Abstract
Dystrophic axons and terminals are common in the myenteric plexus and smooth muscle of the gastrointestinal (GI) tract of aged rats. In young adult rats, alpha-synuclein in its normal state is abundant throughout the myenteric plexus, making this protein--which is prone to fibrillization--a candidate marker for axonopathies in the aged rat. To determine if aggregation of alpha-synuclein is involved in the formation of age-related enteric neuropathies, we sampled the stomach, small intestine and large intestine of adult, middle-aged, and aged virgin male Fischer 344 rats stained for alpha-synuclein in both its normal and pathological states. Alpha-synuclein-positive dystrophic axons and terminals were present throughout the GI tract of middle-aged and aged rats, with immunohistochemical double-labeling demonstrating co-localization within nitric oxide synthase-, calretinin-, calbindin-, or tyrosine hydroxylase-positive markedly swollen neurites. However, other dystrophic neurites positive for each of these four markers were not co-reactive for alpha-synuclein. Similarly, a subpopulation of alpha-synuclein inclusions contained deposits immunostained with an anti-tau phospho-specific Ser262 antibody, but not all of these hyperphosphorylated tau-positive aggregates were co-localized with alpha-synuclein. The presence of heteroplastic and potentially degenerating neural elements and protein aggregates both positive and negative for alpha-synuclein suggests a complex chronological relationship between the onset of degenerative changes and the accumulation of misfolded proteins. Additionally, proteins other than alpha-synuclein appear to be involved in age-related axonopathies. Finally, this study establishes the utility of the aging Fischer 344 rat for the study of synucleopathies and tauopathies in the GI tract.
Keywords: Calbindin, Calcitonin Gene-Related Peptide, Calretinin, Enteric Nervous System, Lewy Neurites/Bodies, Nitric Oxide Synthase, Parkinson’s disease, Tau, Tyrosine Hydroxylase
Introduction
Normal aging of the neural circuitry controlling the gastrointestinal (GI) tract involves a progressive loss of neurons in the enteric nervous system (ENS) and a concurrent increase in the presence of swollen and dystrophic (or heteroplastic) neurites in the enteric ganglia and the smooth muscle wall (Camilleri et al., 2008; Cersosimo and Benarroch, 2008; Phillips and Powley, 2007; Phillips et al., in press), and both patterns parallel the increasing prevalence of disturbances in GI function with aging (Crane and Talley, 2007; Hays and Roberts, 2006; Morley, 2007; Newton, 2004; Norton, 2006; O’Mahony et al., 2002; Roach and Christie, 2008; Trinh and Prabhakar, 2007).
The death of neurons in the ENS with age has been reviewed and discussed in detail (Camilleri et al., 2008; Phillips and Powley, 2007; Saffrey, 2004; Wade and Cowen, 2004): In brief, neuron loss progresses linearly with age (Phillips and Powley, 2001; Phillips et al., 2007), occurs throughout the length of the GI tract (Abalo et al., 2005; Cowen et al., 2000; Gabella, 1989; Phillips and Powley, 2001; Santer and Baker, 1988), and is confined to the cholinergic phenotype of enteric neurons (Abalo et al., 2005; Cowen et al., 2000; Phillips et al., 2003). In contrast, dilated and heteroplastic axonal profiles in the aging gut have been repeatedly observed and reported (Baker and Santer, 1988; Braak et al., 2006; Phillips and Powley, 2007; Phillips et al., 2003, 2006, 2007, in press; Walter et al., 2009), but have not been examined and described as thoroughly as neuron loss with age. Thus, the focus of the current study was to begin to more fully describe these age-related dystrophic neuropathies.
Alpha-synuclein, which has been linked provisionally with a variety of neuronal functions including neurotransmission, the regulation of enzymes and transporters, presynaptic vesicle dynamics and neuronal plasticity in the CNS (Adamczyk et al., 2005; Dev et al., 2003), is a reasonable first choice for identifying dystrophic features in the GI tract because it is expressed in the intrinsic neurons and extrinsic projections to the gut (Bloch et al., 2006; Braak et al., 2006; Phillips and Powley, 2007; Phillips et al., 2008, in press; Walter et al., 2009). In fact, an important role for alpha-synuclein in the aging of the enteric and autonomic circuitry of the GI tract might be anticipated because, in contrast to most neural systems where alpha-synuclein expression is restricted to presynaptic terminals (Andringa et al., 2003; Bennett, 2005; Halliday and McCann, 2008; Jakes et al., 1994), alpha-synuclein in the GI tract is expressed--in addition to presynaptic terminals--in the cytoplasm of neuronal somata and the axoplasm of neurites (Andringa et al., 2003; Bloch et al., 2006; Braak et al., , 2006, 2007; Hawkes et al., 2007; Miwa et al., 2006; Phillips and Powley, 2007; Phillips et al., 2008; Wakabayashi et al., 2007). Furthermore, alpha-synuclein has a tendency to fibrillize with itself and other native proteins, including tau (a protein frequently found to accumulate in aging and degenerating axons), it has a propensity to form insoluble intracellular aggregates, and it is prominently involved in synucleinopathies that frequently occur in old age (Agnati et al., 2008; Bennett, 2005; Frasier and Wolozin, 2004; Galpern and Lang, 2006; Goedert, 1999; Halliday and McCann, 2008; Lee, 2008; Mattson and Magnus, 2006), all of which make it a strong candidate to be involved in the formation of age-related inclusions in the gut’s nervous system. Finally, limited observations already indicate that at least some markedly swollen axons in the wall of the GI tract are immunopositive for alpha-synuclein (Phillips and Powley, 2007; Phillips et al., 2008), affirming the need for a more thorough examination of the expression of alpha-synuclein in the heteroplastic autonomic axons in the aging GI tract.
The goals of the present study, therefore, were to 1) ascertain if aggregation of alpha-synuclein occurs in the myenteric plexus of aging rats, 2) begin to establish the neurochemical coding of dystrophic axons containing aggregated alpha-synuclein, and 3) determine if aggregated alpha-synuclein is specific to one particular region of the gut or found throughout the GI tract. Additionally, with a more limited set of samples, we also sought to learn if any axonopathies immunoreactive for alpha-synuclein are also co-reactive for a second protein that has a similar propensity to form aggregates with age; the hyperphosphorylated form of tau (Ferrer et al., 2002). Such observations on the molecular and anatomical mechanisms implicated in the nonpathological degenerative processes involved in aging will provide insight into how the normal processes of aging progress and how such changes might evolve into pathological aging.
Materials and Methods
Subjects
Virgin male Fischer 344 (F344; n = 38) rats were purchased, at the ages sampled, from the National Institute on Aging colony maintained by Harlan Laboratory (Indianapolis, IN). Groups of adult (9-10 months of age; n = 14), middle-aged (18 months of age; n = 6), and aged (24-25 months of age; n = 18) rats were used. Rats were group housed (n=2/cage) in polypropylene cages containing sterilized Tek-Fresh bedding (Harlan Teklad, Madison, WI) in a room kept at 22-24°C on a 12:12 hour light:dark schedule. Solid chow (NIH-31; Harlan Teklad) and tap water were available ad libitum. Conditions in the AAALAC-approved colony approximated the housing, husbandry, and barrier conditions at Harlan, but did not provide a specific pathogen-free environment. All procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996, and approved by the Purdue University Animal Care and Use Committee. Every effort was made to minimize the number of rats used and their suffering.
