Abstract
This study experimentally tested whether a stressor characterized by social-evaluative threat (SET), a context in which the self can be judged negatively by others, would elicit increases in proinflammatory cytokine activity and alter the regulation of this response. This hypothesis was derived in part from research on immunological responses to social threat in nonhuman animals. Healthy female participants were assigned to perform a speech and a math task in the presence or absence of an evaluative audience (SET or non-SET, respectively). As hypothesized, stimulated production of the proinflammatory cytokine tumor necrosis factor-α (TNF-α) increased from baseline to poststressor in the SET condition, but was unchanged in the non-SET condition. Further, the increases in TNF-α production correlated with participants’ cognitive appraisals of being evaluated. Additionally, the ability of glucocorticoids to shut down the inflammatory response was decreased in the SET condition. These findings underscore the importance of social evaluation as a threat capable of eliciting proinflammatory cytokine activity and altering its regulation.
Preserving a positive social self—maintaining one’s social esteem, status, and acceptance—is central to well-being and survival (Baumeister & Leary, 1995). Therefore, conditions that threaten the social self may elicit psychological, physiological, and behavioral changes to coordinate an appropriate response to the situation (e.g., Dickerson, Gruenewald, & Kemeny, 2004). Acute social self threat, or social-evaluative threat (SET), occurs when an aspect of the self could be negatively judged by others (Dickerson & Kemeny, 2004). Previous work has demonstrated that SET triggers specific psychological and physiological changes. For example, social-evaluative stressors (e.g., delivering a speech in front of an audience) are more likely to elicit production of the hormone cortisol than are otherwise-equivalent stressors without social evaluation (Dickerson & Kemeny, 2004; Dickerson, Mycek, & Zaldivar, 2008; Gruenewald, Kemeny, Aziz, & Fahey, 2004). Additionally, studies have shown that increases in cortisol correlate with the self-evaluative cognitions and emotions experienced under SET, demonstrating patterned psychobiological changes (Dickerson et al., 2008; Gruenewald et al., 2004).
Other physiological responses may also be elicited by SET. Evidence from nonhuman-animal models demonstrates that social threats can increase inflammatory markers; further, there may be specificity with regard to the nature of the threat and the immunological responses observed. Proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), are chemical communication molecules that orchestrate the inflammatory immune response, which is integral for fighting infection and healing from injury. Animals experiencing social threats, such as social subordination or defeat, show greater stimulated production of proinflammatory cytokines and increases in other inflammatory markers compared with animals exposed to either other types of stressors (e.g., physical restraint) or nonstressful control conditions (Avitsur, Stark, & Sheridan, 2001; Quan et al., 2001; Sheridan, Stark, Avitsur, & Padgett, 2000; Stefanski & Engler, 1998). Release of proinflammatory cytokines in the context of social subordination or defeat is thought to be adaptive, for example, to prepare the immune system for potential wounding or infection stemming from an antagonistic social encounter (Dhabhar, 1998), or to support behavioral patterns of submission or disengagement that could be functional in this context (Dickerson, Gruenewald, & Kemeny, 2009).
Social threats may not only increase proinflammatory activity, but also alter the regulation of this response. High levels of glucocorticoids (cortisol or its synthetics, hydrocortisone and dexamethasone) typically inhibit the production and expression of proinflammatory cytokines. Although social threat can elevate glucocorticoids in humans (Dickerson & Kemeny, 2004) and in nonhuman animals (Sapolsky, 2005), certain threats may interfere with the glucocorticoid-driven inhibitory process, thereby leading to simultaneous elevations in glucocorticoid and proinflammatory activity. Indeed, social threat can modulate the ability of glucocorticoids to shut down inflammatory processes in nonhuman animals (Avitsur, Padgett, & Sheridan, 2006). In other words, social stressors can decrease the ability of glucocorticoids to reign in proinflammatory responses (i.e., decrease glucocorticoid sensitivity): As a result of down-regulation of glucocorticoid receptors on immune cells that produce proinflammatory cytokines, glucocorticoids become less able to alter (inhibit) the activity of these cells. Decreases in glucocorticoid sensitivity may be specific to social threat, as other stressors have not induced these immunoregulatory changes (Sheridan et al., 2000).
