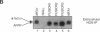Abstract
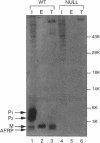
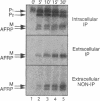
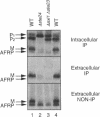
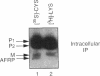
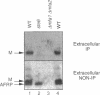
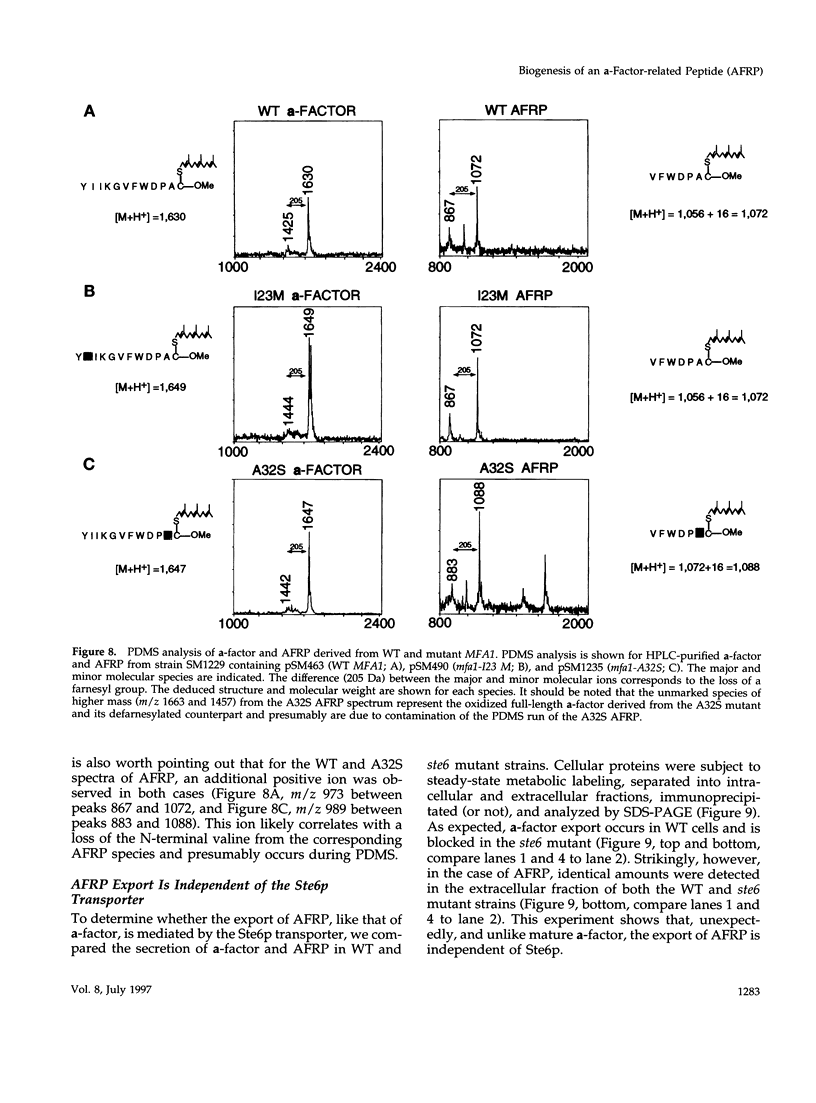
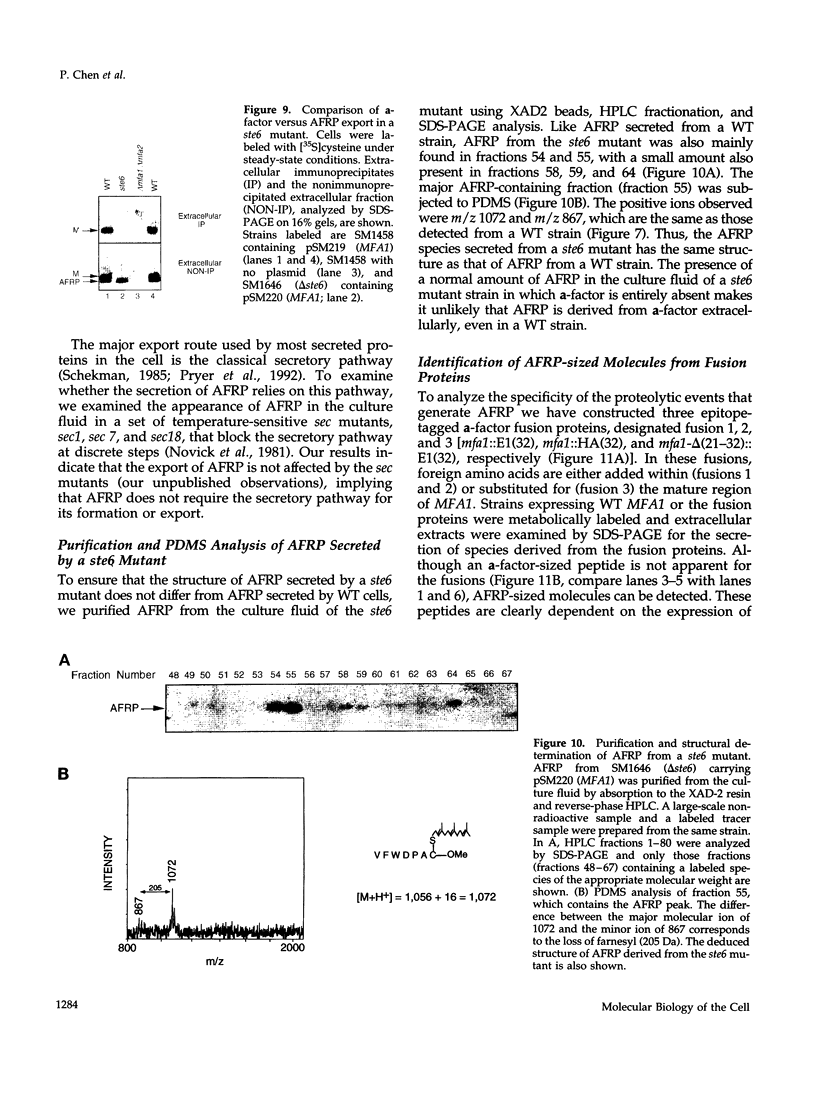
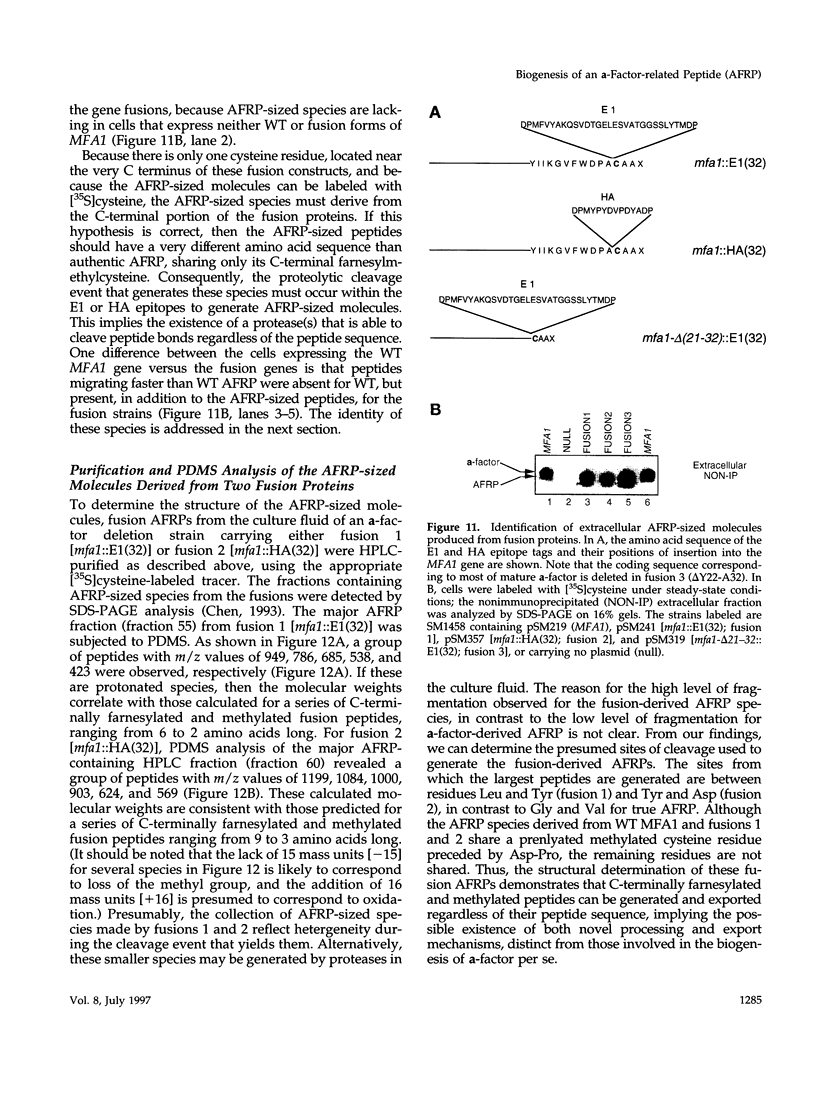
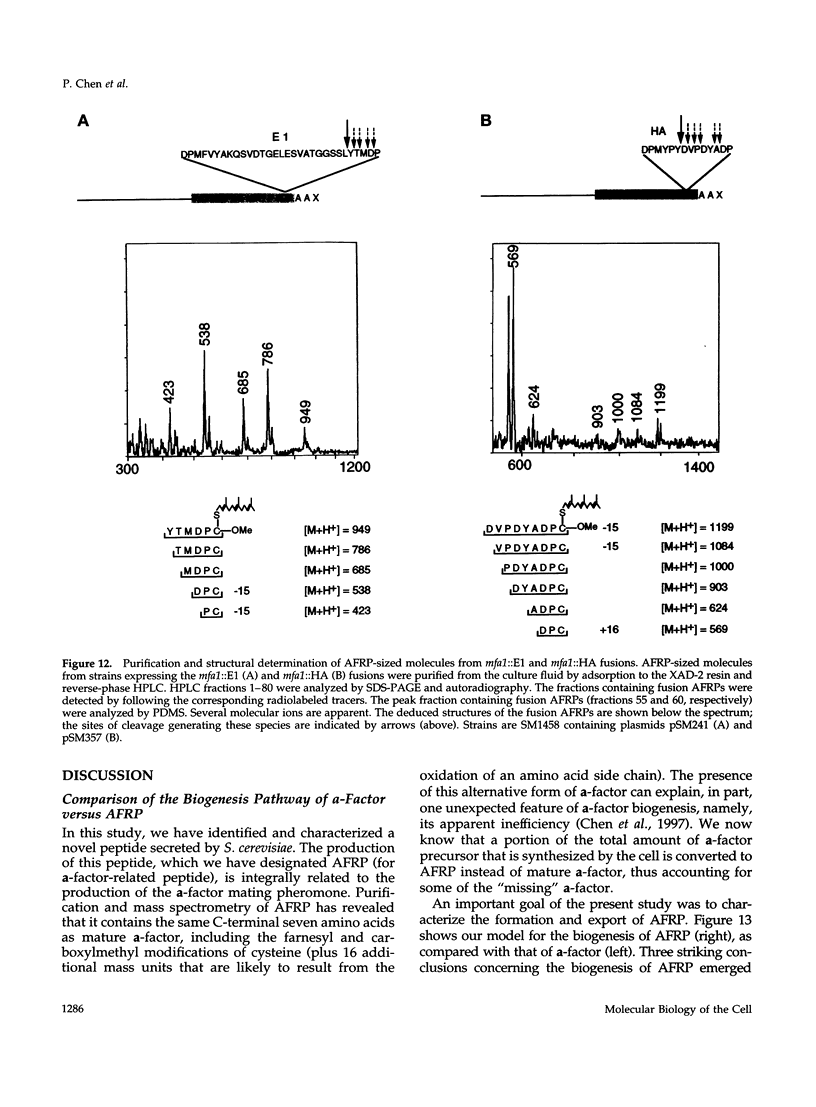
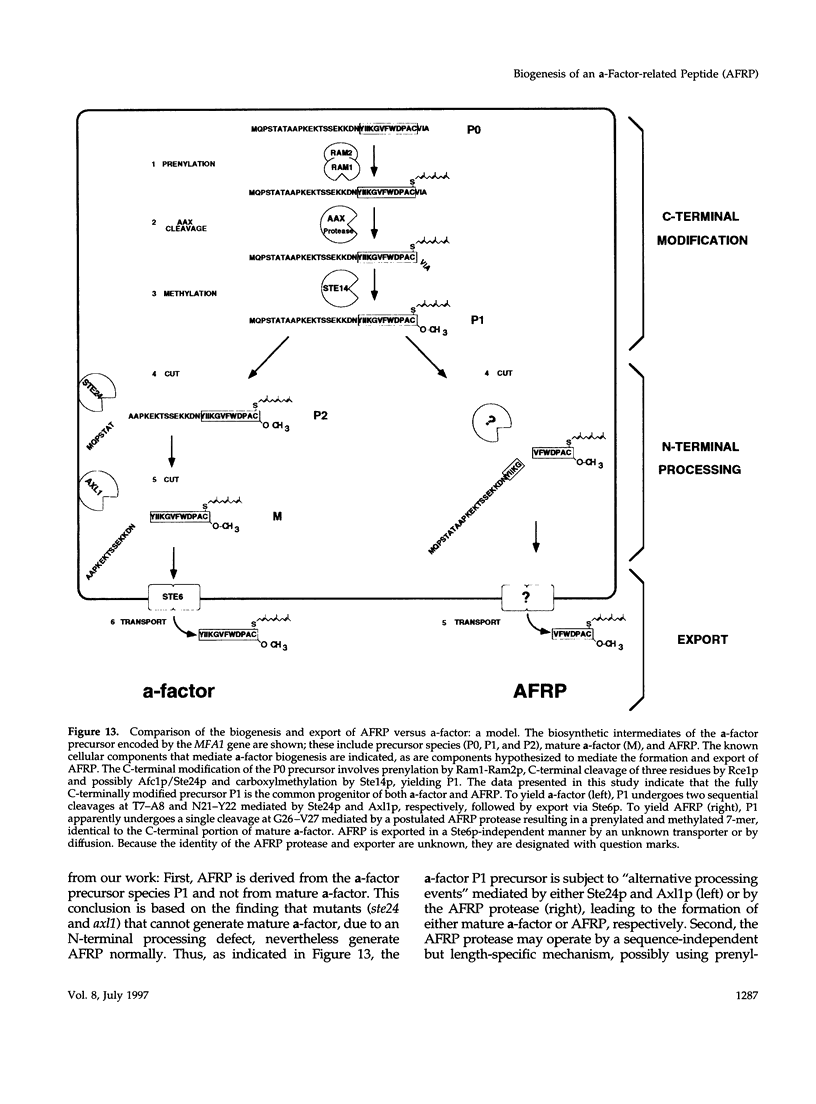
Many secreted signaling molecules are synthesized as precursors that undergo multiple maturation steps to generate their mature forms. The Saccharomyces cerevisiae mating pheromone a-factor is a C-terminally isoprenylated and carboxylmethylated dodecapeptide that is initially synthesized as a larger precursor containing 36 or 38 amino acids. We have previously shown that the maturation of a-factor occurs by an ordered biogenesis pathway involving 1) three C-terminal modification steps, 2) two N-terminal proteolytic processing events, and 3) a nonclassical export mechanism mediated by the ATP-binding-cassette (ABC) transporter Ste6p. In the present study, we demonstrate that an unexpected and abundant a-factor-related peptide (AFRP) exists in the culture fluid of MATa cells and that its biogenesis is integrally related to that of mature a-factor itself. We show by purification followed by mass spectrometry that AFRP corresponds to the C-terminal 7 amino acids (VFWDPAC) of mature a-factor (YIIKGVFWDPAC), including both the farnesyl- and carboxylmethylcysteine modifications. The formation and export of AFRP displays three striking features. First, we show that AFRP is produced intracellularly and that mutants (ste24 and axl1) that cannot produce