Fixation Protocol and Tissue Samples
Rats were weighed and then killed with a lethal dose of sodium pentobarbital (180 mg/kg, i.p.) and perfused through the left ventricle of the heart with 200 ml of 0.01 M PBS followed by 500 ml of 4% paraformaldehyde. The stomach, small intestine, and large intestine were sampled. Intestinal whole mounts consisted of two 3 cm whole mounts from the duodenum (the first 6 cm anal to the pyloric sphincter), jejunum (6 cm from the mid-jejunum), ileum (the first 6 cm oral to the ileocaecal junction), proximal colon (the first 6 cm distal to the cecum), and distal colon (6 cm of tissue starting approximately 3 cm proximal to the pelvic brim). The mucosa, submucosa, and circular muscle were removed leaving specimens consisting of the myenteric plexus adhering to the longitudinal muscle layer. Whole mounts were further fixed for an additional 2 h in the same fixative.
Permanent Immunohistochemistry
Whole mounts were rinsed in PBS and then incubated for 30 min in a hydrogen peroxide:methanol (1:4) solution to quench endogenous peroxidase. Following several more PBS rinses, whole mounts were soaked for 72 h at room temperature in PBS containing 5% normal horse serum, 2% bovine serum albumin, 0.5% Triton X-100, and 0.08% Na Azide. Free-floating whole mounts were then incubated for 24 h at room temperature with a mouse antiserum raised against alpha-synuclein (1:5,000; 610787; BD Biosciences, San Jose, CA) diluted with PBS containing 2% normal horse serum, 2% bovine serum albumin, 0.3% Triton X-100, and 0.08% Na Azide. The Biosciences antibody was used because it has been extensively and rigorous characterized by several independent laboratories and consistently demonstrated to not cross-react with other synuclein isoforms (Andringa et al., 2003; Bennett, 2005; Cabin et al., 2002; Perrin et al., 2003; van der Putten et al., 2000). In addition, in the present experimental series, negative controls were run by omitting the primary antibody on additional whole mounts randomly taken from the jejunum and an absence of spurious staining was verified. Finally, since we have previously employed the antibody to characterize the normative pattern in the GI tract of adult rats (Phillips et al., 2008), the Biosciences antibody was used to facilitate between-experiment comparisons.
Next, whole mounts were rinsed in PBS and incubated for 1 h at room temperature with biotinylated anti-mouse IgG, rat absorbed, raised in horse (diluted as per the recommendations of the supplier; BA-2001; Vector Laboratories, Inc., Burlingame, CA), followed by incubation for 1 h with an avidin-biotin-horseradish peroxidase complex (Vectastain Elite ABC Kit, Standard; Vector Laboratories, Inc.). Horseradish peroxidase was reacted with diaminobenzidine (DAB) and H2O2 for 5 min to yield a permanent deposit. Finally, stained whole mounts were rinsed in distilled water, mounted on gelatin-coated slides, air-dried overnight, dehydrated in alcohol, cleared in xylene, and cover-slipped with cytoseal (Richard-Allan Scientific, Kalamazoo, MI). Before the 72 h soak in blocking buffer, some whole mounts were counterstained for either Cuprolinic Blue (to label all of the myenteric neurons; Phillips et al., 2004) or NADPHd (to label the nitrergic subpopulation of myenteric neurons; Phillips et al., 2003). Negative controls were run by omitting the primary antibody on additional whole mounts randomly taken from the jejunum.
Fluorescent Immunohistochemistry
Whole mounts were rinsed in PBS and then soaked for 5 to 6 days at room temperature in PBS containing 5% normal goat serum, 2% bovine serum albumin, 0.5% Triton X-100, and 0.08% Na Azide followed by incubation for 24 h at room temperature in a cocktail consisting of a mouse primary and a rabbit primary diluted with PBS containing 2% normal goat serum, 2% bovine serum albumin, 0.3% Triton X-100, and 0.08% Na Azide. The cocktail of primary antibodies always consisted of mouse alpha-synuclein (1:2,500; BD Biosciences) and one of the following rabbit antibodies: either calretinin (1:16,000; 7699/4; Swant, Bellinzona, Switzerland), calbindin (1:8,000; CB-38; Swant), nitric oxide synthase (NOS; 1:2,500; SC-648; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), tyrosine hydroxylase (TH; 1:1,000; P40101-0; Pel Freez, Rogers, AR) or calcitonin gene-related peptide (CGRP; 1:2,000; AB5920; Chemicon, Temecula , CA). Whole mounts were then rinsed in PBS and 0.3% Triton X-100 (PBST), and incubated for 2 h at room temperature in a cocktail consisting of goat anti-mouse ALEXA Fluor 488 (1:500; A11029; Molecular Probes, Eugene, OR) and goat anti-rabbit ALEXA Fluor 594 (1:500; A11037; Molecular Probes) diluted with PBST. Finally, labeled whole mounts were rinsed in PBS, mounted on gelatin-coated slides, air-dried overnight, dehydrated in alcohol, cleared in xylene, cover-slipped with DePeX (Electron Microscopy Sciences, Hatfield, PA), and allowed at least one month to cure; whole mounts were stored at room temperature in slide boxes. Using this non-aqueous mounting protocol (Espada et al., 2005), the two fluorochromes were slow to bleach under illumination, and the quality of the ALEXA dyes remained stable for greater than 12 months with no noticeable increase in background fluorescence. Negative controls were run by omitting one of the primary antibodies on whole mounts randomly taken from the jejunal region of the small intestine.
Co-localization of alpha-synuclein with hyperphosphorylated tau was determined as described above using rabbit polyclonal anti-tau phospho-specific Ser262 (1:1000; 577814; Calbiochem, San Diego, CA; Ferrer et al., 2002); however, Zamboni’s fixative was used in place of 4% paraformaldehyde. In addition to the previously mentioned regions sampled, two adult and two aged rats had their entire small intestine double labeled for alpha-synuclein and tau phospho-specific Ser262 to provide a thorough description of the distribution of aggregates positive for both marker along the length of the organ. Finally, a 90 min soak in cupric sulfate followed application of the ALEXA dyes to quench lipofuscin autofluorescence present in the aged tissue (Brehmer et al., 2004); quenching was done on the whole mounts from both the adult and the aged rats. The addition of a lipofuscin quenching step was necessary to discriminate between the dense accumulation of non-specific lipofuscin fluorescence in the whole mounts from aged rats and the fluorescent labeling of alpha-synuclein and tau phospho-specific Ser262 aggregates.
Brightfield Image Capture, Confocal Microscopy, and Image Post-processing
Images were acquired using a LEICA DM microscope with a SPOT RT Slider camera controlled using SPOT Software V4.7 Advanced Plus (Diagnostic Instruments, Sterling Heights, MI). Co-localization of two antigens was verified using an Olympus DSU (Disk Scanning Unit) spinning disk confocal attached to a BX61 motorized microscope (Olympus, Center Valley, PA). The confocal was controlled using SlideBook digital microscopy software (V4.0; Intelligent Imaging Innovations, Denver, CO). Double-labeled sections were imaged by using filter sets appropriate for the specific visualization of ALEXA Fluor 488 (QMAX-Green; Omega Optical, Brattleboro, VT) and ALEXA Fluor 594 (XF102-2; Omega Optical). A 60X oil objective lens (NA = 1.42) was used. Extended-focus images or z-series of up to 30 optical sections at z-increments of either 0.25 or 0.5 μm were created. Images were captured, analyzed, and post-processed using the SlideBook software. In some cases, z-stacks were compressed into one focus plane (i.e., a maximum value projection). To test for co-localization, single sections at the same focal plane were taken out of the z-stacks, and the two channels were merged.