In summary, the nonhuman-animal literature demonstrates that social threat increases proinflammatory cytokine activity and alters the ability of glucocorticoids to regulate this response. These responses may be tied to the social nature of the threat, as not all types of stressors appear to elicit them. However, this hypothesis has not been tested in humans, The majority of the studies examining proinflammatory activity and regulation in humans have focused on whether disease states alter inflammatory processes (e.g., Davis et al., 2008; Miller, Rohleder, Stetler, & Kirschbaum, 2005), on the physiological mechanisms through which these changes occur (e.g., Bierhaus et al., 2003), and on individual differences that explain variability in these effects (e.g., Brydon, Edwards, Mohamed-Ali, & Steptoe, 2004; Rohleder, Schommer, Hellhammer, Engel, & Kirschbaum, 2001). Although qualitative and quantitative reviews demonstrate that, overall, acute psychological stressors can activate inflammatory processes (Segerstrom & Miller, 2004; Steptoe, Hamer, & Chida, 2007) and modulate glucocorticoid sensitivity (Rohleder, Wolf, & Kirschbaum, 2003), no studies have directly compared stressor contexts to test whether certain stressors are more likely than others to elicit these responses. Additionally, little attention has been focused on the social-cognitive processes that may underlie potential changes in inflammatory activity.
Drawing on social-threat research in nonhuman animals, and on our own work demonstrating that the cognitions and emotions often experienced under social-evaluative threat are associated with increased proinflammatory activity (Dickerson, Kemeny, Aziz, Kim, & Fahey, 2004), we hypothesized that, compared with stressors without a social-evaluative component, social-evaluative stressors would elicit greater increases in proinflammatory cytokine activity and reduce the ability of glucocorticoids to suppress proinflammatory responses. Further, we hypothesized that social-evaluative psychological responses (i.e., perceptions of social evaluation) would be associated with the immunological changes.
Our study employed the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993), which involves standardized speech and mental-arithmetic components. Participants were randomly assigned to perform the TSST in its typical form (with an evaluative audience present; SET condition) or in a modified version in which they performed the test alone in a room (non-SET condition). This paradigm has been effective in manipulating social-evaluative threat while holding other factors, such as effort, task difficulty, and participants’ perceptions of their performance, constant across conditions (Gruenewald et al., 2004). Heart rate (HR) and blood pressure were assessed as markers of engagement in the task and arousal (e.g., Blascovich & Tomaka, 1996), which we hypothesized would increase in both conditions (Gruenewald et al., 2004). Proinflammatory cytokine activity was indexed via lipopolysaccharide- (LPS-) stimulated production of the cytokine TNF-α; this measure assesses the ability of cells to produce cytokines upon challenge with a bacterial-wall product. We examined TNF-α because it is a critical mediator of the inflammatory response and has been shown to be sensitive to social threat in humans (Miller et al., 2005) and in nonhuman-animal models (Avitsur et al., 2006). In order to assess relative sensitivity to glucocorticoids, we also assessed LPS-stimulated production of TNF-α after adding three concentrations of hydrocortisone to the blood samples. Because studies have reported gender differences in the reactivity and regulation of inflammatory processes (Rohleder et al., 2001), this investigation was limited to healthy women.
METHOD
Participants
Thirty-nine undergraduate females1 participated in a study described as examining “health responses to laboratory tasks.” Women were screened and were excluded if they had psychiatric disorders, had acute or chronic health problems, engaged in certain health behaviors (e.g., smoking), or used prescription medications (e.g., oral contraceptives) that could alter immune responses. Participants’ average age was 21.0 years (SD = 2.0), and their self-reported ethnic backgrounds were diverse (44% Asian or Pacific Islander, 23% Caucasian, 26% Latina or Chicana, and 7% other ethnicities).
Procedure
Participants were instructed not to exercise, drink alcohol, or take nonprescription medication on the day of their appointment, and not to consume a major meal or caffeine during the hour prior to their afternoon session. After they provided informed consent, a heparinized intravenous catheter was inserted in the nondominant forearm, and a blood pressure cuff was placed on the opposite arm. During the baseline phase, participants completed questionnaires for 20 min, and cardiovascular assessments were collected. Then, the baseline blood sample was taken.