mature a-factor due to an N-terminal processing defect are nevertheless normal for AFRP production. Thus, AFRP is not derived from mature a-factor but, instead, from the P1 form of the a-factor precursor. Second, fusion constructs with foreign amino acids substituted for authentic a-factor residues still yield AFRP-sized molecules; however, the composition of these corresponds to the altered residues instead of to AFRP residues. Thus, AFRP may be generated by a sequence-dependent but length-specific proteolytic activity. Third, a-factor and AFRP use distinct cellular machinery for their secretion. Whereas a-factor export is Ste6p-dependent, AFRP is secreted normally even in a ste6 deletion mutant. Thus, AFRP may exit the cell by another ATP-binding-cassette transporter, a different type of transporter altogether, or possibly by diffusion. Taken together, these studies indicate that the biogenesis of AFRP involves novel mechanisms and machinery, distinct from those used to generate mature a-factor. Because AFRP neither stimulates nor inhibits mating or a-factor halo activity, its function remains an intriguing question.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adames N., Blundell K., Ashby M. N., Boone C. Role of yeast insulin-degrading enzyme homologs in propheromone processing and bud site selection. Science. 1995 Oct 20;270(5235):464–467. doi: 10.1126/science.270.5235.464. [DOI] [PubMed] [Google Scholar]
- Anderegg R. J., Betz R., Carr S. A., Crabb J. W., Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988 Dec 5;263(34):18236–18240. [PubMed] [Google Scholar]