Final figure production was done using both the Spot Imaging software and Photoshop CS4 (Adobe Systems, San Jose, CA). To maximize the depth of field of images taken from thick specimens with uneven thickness resulting from peeling and mounting artifact, Photoshop CS4 was used to merge a series of shots that were taken at different focal distances into a single image with enhanced depth of field (i.e., focus stacking; Gulbins and Gulbins, 2009). Photoshop CS4 was also used to: (1) apply text and scale bars; (2) adjust color, brightness, contrast, and sharpness; (3) remove artifact (i.e., dust and lint); and (4) organize the final layout of the figures.
Results
Body Weight
The Mean (SEM) body weights of the adult, middle-aged, and aged F344 rats did not differ significantly at the time of euthanasia (p = 0.19; one-way ANOVA): 414 (11), 448 (19), and 424 (6) grams, respectively.
Morphology and Distribution of Alpha-Synuclein in the GI tract of Adult Rats
The pattern of expression of alpha-synuclein in the myenteric plexus and smooth muscle of the GI tract of adult rats (i.e., 9-10 month old rats) was consistent with our previous report (i.e., the GI tract of 3-10 month old rats; Phillips et al., 2008), and reports on the protein’s expression in the CNS (Andringa et al., 2003; Yu et al., 2007). Briefly, in adult rats, a subpopulation of myenteric neurons was immunoreactive for alpha-synuclein, with the protein expressed in the cytoplasm as well as the nucleus (Figures 1A, 3A1). Axons immunoreactive for alpha-synuclein, which were especially prominent in the proximal gut, were typically smooth in appearance when running within the connectives, with numerous small swellings becoming evident along their lengths within ganglia (Figures 1C, 3A2). The alpha-synuclein-positive varicosities within a ganglion encircled neurons and were frequently interdigitated with the dendritic arbors of the intrinsic neurons (Figure 1E).
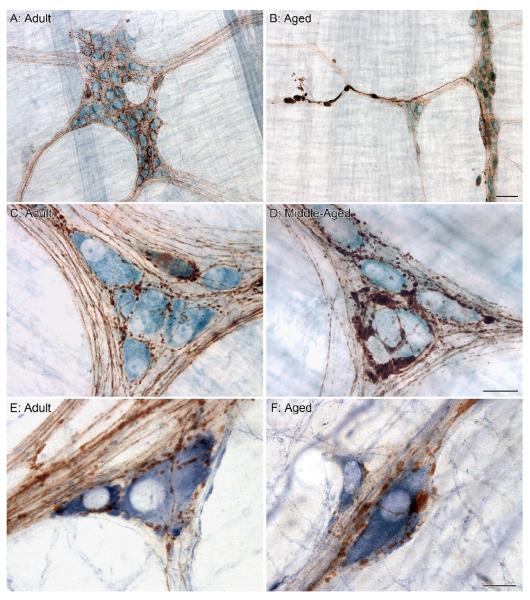
Figure 1.
In the proximal gastrointestinal (GI) tract of adult rats, axons immunoreactive for alpha-synuclein (fine brown fibers) define the connectives between ganglia and form elaborate networks within the ganglion as they encircle individual neurons (blue somata stained with Cuprolinic Blue; A). Upon entering a ganglion, numerous small varicosities become apparent along the length of the alpha-synuclein-positive axons as they encircle individual neurons (C). In aged rats, markedly swollen axons and varicosities positive for alpha-synuclein are easily recognized in the connectives between ganglia (B) and within the ganglion as they form enlarged swellings around individual neurons (D). Alpha-synuclein-positive varicosities interdigitate with the lamellar dendritic arbors of nitrergic myenteric neurons (labeled using the histochemical reaction for NADPHd; E), and in aged rats were often observed swollen and enlarged in comparison to their healthy morphological state (F). Focus stacking was used to create an extended depth of field in panel B (4 merged images). Scale bars = 50 μm in B (applies to A,B); 25 μm in D (applies to C,D); 15 μm in F (applies to E,F).
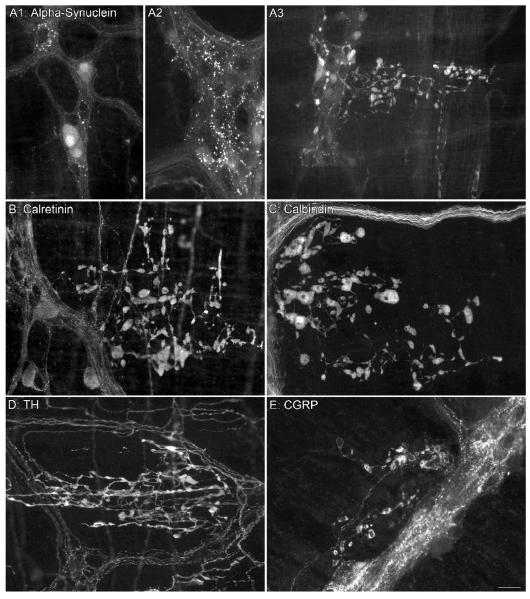
Figure 3.
Alpha-synuclein-positive neurons (A1) and varicosities (A2) are present in the ganglia of adult rats and easily differentiated from the markedly swollen alpha-synuclein-positive varicosities and axons observed in aged rats (A3). Dystrophic axons immunoreactive for either calretinin (B), calbindin (C), TH (D), or calcitonin gene-related peptide (CGRP; E) but negative for alpha-synuclein were found in the myenteric plexus of aged rats; the images in panels B-E come from whole mounts double labeled for alpha-synuclein (not shown). Focus stacking (6 images) was used for panel B. Scale bar in E = 25 μm for A1,A2,B-E; 50 μm for A3.
Morphology and Distribution of Alpha-Synuclein Immunoreactive Inclusions in the Myenteric Plexus of Middle-aged and Aged Rats
In middle-aged and aged rats, distinctive axonopathies were observed throughout the stomach and intestines (Figure 2). These axonopathies consisted of alpha-synuclein-positive markedly swollen axons that were many times larger in diameter than normal axons. Alpha-synuclein-positive dilated varicosities and dystrophic neurites occurred within ganglia (Figures 1D, 2A,F), in regions adjacent to ganglia (Figures 2B-E), and running within connectives between ganglia (Figure 1B). Dystrophic terminals within ganglia encircled individual neurons, forming close associations with their neurites (Figures 1D,F). In some cases, alpha-synuclein inclusions filled an entire ganglion and were as large as neuronal somata (Figure 2A). Dystrophic alpha-synuclein-positive axons located within the non-ganglionic regions adjacent to myenteric ganglia were similarly elaborate, in some cases, forming dense fields of engorged axons that filled an entire region (Figures 2B,C,D). Finally, protein aggregations heavily stained for alpha-synuclein frequently had unstained or lightly stained central regions and/or organelles (Insert in Figure 2A, Figures 2C1,C2).
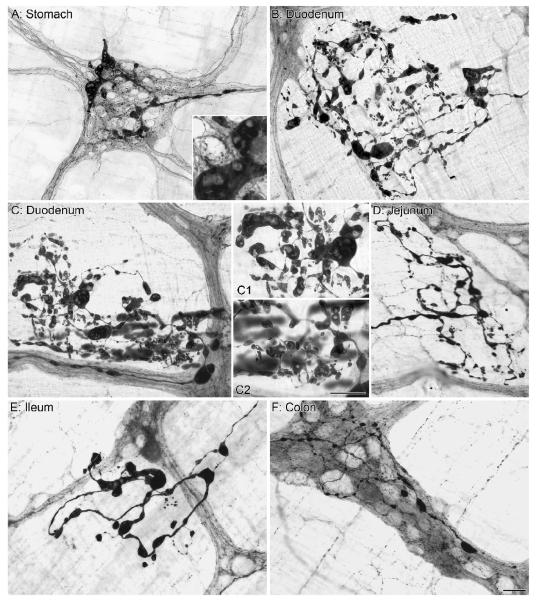
Figure 2.