A modified version of the TSST (Kirschbaum et al., 1993) was used; participants had 10 min to prepare and 5 min to deliver a speech on why they would be the perfect applicant for a job. This speech was followed by a 5-min computerized arithmetic task, which required participants to mentally solve complex arithmetic problems while a noise increasing in volume indicated the time remaining (Pruessner, Hellhammer, & Kirschbaum, 1999).
Prior to hearing the task instructions, participants were randomly assigned to the SET (n = 19) or non-SET (n = 20) condition. In the SET condition, instructions were delivered by two female undergraduate assistants, who explained that they would be present during the tasks. Participants in the non-SET condition received instructions via an audio-recorded message, which stated that they would be performing the tasks alone in a room. Remaining instructions were identical for the two conditions.
Participants completed the tasks in the social context consistent with their condition. In the SET condition, the audience members observed participants’ performance with critical, cold, and rejecting facial expressions (without providing verbal feedback). In the non-SET condition, the participants were alone in the room. Immediately after the stressor, participants completed posttask questionnaires, and a posttask blood sample was collected. A recovery blood sample was taken 40 min later, and then participants were debriefed and compensated ($55).
Assessments
Questionnaires
The Performance Attribution Questionnaire assessed participants’ perceptions of their overall performance, the task’s difficulty level, and how controllable, effortful, and challenging the task was, as well as how much they felt their performance was being evaluated during the task.
The Health Behavior Questionnaire assessed health behaviors during the past week, day, and hour. The questions focused on behaviors that could alter immunological responses, including use of alcohol, drugs, and medication, as well as exercise, eating, sleeping, and smoking. Participants were also asked to indicate the current phase of their menstrual cycle.
Stimulated Proinflammatory Cytokine Production
LPS-stimulated TNF-α production in whole blood was assessed at baseline and 25 min (posttask) and 65 min (recovery) from onset of the stressor. Using standard laboratory procedures (Bloemena, Roos, Van Heijst, Vossen, & Schellekens, 1989), we added 100 pg of LPS (Sigma-Aldrich, St. Louis, MO) to 1 ml of 1:5 diluted whole blood (200 μl of whole blood + 800 μl of 1× phosphate buffered saline) and incubated the mixture for 20 hr. This dose of LPS was used because we previously found that it induces a robust yet submaximal response, allowing for the assessment of individual differences. After incubation, the culture supernatant was collected and stored at − 80 °C for batch testing. All samples were assayed in duplicate, and TNF-α levels were measured using the Quantikine immunoassay kit from R&D Systems (Minneapolis, MN) according to the manufacturer’s instructions. The lower limit of detection for this assay was 4.4 pg/ml, and intra- and interassay coefficients of variation were less than 10%.
Glucocorticoid Sensitivity
Glucocorticoid sensitivity was assessed at baseline and 25 min from onset of the stressor (posttask) in the presence of different concentrations (10−8, 10−7, and 10−6 moles) of hydrocortisone added to 100 pg/ml of LPS and 1:5 diluted whole blood. TNF-α levels were then quantified, as described in the previous paragraph. We used hydrocortisone (or cortisol), rather than dexamethasone, to mimic physiological inhibition of the inflammatory response (Davis et al., 2008). Optimal hydrocortisone concentrations were determined through previous quality-control tests and protocols conducted in the Clinical Immunology Research Laboratory at the University of California, Los Angeles (Bower et al., 2007; Davis et al., 2008).
Cardiovascular Assessments
A Critikon automatic sphygmomanometer (Dinamap Pro100 model; GE Healthcare, Piscataway, NJ) was used to assess systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arteriole pressure (MAP), and HR. Assessments were taken every 5 min during baseline and speech preparation, and every 2 min during the speech and math tasks. Readings were averaged to produce one value for each of these four study phase (mean α = .86).
Statistical Analyses
Primary hypotheses were tested using a mixed-model analysis of variance (ANOVA) with condition (SET vs. non-SET) as a between-subjects variable and time of assessment as a within-subjects variable. For the analyses of glucocorticoid sensitivity, we expanded the mixed-model ANOVA to include hydrocortisone concentration (0, 10−8, 10−7, and 10−6 moles) as an additional within-subjects variable. Univariate ANOVAs tested for differences in TNF-α levels between participants in the two conditions at specific time points or hydrocortisone concentrations.