- Ashby M. N., King D. S., Rine J. Endoproteolytic processing of a farnesylated peptide in vitro. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4613–4617. doi: 10.1073/pnas.89.10.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkower C., Michaelis S. Effects of nucleotide binding fold mutations on STE6, a yeast ABC protein. Soc Gen Physiol Ser. 1993;48:129–146. [PubMed] [Google Scholar]
- Betz R., Crabb J. W., Meyer H. E., Wittig R., Duntze W. Amino acid sequences of a-factor mating peptides from Saccharomyces cerevisiae. J Biol Chem. 1987 Jan 15;262(2):546–548. [PubMed] [Google Scholar]
- Boyartchuk V. L., Ashby M. N., Rine J. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science. 1997 Mar 21;275(5307):1796–1800. doi: 10.1126/science.275.5307.1796. [DOI] [PubMed] [Google Scholar]
- Brizzio V., Gammie A. E., Nijbroek G., Michaelis S., Rose M. D. Cell fusion during yeast mating requires high levels of a-factor mating pheromone. J Cell Biol. 1996 Dec;135(6 Pt 2):1727–1739. doi: 10.1083/jcb.135.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell G. A., Wang S. H., Xue C. B., Jiang Y., Lu H. F., Naider F., Becker J. M. Molecular determinants of bioactivity of the Saccharomyces cerevisiae lipopeptide mating pheromone. J Biol Chem. 1994 Aug 5;269(31):19817–19825. [PubMed] [Google Scholar]
- Chen P., Sapperstein S. K., Choi J. D., Michaelis S. Biogenesis of the Saccharomyces cerevisiae mating pheromone a-factor. J Cell Biol. 1997 Jan 27;136(2):251–269. doi: 10.1083/jcb.136.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- Cleves A. E., Cooper D. N., Barondes S. H., Kelly R. B. A new pathway for protein export in Saccharomyces cerevisiae. J Cell Biol. 1996 Jun;133(5):1017–1026. doi: 10.1083/jcb.133.