Markedly swollen axons positive for alpha-synuclein were observed in the stomach (A), small intestine (B-E) and large intestine (F) of aged rats. These engorged axonal processes contained ovoid, fusiform, club-shaped, and spherical alpha-synuclein immunoreactive inclusions are similar to descriptions of Lewy bodies in the central and peripheral nervous system of Parkinson’s patients, with the central core of these alpha-synuclein inclusions unstained for alpha-synuclein (Inset in A and panels C1 and C2). Interestingly, in the adult rat, alpha-synuclein processes and terminals are typically confined within the borders of the myenteric ganglia, however complex alpha-synuclein-positive heteroplastic processes were often observed outside of the ganglionic cellular complexes (B-E). Focus stacking was used for the Insert in panel A (2 merged images) and for panel C2 (6 merged images). Scale bar in F = 50 μm for A; 25 μm for B-F. Scale bar in C2 = 25 μm for Inset in A and panels C1 and C2.
Neurochemical Coding of Alpha-Synuclein-Positive Axonopathies
The morphology of markedly swollen axons positive for alpha-synuclein (Figure 3A3) was easily distinguishable from that of the normative pattern of innervations by alpha-synuclein (Figure 3A1,A2). Qualitative comparison of the morphology of the swollen alpha-synuclein fibers innervating the non-ganglionic regions adjacent to myenteric ganglia (Figures 2B-E, 3A3) with that of swollen fibers originating from intrinsic neurons (i.e., calretinin and calbindin; Figures 3B,C) and extrinsic sources (i.e., TH; Figure 3D) indicated that the axonopathies shared more similarities with intrinsic dystrophic axons than with extrinsic axons.
Cholinergic neurons (i.e., neurons expressing either calbindin or calretinin) and nitrergic neurons (expressing NOS) are the two non-overlapping chemical phenotypes that collectively account for almost the entire population of neurons in the myenteric plexus, and a subpopulation of each phenotype is co-reactive for alpha-synuclein (Phillips et al., 2008). In the present aged series, instances in which the markedly swollen axons positive for either calretinin (Figure 4), calbindin, or NOS that were co-reactive for alpha-synuclein were observed. In whole mounts of the small and large intestines, calretinin-positive dystrophic axons were frequently positive for alpha-synuclein, whereas the calbindin-positive and NOS-positive processes were less likely to be co-reactive for alpha-synuclein. For both cholinergic subtypes and NOS neurons, though, we observed markedly swollen axons that were negative for alpha-synuclein (Figure 3B,C).
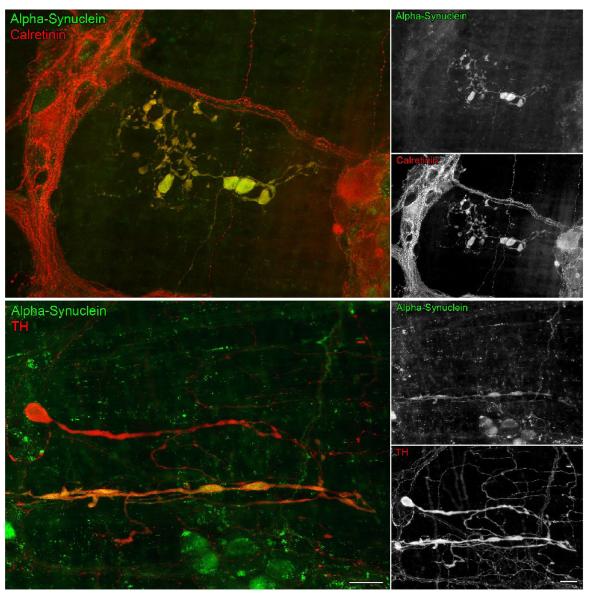
Figure 4.
In aged rats, there were markedly swollen axons positive for alpha-synuclein (green) that were co-reactive (yellow) for the calcium binding protein calretinin (red), or in different cases, co-reactive (yellow) for tyrosine hydroxylase (TH; red). In the bottom panel, of particular importance, is the occurrence of a markedly swollen axon that is TH-positive and alpha-synuclein-negative (red) running parallel and near a swollen fiber that is co-reactive for TH and alpha-synuclein (yellow). Focus stacking (2 merged images) was used in the images showing double labeling for alpha-synuclein and TH. Scale bars = 25 μm in the bottom color panel (applies to both color panels); 25 μm in the bottom B&W panel (applies to all B&W panels).
The sympathetic innervation of the myenteric plexus, selectively visualized using an antibody to TH, revealed a very pronounced age-related axonopathy consisting of markedly swollen TH-positive axons located throughout the GI tract of aged rats. Although rare, some swollen sympathetic axons were positive for alpha-synuclein (Figure 4), however the majority were negative for alpha-synuclein (Figure 3D). Age-related swellings and dystrophic features were seldom seen in calcitonin gene-related peptide-positive neurons (CGRP; a marker for extrinsic primary visceral afferent innervation of the myenteric plexus), and the infrequent axonopathies of CGRP neurites were not co-reactive for alpha-synuclein. (Figure 3E).
Hyperphosphorylated Tau Aggregates and Dystrophic Alpha-Synuclein Axons and Terminals
Hyperphosphorylated tau phospho-specific Ser262-positive aggregates were extremely rare within the myenteric plexus of adult rats, but present throughout the plexus of aged rats. Aging-associated tau phospho-specific Ser262-positive aggregates were particularly common in the proximal small intestine; an observation verified in the two aged rats whose entire small intestine was labeled for tau phospho-specific Ser262.
In the aged animals, hyperphosphorylated tau aggregates occurred within the ganglia and the connectives of the myenteric plexus (Figure 5), but were not observed in the interganglionic regions adjacent to ganglia. Tau phospho-specific Ser262 aggregates varied in size, ranging from small granules to large chunks that were similar in diameter to myenteric neurons (Figure 5). Unlike alpha-synuclein, hyperphosphorylated tau aggregates did not fill the entire processes of swollen axons and varicosities, but instead were localized to discrete regions of these dystrophic axons. We observed heteroplastic alpha-synuclein-positive axons with numerous engorged varicosities along their length and with every swelling containing aggregated hyperphosphorylated tau; whereas, others axons were observed to have swollen varicosities along their length, with some of those varicosities being positive and some negative for the presence of hyperphosphorylated tau aggregates. Similarly, tau phospho-specific Ser262 aggregates that were not co-localized within alpha-synuclein inclusions were frequently observed within ganglia and fiber tracts.
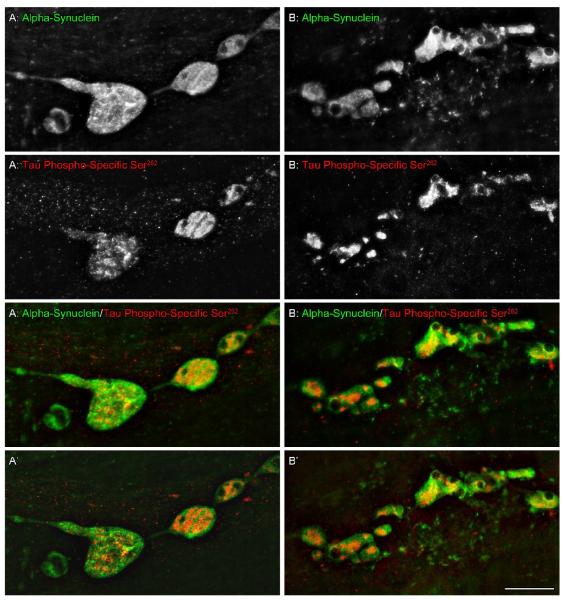
Figure 5.