RESULTS
Preliminary Analyses
Participants in the SET and non-SET conditions did not differ significantly in demographics (ethnicity, age, year in school; ps > .14) or baseline physiological measures (TNF-α production at the four levels of hydrocortisone concentrations: ps > .20; SBP, DBP, MAP, and HR: ps > .13). We tested whether health behaviors (e.g., sleep, medication use, exercise) or phase of the menstrual cycle was associated with TNF-α production at baseline or in response to the stressor, as this could potentially confound the results. The correlations were not significant (all ps > .13).
Task Appraisals and Manipulation Checks
Posttask ratings demonstrated that the SET manipulation was successful. As Table 1 shows, participants in the SET condition believed their performance on the tasks was being evaluated more than did participants in the non-SET condition. However, the two conditions did not differ significantly in ratings of task difficulty, effort expended, task challenge, or task controllability, or in participants’ perceptions of how well they performed. Thus, participants in the two conditions perceived the stressor similarly along these dimensions.
Table 1.
Comparison of Posttask Appraisals in the Social-Evaluative Threat (SET) and Non-SET Conditions
| Posttask appraisal | Condition |
|||||
|---|---|---|---|---|---|---|
| SET (n = 19) | Non-SET (n = 20) | Difference | ||||
| M | SD | M | SD | F | p | |
| Others evaluated performance | 5.7 | 1.5 | 2.5 | 1.9 | 32.7 | < .001 |
| Task difficulty | 4.9 | 1.2 | 4.4 | 1.3 | 1.5 | > .20 |
| Effort expended | 2.8 | 1.6 | 2.3 | 1.3 | 0.9 | > .20 |
| Task challenge | 4.8 | 1.6 | 5.0 | 1.2 | 0.2 | > .20 |
| Task controllability | 4.1 | 1.4 | 4.0 | 1.7 | 0.1 | > .20 |
| Performance | 3.5 | 1.3 | 3.4 | 1.3 | 0.0 | > .20 |
Note. All ratings were made on 7-point scales. For evaluation by others, task difficulty, and task challenge, 1 = not at all and 7 = very much; for effort, 1 = tried hard and 7 = didn’t try hard; for task controllability, 1 = out of control and 7 = in control; and for performance 1 = very poorly and 7 = very well.
Participants in both conditions showed elevations in cardiovascular parameters in response to the stressor; there were significant multivariate effects of time for SBP, DBP, MAP, and HR (all ps < .001). However, increases in these parameters were similar for the SET and non-SET conditions. There were no significant Time × Condition interactions — SBP: F(3, 30) = 1.4, p > .20; DBP: F(3, 30) = 0.8, p > .20; MAP: F(3, 30) = 0.4, p > .20; HR: F(3, 30) = 2.3, p > .09. These results indicate that levels of engagement and arousal were comparable in the SET and non-SET conditions
Stimulated TNF-α Production
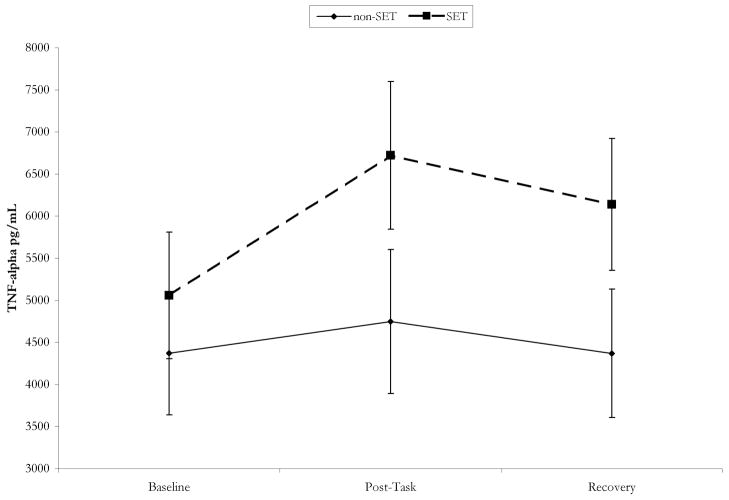
Consistent with hypotheses, analyses revealed a significant Time × Condition interaction for TNF-α production, F(2, 34) = 3.95, p < .05. As depicted in Figure 1, participants in the SET condition showed a significant increase in TNF-α production from baseline to posttask, t(18) = 4.35, p < .001, and this increase was maintained at the 40-min recovery time point, t(18) = 2.36, p < .05. However, participants in the non-SET condition showed no changes in TNF-α production from baseline to posttask, t(18) = 1.51, p > .14, or from baseline to recovery, t(18) = 0.01, p > .20. Furthermore, the differences in TNF-α production between the SET and non-SET conditions were significant at posttask, F(1, 35) = 7.34, p < .01, and at recovery, F(1, 35) = 4.67, p < .05 (controlling for baseline TNF-α production).
