/ajpregu.1987.253.2.R321. [DOI] [PubMed] [Google Scholar]
- Payne AP, Swanson HH. The effect of sex hormones on the aggressive behavior of the female golden hamsters (Mesocricetus auratus waterhouse) Anim. Behav. 1972;20:782–787. doi: 10.1016/s0003-3472(72)80152-0. [DOI] [PubMed] [Google Scholar]
- Pieper DR, Lobocki CA. Characterization of serum dehydroepiandrosterone secretion in golden hamsters. Proc. Soc. Exp. Biol. Med. 2000;224:278–284. doi: 10.1046/j.1525-1373.2000.22432.x. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Adelson JD, Nelson RJ. Short days increase hypothalamic-pituitary-adrenal axis responsiveness. Endocrinology. 2007;148:3402–3409. doi: 10.1210/en.2006-1432. [DOI] [PubMed] [Google Scholar]
- Ronchi E, Spencer RL, Krey LC, McEwen BS. Effects of photoperiod on brain corticosteroid receptors and the stress response in the golden hamster (Mesocricetus auratus) Brain Res. 1998;780:348–351. doi: 10.1016/s0006-8993(97)01303-6. [DOI] [PubMed] [Google Scholar]
- Scotti MA, Place NJ, Demas GE. Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus) Horm. Behav. 2007;52:183–190. doi: 10.1016/j.yhbeh.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Goldman BD. Effects of photoperiod on cyclicity and serum gonadotropins in the Syrian hamster. Biol. Reprod. 1975;12:223–31. doi: 10.1095/biolreprod12.2.223. [DOI] [PubMed] [Google Scholar]
- Soma KK, Wingfield JC. Dehydroepiandrosterone in songbird plasma: Seasonal regulation and relationship to territorial aggression. Gen. Comp. Endocrinol. 2001;123:144–155. doi: 10.1006/gcen.2001.7657. [DOI] [PubMed] [Google Scholar]
- Soma KK, Sullivan KA, Tramontin AD, Saldanha CJ, Schlinger BA, Wingfield JC. Acute and chronic effects of an aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrows. J. Comp. Physiol. A. 2000;186:759–769. doi: 10.1007/s003590000129. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Lisk RD. Organization and expression of agonistic and socio-sexual behavior in golden hamsters over the estrous cycle and after ovariectomy. Physiol. Behav. 1983;31:447–482. doi: 10.1016/0031-9384(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Turek FW, Elliott J, Alvis J, Menaker M. Effects of prolonged exposure to nonstimulatory photoperiods on the activity of the neuroendocrine-testicular axis of golden hamsters. Biol. Reprod. 1975;13:475–481. doi: 10.1095/biolreprod13.4.475. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. The effects of gonadal hormones on the aggressive behavior of adult golden hamsters (Mesocricetus auratus) Anim. Behav. 1971;19:589–594. doi: 10.1016/s0003-3472(71)80116-1. [DOI] [PubMed] [Google Scholar]