In aged rats, intestinal whole mounts double immunostained for alpha-synuclein (green) and tau phospho-specific Ser262 (red) revealed tau phospho-specific Ser262-positive aggregates within some of the markedly swollen alpha-synuclein-positive axons and varicosities; confocal images deconvoluted (nearest neighbors function) and merged to create all-in-focus projections of 26 (A) and 14 (B) single optical sections. Two notable features of the co-localization of the two proteins were that 1) hyperphosphorylated tau occurred internally or centrally within more extensive masses or aggregates of alpha-synuclein, not around the peripheries of the alpha-synuclein aggregates, and 2) hyperphosphorylated tau appeared not so much to mix or combine with alpha-synuclein, as to precipitate as concentrated deposits surrounded by alpha synuclein material. Single optical sections are shown in A’ and B’ which were taken from the center of each respective stack illustrating minimal color mixing. Scale bar = 20 μm.
Tau phospho-specific Ser262-positive deposits localized within the swollen varicosities of dystrophic alpha-synuclein axons were surrounded by extensive aggregations of alpha-synuclein (Figure 5A-D). When the two proteins were co-localized in the same swollen varicosities, serial confocal reconstructions of varicosities double labeled for tau phospho-specific Ser262 and alpha-synuclein revealed that the hyperphosphorylated tau formed internal deposits within these enlarged regions with alpha-synuclein forming the periphery or outer “shell.” Additionally, examination of single optical sections, 0.25 μm apart in the z-axis, revealed that the two proteins were not intermingled, but rather that the alpha-synulcein-positive swellings formed chambers or pockets within which tau phospho-specific Ser262 aggregates where sequestered.
Distribution of Alpha-Synuclein and Tau Phospho-Specific Ser262 throughout the GI Tract
In the aged rats, alpha synuclein axonopathies were observed in the stomach, duodenum, jejunum, ileum and colon (Figure 2). Deposits of tau phospho-specific Ser262 were also observed in both ganglia and connectives of the myenteric plexus throughout the GI tract of aged rats, but the relative frequencies of tau phospho-specific Ser262-positive aggregates did not completely parallel that of alpha-synuclein: In the two aged rats whose entire intestines were stained for tau phospho-specific Ser262, the greatest density of hyperphosphorylated tau aggregates occurred in the proximal 14 cm of the small intestine. The density then decreased in samples nearer the ileum. Then, still more caudally, the density increased somewhat in the distal colon.
Discussion
The present experiment was designed to: 1) assess whether alpha-synuclein (and also, in a subset of whole mounts, tau phospho-specific Ser262) aggregation occurs in the axonal swellings and heteroplastic profiles that characterize aging of the enteric and autonomic innervation of the gut, 2) identify which of the common neuronal chemical phenotypes comprising the circuitry of the GI tract form aggregates of alpha-synuclein, and 3) determine whether the observed alpha-synuclein aggregates are widely distributed throughout the GI tract or confined to a limited region of the gut. The results can be considered according to the three issues.
In addition to shedding some light on how the nervous system of the GI tract normally ages, the experimental observations were also designed to generate predictions as to how neuronal changes during normal aging might evolve into pathological aging processes or disease. Such implications are discussed below, after the basic pattern of aging in healthy F344 rats is considered.
Alpha-synuclein aggregates occur in dystrophic enteric and autonomic axons in the aging GI tract
A key finding of the present experiment is that aggregates of alpha-synuclein appear regularly in axonal swellings and distorted terminal fields located within the myenteric ganglia, the fiber tracts connecting the ganglia, and the non-ganglionic regions adjacent to the ganglia throughout the GI tract with aging. These aggregates observed in the myenteric plexus of middle-aged and aged rats were masses consisting of darkly stained alpha-synuclein forming immunoreactive halos surrounding unstained or lightly stained inner cores (e.g., Figures 2 and 5). Such profiles are comparable to the classical CNS (Bennett, 2005; Chua and Tang, 2006; Wakabayashi et al., 2007) and ENS (Cersosimo and Benarroch, 2008; Natale et al., 2008; Wakabayashi et al., 1989, 1990) aggregates of Lewy body material present in human patients diagnosed with Parkinson’s disease: a halo, at the ultrastructural level, that consists of filamentous material forming fibrillary elements immunoreactive for alpha-synuclein, with a central core composed of a granular dense material that does not stain for alpha-synuclein. Thus, the alpha-synuclein aggregates that we observed in the myenteric plexus of aging rats are strikingly similar in appearance and content to CNS Lewy material that can occur in both somata, i.e., “Lewy bodies,” and neurites, i.e., “Lewy neurites.”
Another finding in the present results that bears on an understanding of alpha-synuclein aggregates in the myenteric plexus is that hyperphosphorylated (and presumably fibrillized) tau forms deposits within some alpha-synuclein inclusions. Similar co-localization of fibrils of alpha-synuclein with hyperphosphorylated tau has been reported in the CNS (Ishizawa et al., 2003; Muntane et al., 2008), and evidence suggests that under the right conditions the two proteins can reciprocally promote each other’s fibrillization (Frasier and Wolozin, 2004; Galpern and Lang, 2006; Goedert, 1999), with hyperphosphorylated tau enhancing the aggregation of alpha-synuclein (Giasson et al., 2003) and vice versa (Geddes, 2005). Thus, the tau phospho-specific Ser262-positive deposits within alpha-synuclein aggregates that we observed in the aging nervous system of the GI tract are reminiscent of tangles and protein aggregates seen in the CNS. At the same time, though, the association of alpha-synuclein and hyperphosphorylated tau was by no means complete. In many cases, alpha-synuclein aggregates occurred without stained tau phospho-specific Ser262-material; tau phospho-specific Ser262-positive deposits occurred in other sites that were not positive for alpha-synuclein; and the distribution of hyperphosphorylated tau in the aging gut was more limited and restricted (e.g. to the proximal intestine) than the distribution of alpha-synuclein. Similar dissociations are also common in the CNS (Galpern and Lang, 2006; Ishizawa et al., 2003). Furthermore, it is also known that different antibodies recognizing different species of phosphorylated tau and synuclein can yield different staining profiles (Andringa et al., 2003; Ferrer et al., 2002; Muntane et al., 2008; Yu et al., 2007) and, thus, could explain some of the absence--or low levels--of expression observed in the present series.
Regardless, though, the presence, in many cases, of the two aggregated proteins co-localized in the same dystrophic neurites in the aged animals in the present experiment strongly supports the assumption that there is impaired synaptic function and plasticity in the affected neurites (Muntane et al., 2008) that would most likely result in degeneration (Goedert, 1999). Finally, it is worth noting that oxidation directly stimulates tau and alpha-synuclein phosphorylation and fibrillization, and the presence of both in the aging gut is consistent with oxidative stress playing a significant role in age-related loss of myenteric neurons by impairing normal function and rendering neurons vulnerable to excitotoxicity and apoptosis (Thrasivoulou et al., 2006).
An aspect of the pattern of alpha-synuclein expression that merits emphasis is that not all swollen or dystrophic axonal features identified by the different phenotypic markers (see below) contained aggregates of the protein. This fact that neither all cholinergic (i.e., calretinin-positive or calbindein-postive) nor all nitrergic (i.e., NOS-positive) markedly swollen axons contained alpha-synuclein aggregates suggests two possible scenarios. First, synuclein-negative swellings and axonopathies may be produced by other proteins. Clearly numerous proteins other than alpha-synuclein are prone to misfolding, fibrillization, and aggregation during aging, so it could be that those dystrophic axons negative for alpha-synuclein had aggregates consisting of ubiquitin or tau (Wakabyashi et al., 2007) or other proteins. Second, and alternatively, a more complex scenario would be that there is a critical chronological or sequential relationship between an early occurrence of axonopathies or swellings that are first synuclein-negative and that only later accumulate aggregates of alpha-synuclein. Specifically, axons might at first become dystrophic and dilated, and aggregates of the protein might then slowly accumulate. And, of course, the two scenarios are not mutually exclusive.