Fig. 1.
Mean tumor necrosis factor-α (TNF-α) production at baseline, posttask, and 40-min recovery for participants in the social-evaluative threat (SET) and non-SET conditions. Error bars represent standard errors of the mean.
Because TNF-α production was sensitive to the social-evaluative nature of the stressor, we tested whether participants’ perceptions of being evaluated during the task were associated with the changes in TNF-α production.2 Across conditions, greater perceptions of being evaluated significantly predicted greater increases in TNF-α production, b = 140, SE = 50, p < .01. In contrast, changes in TNF-α production were not predicted by other posttask cognitive appraisals—task difficulty: b = 4.30, SE = 86.8, p > .20; effort expended: b = 132, SE = 91, p > .14; task controllability: b = 17, SE = 74, p > .20; task challenge: b = −135.3, SE = 104, p > .19; and perceived performance: b = 69, SE = 111, p > .20. Furthermore, perceptions of being evaluated remained a significant predictor of increased TNF-α production when we controlled for each of the other task appraisals (ps < .05). This suggests that changes in TNF-α production may be driven by perceptions of social evaluation, rather than by perceptions of effort, difficulty, controllability, challenge, or performance.
Glucocorticoid Inhibition of Stimulated TNF-α Production
Across all participants, there was a significant effect of hydrocortisone concentration on stimulated TNF-α production, F(3, 35) = 23.30, p < .001; thus, the data showed the known suppressive effects of glucocorticoids on proinflammatory cytokine activity. As predicted, the Time × Condition × Hydrocortisone Concentration interaction was significant, F(3, 34) = 3.07, p < .05; the suppressive effects of hydrocortisone varied by condition and time. To decompose this interaction, we examined the effects of condition and hydrocortisone concentration on TNF-α production separately for each time point.
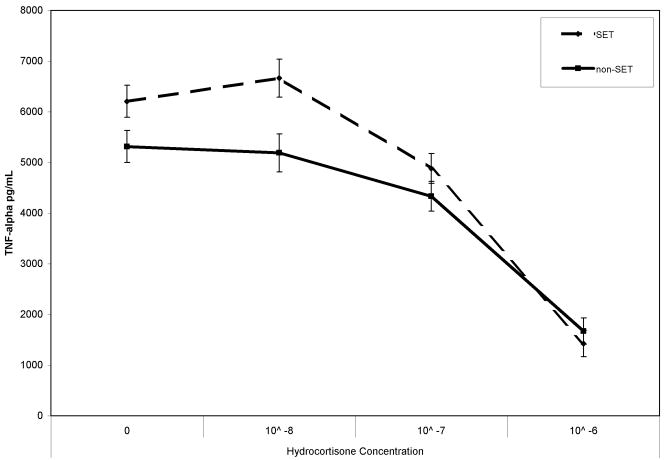
At baseline, the Condition × Hydrocortisone Concentration interaction was not significant, F(3, 35) = 1.02, p > .20; TNF-α production across the different hydrocortisone concentrations did not differ between the SET and non-SET conditions prior to the stressor. Posttask, however, the Condition × Hydrocortisone Concentration interaction was significant, F(3, 30) = 3.73, p < .05, controlling for baseline TNF-α production levels at the four hydrocortisone concentrations (see Fig. 2). Post hoc analyses revealed that TNF-α production was significantly greater in the SET condition than in the non-SET condition when hydrocortisone was not added to the posttask samples (see the previous section) and when the 10−8-mole hydrocortisone concentration was used, F(1, 37) = 5.48, p < .05; the between-condition difference in TNF-α production approached significance for the 10−7-mole hydrocortisone concentration, F(1, 37) = 3.26, p = .079. TNF-α production did not differ between the SET and non-SET conditions at the highest hydrocortisone concentration (10−6 mole), F(1, 37) = 0.05, p > .20. These findings of higher levels of stimulated TNF-α production in the SET condition suggest that sensitivity to the suppressive effects of glucocorticoids on TNF-α production was decreased in this condition relative to the non-SET condition.