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura-Kamada K., Nouvet F. J., Michaelis S. A novel membrane-associated metalloprotease, Ste24p, is required for the first step of NH2-terminal processing of the yeast a-factor precursor. J Cell Biol. 1997 Jan 27;136(2):271–285. doi: 10.1083/jcb.136.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- He B., Chen P., Chen S. Y., Vancura K. L., Michaelis S., Powers S. RAM2, an essential gene of yeast, and RAM1 encode the two polypeptide components of the farnesyltransferase that prenylates a-factor and Ras proteins. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11373–11377. doi: 10.1073/pnas.88.24.11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekes H., Nigg E. A. The role of isoprenylation in membrane attachment of nuclear lamins. A single point mutation prevents proteolytic cleavage of the lamin A precursor and confers membrane binding properties. J Cell Sci. 1994 Apr;107(Pt 4):1019–1029. doi: 10.1242/jcs.107.4.1019. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M., Ellison M. J., Chau V., Varshavsky A. The short-lived MAT alpha 2 transcriptional regulator is ubiquitinated in vivo. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4606–4610. doi: 10.1073/pnas.88.11.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Opin Cell Biol. 1995 Apr;7(2):215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- Hrycyna C. A., Clarke S. Maturation of isoprenylated proteins in Saccharomyces cerevisiae. Multiple activities catalyze the cleavage of the three carboxyl-terminal amino acids from farnesylated substrates in vitro. J Biol Chem. 1992 May 25;267(15):10457–10464. [PubMed] [Google Scholar]