Alpha-synuclein aggregates occur in multiple ENS and ANS phenotypes with aging
The use of double immunofluoro-labeled protocols in the present experiment to evaluate which specific chemical phenotypes in gut neural circuitry produce alpha-synuclein aggregates (and, conversely, which ones potentially did not) indicated that excess masses of the protein can be found in several phenotypically distinct subpopulations within the ENS and its extrinsic inputs. Specifically, alpha-synuclein aggregates were found in dystrophic calretinin-, calbindin-, NOS-, and TH-positive neurons. Without an unambiguous inference as to the role(s) alpha-synuclein plays in the axonopathies of aging in the GI tract, these patterns cannot be fully evaluated. They do, however, constrain certain inferences or models.
For example, it might reasonably be hypothesized that the swollen, dystrophic or heteroplastic axons that occur with aging and that develop aggregates of fibrillated alpha-synuclein are undergoing degeneration and would eventually progress to neurite dissolution and cell death. This hypothesis is not readily reconciled, however, with mounting evidence that age-related ENS cell loss is specific to cholinergic neurons (and that the complementary NOS population, which when combined with cholinergic neurons collectively account for practically 100% of the ENS neuron pool, is spared; Abelo et al., 2005; Phillips et al., 2003; Santer, 1994; Thrasivoulou et al., 2006) and with the double labeling patterns in the present experiment that demonstrated the presence of alpha-synuclein in both cholinergic and nitrergic phenotypes. Specifically, since cell loss in the ENS is limited to the cholinergic population of enteric neurons, a degeneration/cell death hypothesis would presumably predict the counterfactual that alpha-synuclein aggregates and axonopathies should not have been seen in the NOS-positive axons, but this was not the case. Therefore, the presence of alpha-synuclein aggregates appears not to be a good indicator of which neurons succumb, so it appears necessary to consider the more complicated alternative that instead of alpha-synuclein presence determining a neuron’s fate it is instead differences between neuronal subpopulations in their ability to clear abnormal accumulation of aggregated alpha-synuclein that determines which survives and which dies. Thus, in the myenteric plexus where both cholinergic and nitrergic myenteric neurons appear to amass similar amounts of damaged alpha-synuclein, it is something about the cholinergic population of neurons that makes them more vulnerable and prone to dysfunction and death in the presence of proteins modified by age while the nitrergic neurons are able to accommodate similar accumulations of damaged proteins and survive; possibly through more effective clearance of abnormal proteins (Mattson and Magnus, 2006).
A paucity or absence of alpha-synuclein expression and aggregates in other neuronal phenotypes is also instructive. In the present survey, CGRP was used as a marker for dorsal root afferents, and the peptide was only rarely seen co-localized with alpha-synuclein concentrations. Similarly, in other observations on vagal visceral afferents (identified by their terminal morphology), we have seen swollen neurites, but we have seen little evidence that vagal afferent terminals in the periphery express alpha-synuclein (Phillips et al., in press). More systematic observations on vagal afferents are still needed, but these initial results would suggest that in the neural circuitry of the gut, alpha synuclein is expressed in preganglionic efferents (vagal efferents--Phillips et al., 2008; sympathetic TH-positive efferents--present results) and some ENS postganglionic neurons, but not visceral afferents (Phillips et al., 2008, in press).
Alpha-synuclein aggregates occur throughout the GI tract with aging
The present results also provide information as to the distribution of alpha-synuclein aggregation along the longitudinal dimension of the GI tract. Paralleling, perhaps, one earlier observation that age-related neuronal loss occurs along the entire length of the GI tract (Phillips and Powley, 2001; Phillips et al., 2003), the present results indicate that aggregates of alpha-synuclein are also found along the entire rostrocaudal reach of the GI tract. In something of a contrast, hyperphosphorylated (presumably fibrillized) tau material was primarily expressed in the proximal small intestine, with only limited and sparse expression observed in the more distal gut.
Alpha-synuclein: mechanism of normal aging; protein of pathological aging
Deciding how, when, and why normal aging processes sometimes transition to pathological aging are major challenges in neurogerontology. Though far more research has been done on CNS--compared to ENS--aging, the issues remain unresolved, even in the CNS (Yankner et al., 2008). And certainly, in the ENS the issues are far from resolved. From the perspective of these questions, however, the present observations on aggregation and deposition of alpha-synuclein (and hyperphosphorylated tau) in the neural circuitry of the gut may provide not only a window on the processes of normal aging in the gut, but also a perspective on how such processes could be points of vulnerability and mechanisms of pathology that can eventually compromise the GI tract, especially in aged individuals.
With regard to this general issue, though, it is important to emphasize that the F344 strain of rats used in the present analysis was developed and has been maintained as a model of “normal” and “nonpathological” aging, and, in particular, the tissue examined in the present series was taken from rats that appeared (based on body weight and general appearance) to be healthy. Thus, the focal axonopathies, heteroplasias, and protein aggregations examined in the present experiment as well as the neuronal losses described for the ENS in earlier studies (Phillips and Powley, 2001; Phillips et al., 2003) on the aging F344 are presumably within the limits of the “normal” aging of the GI tract. Furthermore, in the case of alpha-synuclein aggregation, it needs to be remembered that the role(s) of the protein are still unclear and that some of its functional roles could be restorative (Halliday and McCann, 2008; Dachsel et al., 2007). It has also been suggested that aggregates of the protein at dystrophic axonal sites might reflect a reactive response mobilized to repair afflicted axons. Conversely, over-expression of the protein has been repeatedly shown to produce synucleinopathies and, in the CNS, Parkinson’s disease (Bennett, 2005; Chua and Tang, 2006; Goedert, 1999; Leroy et al., 2007).
Indeed, considering the present results in terms of the pathologies of Lewy body disease, and of Parkinson’s disease specifically, may yield an instructive example of how normal aging can evolve into pathological aging. And such a consideration may indicate the type of molecular pathways that account for the truism that aging is a major risk factor for the pathologies and diseases of old age.
Alpha-synuclein is widely distributed throughout the nerve networks of the gut (Bloch et al., 2006; Braak et al., 2007; Phillips et al., 2008). Such a conspicuous presence may reflect the need for neuronal plasticity or protective responses to the “wear and tear” (oxidative stress; immunological challenge; etc.) that the GI tract must continuously address (Cersosimo and Benarroch, 2008; Frasier and Wolozin, 2004; Hinault et al., 2006; Mattson et al., 1999; Vasina et al., 2006). Whatever the cause(s), it is particularly relevant that the prominent presence of the protein leaves the elements of the circuitry more vulnerable to developing “subclinical” focal synucleinopathies observed in normal aging (the present results). Amplification or exaggeration of such low-grade synucleinopathies by pathogens and disease-related events, though, might shift the aggregation of alpha synuclein from normal “subclinical” to pathological and “clinical” conditions (Andringa et al., 2003; Mattson and Magnus, 2006).