Fig. 2.
Mean posttask tumor necrosis factor-α (TNF-α) production in the social-evaluative threat (SET) and non-SET conditions, for assays at four hydrocortisone concentrations. The means have been adjusted for baseline TNF-α production values. Error bars represent standard errors of the mean.
DISCUSSION
This study tested whether a social-evaluative stressor, a test in which the self could be negatively judged by others, would elicit greater increases in proinflammatory cytokine activity and alter the regulation of this response compared with an otherwise-equivalent stressor without social evaluation. As predicted, participants who performed speech and math tasks in front of an evaluative audience (SET condition) showed increases in production of the proinflammatory cytokine TNF-α immediately poststressor and after a 40-min recovery period. Participants completing the same tasks alone in a room (non-SET condition) showed no changes in TNF-α; this flat, stable pattern is similar to that observed under resting, nonstressful conditions (e.g., Edwards, Burns, Ring, & Carroll, 2006; O’Connor, Motivala, Valladares, Olmstead, & Irwin, 2007). Additionally, the social-evaluative context influenced the regulation of the inflammatory response. Cells from the participants in the SET condition showed decreased sensitivity to the suppressive effects of glucocorticoids compared with cells from the participants in the non-SET condition; this indicates that glucocorticoids were less effective in reigning in the inflammatory response under SET.
Our manipulation was successful in inducing social-evaluative threat in the SET condition while keeping other factors (perceptions of effort, task difficulty, controllability, challenge, and performance) comparable in the SET and non-SET conditions. Participants in the two conditions also demonstrated similar levels of arousal and task engagement, as indexed by increases in cardiovascular parameters. Therefore, the pattern of our results—an increase in TNF-α production only in the SET condition—was likely driven by differences in social evaluation between the groups and cannot be fully explained by alternative explanations (the SET condition was more difficult, required more effort, etc.). This argument is further bolstered by our finding that participants’ ratings of how much they felt they were evaluated during the stressor correlated with increases in TNF-α activity, whereas this cytokine change was unrelated to other cognitive appraisals (e.g., perceived difficulty, effort, and performance). Our study adds to a growing literature demonstrating that the social-evaluative cognitive and affective states experienced under SET are associated with physiological changes (Dickerson, Kemeny, et al., 2004; Dickerson et al., 2008; Gruenewald et al., 2004); this association may reflect a coordinated psychobiological response to this threat.
Our results demonstrate that researchers should consider the social milieu of the stressors employed in laboratory research in order to understand the conditions capable of increasing proinflammatory cytokine activity and regulating this response. Recent meta-analyses have shown that, overall, acute psychological stressors can activate inflammatory pathways (Segerstrom & Miller, 2004; Steptoe et al., 2007). However, there has been variability in these effects; some studies have reported increases in inflammatory activity, whereas others have not. Examination of the social-evaluative context of stressors has been fruitful for clarifying the specific conditions that can elicit cortisol reactivity (Dickerson & Kemeny, 2004); this approach may also be useful for delineating stressors that trigger and regulate inflammatory responses.
Our findings replicate and extend previous research with nonhuman animals, which has demonstrated that social threats increase proinflammatory cytokine production and other markers of inflammation and, further, can decrease the sensitivity of immune cells to the suppressive effects of glucocorticoids (Avitsur et al., 2006). That work has also found that not all stressors are capable of activating these immunoregulatory changes; specific social threats (e.g., social subordination, defeat) induce these responses, whereas other threats (e.g., physical restraint) do not. Our findings are consistent with these results: Only the SET condition led to increases in proinflammatory activity and altered the regulation of this process. Taken together, these studies suggest that all stressors do not have uniform effects on proinflammatory cytokine activity and regulation; instead, changes in production and regulation may occur for a subset of stressful conditions, including those that involve specific social threats. These responses could be adaptive in initiating biological changes to deal with the acute demands of a threat (e.g., a wound or infection following an antagonistic encounter), or to reinforce functional behavioral strategies such as submission or disengagement (Dickerson et al., 2009). Proinflammatory cytokines have been shown to induce behavior consistent with a disengaged motivational state (e.g., Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008), and disengagement could be an adaptive response to relatively uncontrollable social threat.