- Hrycyna C. A., Sapperstein S. K., Clarke S., Michaelis S. The Saccharomyces cerevisiae STE14 gene encodes a methyltransferase that mediates C-terminal methylation of a-factor and RAS proteins. EMBO J. 1991 Jul;10(7):1699–1709. doi: 10.1002/j.1460-2075.1991.tb07694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralli A., Bohen S. P., Yamamoto K. R. LEM1, an ATP-binding-cassette transporter, selectively modulates the biological potency of steroid hormones. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4701–4705. doi: 10.1073/pnas.92.10.4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchler K., Sterne R. E., Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 1989 Dec 20;8(13):3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Macfarlane R. D. 252Californium plasma desorption mass spectrometry. Biomed Mass Spectrom. 1981 Sep;8(9):449–453. doi: 10.1002/bms.1200080918. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. A specific transmembrane domain of a coronavirus E1 glycoprotein is required for its retention in the Golgi region. J Cell Biol. 1987 Sep;105(3):1205–1214. doi: 10.1083/jcb.105.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Herskowitz I. The a-factor pheromone of Saccharomyces cerevisiae is essential for mating. Mol Cell Biol. 1988 Mar;8(3):1309–1318. doi: 10.1128/mcb.8.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S. STE6, the yeast a-factor transporter. Semin Cell Biol. 1993 Feb;4(1):17–27. doi: 10.1006/scel.1993.1003. [DOI] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Parham P. Antigen presentation. Flying the first class flag. Nature. 1992 May 21;357(6375):193–194. doi: 10.1038/357193a0. [DOI] [PubMed] [Google Scholar]
- Powers S., Michaelis S., Broek D., Santa Anna S., Field J., Herskowitz I., Wigler M. RAM, a gene of yeast required for a functional modification of RAS proteins and for production of mating pheromone a-factor. Cell. 1986 Nov 7;47(3):413–422. doi: 10.1016/0092-8674(86)90598-2. [DOI] [PubMed] [Google Scholar]