A case in point is Braak and colleagues’ (Braak et al., 2003, 2006; Del Tredici et al., 2002; Hawkes et al., 2007) hypothesis to account for progressive spread of Parkinson’s disease damage through the nervous system. On the basis of extensive analysis of post mortem material rated for the different stages of Parkinson’s disease, Braak and colleagues noted that the neuropathy appears to begin in the ENS, moves centrally by passing retrogradely in vagal efferents to the brainstem, and then distributes to vulnerable CNS sites. The investigators postulated that an as-yet-unidentified agent or pathogen affects or “infects” alpha-synuclein elements of the ENS, and that the resulting synucleinopathy then spreads progressively and centripetally through a network of alpha-synuclein-positive neurons to the CNS. In results that would be consistent with such a mechanisms, we demonstrated that vagal preganglionic axons strongly express alpha-synuclein and that at least some of these axons do form appositions with myenteric ganglion neurons that are also positive for the protein (Phillips et al., 2008). The observations of the present experiment indicate that this synuclein-positive network does, even in normal “healthy” aging, develop axonopathies and “subclinical” Lewy neurites. Additionally, of course, Braak and colleagues (Braak et al., 2003, 2006, 2007; Del Tredici et al., 2002) and others (Bloch et al., 2006; Cersosimo and Benarroch, 2008; Natale et al., 2008; Wakabayashi and Takahashi, 1997; Wakabayashi et al., 1989) have reported Lewy body material in the myenteric plexus of patients with Parkinson’s disease. Given the deposits of hyperphosphorylated tau and “shells” of alpha-synuclein we observed in the normal aging nervous system of the gut, and given the striking similarities of these proteinaceous masses to conventionally described Lewy bodies, it is easy to envision how axonopathies of aging might progress into pathological conditions such as Parkinson’s disease. In light of these different observations, it is reasonable to hypothesize that alpha-synuclein-positive elements of the enteric and autonomic innervation of the gut may well be susceptible to compromise or infection by pathogens that can convert normal aging into pathological aging associated with Parkinson’s disease and other synucleinopathies.
Acknowledgements
We thank Sarah Wilder for expert technical assistance. This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIH DK61317 and DK27627).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abalo R, Rivera A. Jose, Vera G, Martin M. Isabel. Ileal myenteric plexus in aged guinea-pigs: loss of structure and calretinin-immunoreactive neurones. Neurogastroenterol Motil. 2005;17:123–132. doi: 10.1111/j.1365-2982.2004.00612.x. [DOI] [PubMed] [Google Scholar]
- Adamczyk A, Solecka J, Strosznajder JB. Expression of alpha-synuclein in different brain parts of adult and aged rats. J Physiol Pharmacol. 2005;56:29–37. [PubMed] [Google Scholar]
- Agnati LF, Baldelli E, Andreoli N, Woods AS, Vellani V, Marcellino D, Guidolin D, Fuxe K. On the key role played by altered protein conformation in Parkinson’s disease. J Neural Transm. 2008;115:1285–1299. doi: 10.1007/s00702-008-0072-1. [DOI] [PubMed] [Google Scholar]
- Andringa G, Du F, Chase TN, Bennett MC. Mapping of rat brain using the Synuclein-1 monoclonal antibody reveals somatodendritic expression of alpha-synuclein in populations of neurons homologous to those vulnerable to Lewy body formation in human synucleopathies. J Neuropathol Exp Neurol. 2003;62:1060–1075. doi: 10.1093/jnen/62.10.1060. [DOI] [PubMed] [Google Scholar]
- Baker DM, Santer RM. A quantitative study of the effects of age on the noradrenergic innervation of Auerbach’s plexus in the rat. Mech Ageing Dev. 1988;42:147–158. doi: 10.1016/0047-6374(88)90070-x. [DOI] [PubMed] [Google Scholar]
- Bennett MC. The role of alpha-synuclein in neurodegenerative diseases. Pharmacol Ther. 2005;105:311–331. doi: 10.1016/j.pharmthera.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Steur E.N. Jansen, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol (Berl) 2007;113:421–429. doi: 10.1007/s00401-007-0193-x. [DOI] [PubMed] [Google Scholar]
- Brehmer A, Blaser B, Seitz G, Schrodl F, Neuhuber W. Pattern of lipofuscin pigmentation in nitrergic and non-nitrergic, neurofilament immunoreactive myenteric neuron types of human small intestine. Histochem Cell Biol. 2004;121:13–20. doi: 10.1007/s00418-003-0603-7. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Cowen T, Koch TR. Enteric neurodegeneration in ageing. Neurogastroenterol Motil. 2008;20:418–429. doi: 10.1111/j.1365-2982.2008.01134.x. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord. 2008;23:1065–1075. doi: 10.1002/mds.22051. [DOI] [PubMed] [Google Scholar]
- Chua CE, Tang BL. alpha-synuclein and Parkinson’s disease: the first roadblock. J Cell Mol Med. 2006;10:837–846. doi: 10.1111/j.1582-4934.2006.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Cowen T, Johnson RJ, Soubeyre V, Santer RM. Restricted diet rescues rat enteric motor neurones from age related cell death. Gut. 2000;47:653–660. doi: 10.1136/gut.47.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane SJ, Talley NJ. Chronic gastrointestinal symptoms in the elderly. Clin Geriatr Med. 2007;23:721–734. v. doi: 10.1016/j.cger.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Dachsel JC, Lincoln SJ, Gonzalez J, Ross OA, Dickson DW, Farrer MJ. The ups and downs of alpha-synuclein mRNA expression. Mov Disord. 2007;22:293–295. doi: 10.1002/mds.21223. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- Dev KK, Hofele K, Barbieri S, Buchman VL, van der Putten H. Part II: alpha-synuclein and its molecular pathophysiological role in neurodegenerative disease. Neuropharmacology. 2003;45:14–44. doi: 10.1016/s0028-3908(03)00140-0. [DOI] [PubMed] [Google Scholar]
- Espada J, Juarranz A, Galaz S, Canete M, Villanueva A, Pacheco M, Stockert JC. Non-aqueous permanent mounting for immunofluorescence microscopy. Histochem Cell Biol. 2005;123:329–334. doi: 10.1007/s00418-005-0769-2. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Barrachina M, Puig B. Anti-tau phospho-specific Ser262 antibody recognizes a variety of abnormal hyper-phosphorylated tau deposits in tauopathies including Pick bodies and argyrophilic grains. Acta Neuropathol. 2002;104:658–664. doi: 10.1007/s00401-002-0600-2. [DOI] [PubMed] [Google Scholar]
- Frasier M, Wolozin B. Following the leader: fibrillization of alpha-synuclein and tau. Exp Neurol. 2004;187:235–239. doi: 10.1016/j.expneurol.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Gabella G. Fall in the number of myenteric neurons in aging guinea pigs. Gastroenterology. 1989;96:1487–1493. doi: 10.1016/0016-5085(89)90516-7. [DOI] [PubMed] [Google Scholar]
- Galpern WR, Lang AE. Interface between tauopathies and synucleinopathies: a tale of two proteins. Ann Neurol. 2006;59:449–458. doi: 10.1002/ana.20819. [DOI] [PubMed] [Google Scholar]
- Geddes JW. alpha-Synuclein: a potent inducer of tau pathology. Exp Neurol. 2005;192:244–250. doi: 10.1016/j.expneurol.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, Trojanowski JQ, Lee VM. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300:636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- Goedert M. Filamentous nerve cell inclusions in neurodegenerative diseases: tauopathies and alpha-synucleinopathies. Philos Trans R Soc Lond B Biol Sci. 1999;354:1101–1118. doi: 10.1098/rstb.1999.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbins J, Gulbins R. Photographic multishot techniques : high dynamic range, super-resolution, extended depth of field, stitching. Rocky Nook; Santa Barbara, CA: 2009. [Google Scholar]