Several limitations of this study warrant comment. First, all the participants were women. It will be important for future studies to determine the degree to which these findings generalize to men, and, further, if there are gender differences in immunoregulatory responses to SET versus non-SET contexts. There is some evidence that, compared with men, women may show a greater decrease in glucocorticoid sensitivity in response to SET (Rohleder et al., 2001), and this could be a mechanism underlying gender differences in the incidence of inflammatory disorders (e.g., rheumatoid arthritis).
Second, we did not test whether the increases in TNF-α production observed under SET were due to changes in the amount of cytokine produced or in the numbers of monocytes or immunoregulatory cells from baseline to posttask. Given that monocytes are a major source of stimulated TNF-α production, it is possible that the increase in TNF-α production from baseline to posttask was due to an increase in the number of monocytes, although a meta-analysis demonstrated that monocyte number is typically unresponsive to acute psychological stressors (Segerstrom & Miller, 2004). Research that also examines the number of monocytes or evaluates the production of TNF-α at the cellular level is needed to address these additional mechanistic considerations.
Third, the current study focused on one proinflammatory cytokine, TNF-α. Future research should assess a wider range of proinflammatory and anti-inflammatory cytokines, and other physiological systems, to further delineate the full pattern of psychobiological changes that may be elicited or regulated by SET.
This study compared a condition in which no observers were present with a condition in which observers were both present and negatively evaluating the participant’s performance. Therefore, it is unclear whether the mere presence of others or the social-evaluative component was responsible for eliciting the immunological changes observed. However, previous work has shown that explicit social evaluation, but not the mere presence of others, triggers increases in cortisol (Dickerson et al., 2008). Our finding that perceptions of being evaluated correlated with increases in TNF-α also supports the notion that evaluation may have driven the proinflammatory changes. Testing this experimentally will be an important next step. Examining factors that could heighten the impact of evaluation would also programmatically extend this work.
Our results underscore the importance of considering the social context in identifying conditions capable of eliciting and regulating immunological responses. The vast majority of research within health psychology has adopted a general approach, in regard to both an eliciting condition and the physiological responses engendered. These findings highlight the utility of focusing on specific eliciting conditions and social-cognitive processes in psychobiological research (Kemeny, 2003; Weiner, 1992).
Identifying the specific conditions that alter inflammatory processes could have important implications for health. Decreased glucocorticoid sensitivity could lead a proinflammatory response to “overshoot”: If stressors are frequent or prolonged, sustained exposure to proinflammatory mediators could result. This could put individuals at risk, as sustained inflammation has been linked to the initiation and progression of a number of diseases, including cardiovascular disease, rheumatoid arthritis, metabolic syndrome, and depression (e.g., Kronfol & Remick, 2000; Miller & Blackwell, 2006), as well as to mortality (Harris et al., 1999). Understanding the specific social conditions and accompanying cognitive-emotional processes that elicit and prolong proinflammatory responses could help researchers delineate the threats that, if chronically experienced, could lead to negative health effects.
Acknowledgments
Funding for this study was provided by the Cousins Center for Psychoneuroimmunology and by the General Clinical Research Center at the University of California, Los Angeles (National Institutes of Health/National Center for Research Resources M01-RR00865).
Footnotes
Forty-two women were initially recruited. One withdrew after hearing the experimental instructions, and another became physically ill during the session; an additional woman’s scores on baseline questionnaires fell more than 4 standard deviations above the mean. These participants were not included in analyses.
These analyses were conducted using multilevel modeling, as this regression technique allowed continuous covariates (i.e., appraisals) as predictors of the immunological changes. Time of assessment was the Level 1 factor (baseline, posttask, recovery), and task appraisals were the Level 2 predictors. Contrast coefficients compared the baseline (pretask) time point with the posttask and recovery time points. Therefore, positive slopes (bs) represent increases in TNF-α production from baseline to poststressor.