- Pryer N. K., Wuestehube L. J., Schekman R. Vesicle-mediated protein sorting. Annu Rev Biochem. 1992;61:471–516. doi: 10.1146/annurev.bi.61.070192.002351. [DOI] [PubMed] [Google Scholar]
- Resnick N., Zasloff M. A. Novel proteases with unusual specificities. Curr Opin Cell Biol. 1992 Dec;4(6):1032–1036. doi: 10.1016/0955-0674(92)90136-z. [DOI] [PubMed] [Google Scholar]
- Sapperstein S., Berkower C., Michaelis S. Nucleotide sequence of the yeast STE14 gene, which encodes farnesylcysteine carboxyl methyltransferase, and demonstration of its essential role in a-factor export. Mol Cell Biol. 1994 Feb;14(2):1438–1449. doi: 10.1128/mcb.14.2.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R. Protein localization and membrane traffic in yeast. Annu Rev Cell Biol. 1985;1:115–143. doi: 10.1146/annurev.cb.01.110185.000555. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia S. S. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazdis J. R., MacKay V. L. Reproducible and rapid methods for the isolation and assay of a-factor, a yeast mating hormone. J Bacteriol. 1982 Sep;151(3):1153–1161. doi: 10.1128/jb.151.3.1153-1161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Traub P. Maturation of nuclear lamin A involves a specific carboxy-terminal trimming, which removes the polyisoprenylation site from the precursor; implications for the structure of the nuclear lamina. FEBS Lett. 1989 Nov 6;257(2):411–414. doi: 10.1016/0014-5793(89)81584-4. [DOI] [PubMed] [Google Scholar]
- Zhang F. L., Casey P. J. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]