- Halliday GM, McCann H. Human-based studies on alpha-synuclein deposition and relationship to Parkinson’s disease symptoms. Exp Neurol. 2008;209:12–21. doi: 10.1016/j.expneurol.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays NP, Roberts SB. The anorexia of aging in humans. Physiol Behav. 2006;88:257–266. doi: 10.1016/j.physbeh.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Hinault MP, Ben-Zvi A, Goloubinoff P. Chaperones and proteases: cellular fold-controlling factors of proteins in neurodegenerative diseases and aging. J Mol Neurosci. 2006;30:249–265. doi: 10.1385/JMN:30:3:249. [DOI] [PubMed] [Google Scholar]
- Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Origins and effects of extracellular alpha-synuclein: implications in Parkinson’s disease. J Mol Neurosci. 2008;34:17–22. doi: 10.1007/s12031-007-0012-9. [DOI] [PubMed] [Google Scholar]
- Leroy K, Bretteville A, Schindowski K, Gilissen E, Authelet M, De Decker R, Yilmaz Z, Buee L, Brion JP. Early axonopathy preceding neurofibrillary tangles in mutant tau transgenic mice. Am J Pathol. 2007;171:976–992. doi: 10.2353/ajpath.2007.070345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Ann N Y Acad Sci. 1999;893:154–175. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- Miwa H, Kubo T, Suzuki A, Kondo T. Intragastric proteasome inhibition induces alpha-synuclein-immunopositive aggregations in neurons in the dorsal motor nucleus of the vagus in rats. Neurosci Lett. 2006;401:146–149. doi: 10.1016/j.neulet.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Morley JE. Constipation and irritable bowel syndrome in the elderly. Clin Geriatr Med. 2007;23:823–832. vi–vii. doi: 10.1016/j.cger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Muntane G, Dalfo E, Martinez A, Ferrer I. Phosphorylation of tau and alpha-synuclein in synaptic-enriched fractions of the frontal cortex in Alzheimer’s disease, and in Parkinson’s disease and related alpha-synucleinopathies. Neuroscience. 2008;152:913–923. doi: 10.1016/j.neuroscience.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Natale G, Pasquali L, Ruggieri S, Paparelli A, Fornai F. Parkinson’s disease and the gut: a well known clinical association in need of an effective cure and explanation. Neurogastroenterol Motil. 2008;20:741–749. doi: 10.1111/j.1365-2982.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- Newton JL. Changes in upper gastrointestinal physiology with age. Mech Ageing Dev. 2004;125:867–870. doi: 10.1016/j.mad.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Norton C. Constipation in older patients: effects on quality of life. Br J Nurs. 2006;15:188–192. doi: 10.12968/bjon.2006.15.4.20542. [DOI] [PubMed] [Google Scholar]
- O’Mahony D, O’Leary P, Quigley EM. Aging and intestinal motility: a review of factors that affect intestinal motility in the aged. Drugs Aging. 2002;19:515–527. doi: 10.2165/00002512-200219070-00005. [DOI] [PubMed] [Google Scholar]
- Perrin RJ, Payton JE, Barnett DH, Wraight CL, Woods WS, Ye L, George JM. Epitope mapping and specificity of the anti-alpha-synuclein monoclonal antibody Syn-1 in mouse brain and cultured cell lines. Neurosci Lett. 2003;349:133–135. doi: 10.1016/s0304-3940(03)00781-x. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods. 2004;133:99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci. 2003;106:69–83. doi: 10.1016/S1566-0702(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Pairitz JC, Powley TL. Age-related neuronal loss in the submucosal plexus of the colon of Fischer 344 rats. Neurobiol Aging. 2007;28:1124–1137. doi: 10.1016/j.neurobiolaging.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. As the gut ages: timetables for aging of innervation vary by organ in the Fischer 344 rat. J Comp Neurol. 2001;434:358–377. doi: 10.1002/cne.1182. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1–19. doi: 10.1016/j.autneu.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Rhodes BS, Powley TL. Effects of age on sympathetic innervation of the myenteric plexus and gastrointestinal smooth muscle of Fischer 344 rats. Anat Embryol (Berl) 2006;211:673–683. doi: 10.1007/s00429-006-0123-z. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Walter GC, Powley TL. Age-related changes in vagal afferents innervating the gastrointestinal tract. Auton Neurosci. 2009 doi: 10.1016/j.autneu.2009.07.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Walter GC, Wilder SL, Baronowsky EA, Powley TL. Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: Autonomic pathway implicated in Parkinson’s disease? Neuroscience. 2008;153:733–750. doi: 10.1016/j.neuroscience.2008.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach M, Christie JA. Fecal incontinence in the elderly. Geriatrics. 2008;63:13–22. [PubMed] [Google Scholar]
- Saffrey MJ. Ageing of the enteric nervous system. Mech Ageing Dev. 2004;125:899–906. doi: 10.1016/j.mad.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Santer RM. Survival of the population of NADPH-diaphorase stained myenteric neurons in the small intestine of aged rats. J Auton Nerv Syst. 1994;49:115–121. doi: 10.1016/0165-1838(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Santer RM, Baker DM. Enteric neuron numbers and sizes in Auerbach’s plexus in the small and large intestine of adult and aged rats. J Auton Nerv Syst. 1988;25:59–67. doi: 10.1016/0165-1838(88)90008-2. [DOI] [PubMed] [Google Scholar]
- Thrasivoulou C, Soubeyre V, Ridha H, Giuliani D, Giaroni C, Michael GJ, Saffrey MJ, Cowen T. Reactive oxygen species, dietary restriction and neurotrophic factors in age-related loss of myenteric neurons. Aging Cell. 2006;5:247–257. doi: 10.1111/j.1474-9726.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- Trinh C, Prabhakar K. Diarrheal diseases in the elderly. Clin Geriatr Med. 2007;23:833–856. vii. doi: 10.1016/j.cger.2007.06.005. [DOI] [PubMed] [Google Scholar]
- van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, Lin S, Caroni P, Sommer B, Tolnay M, Bilbe G. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20:6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasina V, Barbara G, Talamonti L, Stanghellini V, Corinaldesi R, Tonini M, De Ponti F, De Giorgio R. Enteric neuroplasticity evoked by inflammation. Auton Neurosci. 2006:126–127. 264–272. doi: 10.1016/j.autneu.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Wade PR, Cowen T. Neurodegeneration: a key factor in the ageing gut. Neurogastroenterol Motil. 2004;16(Suppl 1):19–23. doi: 10.1111/j.1743-3150.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Lewy bodies in the enteric nervous system in Parkinson’s disease. Arch Histol Cytol. 1989;52(Suppl):191–194. doi: 10.1679/aohc.52.suppl_191. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H. Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol. 1997;38(Suppl 2):2–7. doi: 10.1159/000113469. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol (Berl) 1990;79:581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Tanji K, Mori F, Takahashi H. The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology. 2007;27:494–506. doi: 10.1111/j.1440-1789.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- Walter GC, Phillips RJ, Baronowsky EA, Powley TL. Versatile, high-resolution anterograde labeling of vagal efferent projections with dextran amines. J Neurosci Methods. 2009;178:1–9. doi: 10.1016/j.jneumeth.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- Yu S, Li X, Liu G, Han J, Zhang C, Li Y, Xu S, Liu C, Gao Y, Yang H, Ueda K, Chan P. Extensive nuclear localization of alpha-synuclein in normal rat brain neurons revealed by a novel monoclonal antibody. Neuroscience. 2007;145:539–555. doi: 10.1016/j.neuroscience.2006.12.028. [DOI] [PubMed] [Google Scholar]