References
- Avitsur R, Padgett DA, Sheridan JF. Social interactions, stress, and immunity. Neurology Clinics. 2006;24:483–491. doi: 10.1016/j.ncl.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Hormones and Behavior. 2001;39:247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences, USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blascovich J, Tomaka J. The biopsychosocial model of arousal regulation. Advances in Experimental Social Psychology. 1996;28:1–51. [Google Scholar]
- Bloemena E, Ross MT, Van Heijst JL, Vossen JM, Schellekens PT. Whole blood lymphocyte cultures. Journal of Immunology Methods. 1989;122:161–167. doi: 10.1016/0022-1759(89)90260-3. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: Relationship to glucocorticoids. Brain, Behavior, and Immunity. 2007;21:251–258. doi: 10.1016/j.bbi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Brydon L, Edwards S, Mohamed-Ali V, Steptoe A. Socioeconomic status and stress-induced increases in interleukin-6. Brain, Behavior, and Immunity. 2004;18:281–290. doi: 10.1016/j.bbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Zautra AJ, Younger J, Motivala SJ, Attrep J, Irwin MR. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: Implications for fatigue. Brain, Behavior, and Immunity. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS. Stress-induced enhancement of cell-mediated immunity. Annals of the New York Academy of Sciences. 1998;840:359–372. doi: 10.1111/j.1749-6632.1998.tb09575.x. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. When the social self is threatened: Shame, physiology, and health. Journal of Personality. 2004;72:1192–1216. doi: 10.1111/j.1467-6494.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. Psychobiological responses to social self threat: Functional or detrimental? Self and Identity. 2009;8:270–285. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosomatic Medicine. 2004;66:124–131. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Mycek PJ, Zaldivar F. Negative social evaluation, but not mere social presence, elicits cortisol responses to a laboratory stressor task. Health Psychology. 2008;27:116–121. doi: 10.1037/0278-6133.27.1.116. [DOI] [PubMed] [Google Scholar]
- Edwards KM, Burns VE, Ring C, Carroll D. Individual differences in the interleukin-6 response to maximal and submaximal exercise tasks. Journal of Sport Sciences. 2006;24:855–862. doi: 10.1080/02640410500245645. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Kemeny ME, Aziz N, Fahey JL. Acute threat to the social self: Shame, social self-esteem, and cortisol activity. Psychosomatic Medicine. 2004;66:915–924. doi: 10.1097/01.psy.0000143639.61693.ef. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, et al. Association of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. American Journal of Medicine. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Kemeny ME. The psychobiology of stress. Current Directions in Psychological Science. 2003;12:124–129. [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kronfol Z, Remick DG. Cytokines and the brain: Implications for clinical psychiatry. American Journal of Psychiatry. 2000;157:683–694. doi: 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- Miller GE, Blackwell E. Turning up the heat: Inflammation as a mechanism linking chronic stress, depression, and heart disease. Current Directions in Psychological Science. 2006;15:269–272. [Google Scholar]
- Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosomatic Medicine. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Motivala SJ, Valladares EM, Olmstead R, Irwin MR. Sex differences in monocyte expression of IL-6: Role of autonomic mechanisms. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2007;293:R145–151. doi: 10.1152/ajpregu.00752.2006. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Low self-esteem, induced failure and the adrenocortical stress response. Personality and Individual Differences. 1999;27:477–489. [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, et al. Social stress increases the susceptibility to endotoxic shock. Journal of Neuroimmunology. 2001;115:36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosomatic Medicine. 2001;63:966–972. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Wolf JM, Kirschbaum C. Glucocorticoid sensitivity in humans: Inter-individual differences and acute stress effects. Stress. 2003;6:207–222. doi: 10.1080/1025389031000153658. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan JF, Stark JL, Avitsur R, Padgett DA. Social disruption, immunity, and susceptibility to viral infection. Role of glucocorticoid insensitivity and NGF. Annals of the New York Academy of Sciences. 2000;917:894–905. doi: 10.1111/j.1749-6632.2000.tb05455.x. [DOI] [PubMed] [Google Scholar]
- Stefanski V, Engler H. Effects of acute and chronic social stress on blood cellular immunity in rats. Physiology and Behavior. 1998;64:733–741. doi: 10.1016/s0031-9384(98)00127-9. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behavior, and Immunity. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Weiner H. Perturbing the organism: The biology of stressful experience. Chicago: University of Chicago Press; 1992. [Google Scholar]