Abstract
Aspergillus terreus was identified in an intra-dural spinal biopsy specimen from an African female with recurrent headache and hydrocephalus. Prior laboratory testing of cerebrospinal fluid (CSF) was non-diagnostic, despite extensive central nervous system (CNS) involvement. CNS Aspergillus infection presents a diagnostic and therapeutic challenge and is reviewed in the context of this particularly instructive and difficult case.
CASE PRESENTATION AND DISCUSSION
The patient was a 37 year old West African female who presented to our emergency department with headache, blurry vision, and numbness in the left lower extremity. Twelve months prior to admission, while still living in Africa, she underwent a cesarean section accompanied by epidural analgesia. Her post-partum course was notable for episodes of recurrent headache. She subsequently moved to the United States where her headaches continued.
The patient returned to Africa three months prior to the present admission. During that time she visited an emergency department with symptoms of severe headache, and a computed tomography (CT) scan demonstrated dilation of the lateral, third, and fourth cerebral ventricles. She returned to the United States and presented to our emergency department shortly thereafter.
The patient had an admission temperature of 37.4°C. Bilateral papilledema, mild oral thrush, and brisk deep tendon reflexes in the lower extremities were noted on physical examination. Brain magnetic resonance imaging (MRI) suggested communicating hydrocephalus (Figure 1A), although slight abnormal enhancement anterior to the pons and encasing the basilar artery was also noted (not shown).
Figure 1. Magnetic Resonance Imaging (MRI).
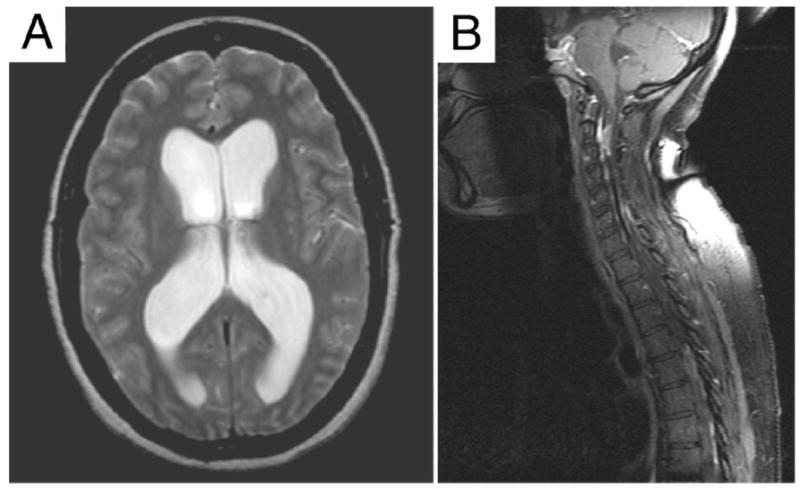
A. Lateral ventricular dilation is evident in this horizontal scan (Day 1). B. Mid-sagittal section from a spine MRI (Day 9) shows multiple signal abnormalities including intradural mass lesions and compression along the cervical and thoracic regions of the spinal cord.
Initial complete blood count (CBC), erythrocyte sedimentation rate (ESR), and serum chemistries were in the normal range. A lumbar puncture on Day 1 (Table 1) was notable for increased cerebrospinal fluid (CSF) red blood cells, nucleated cells, protein, albumin (48.1 mg/dl; nl <25), IgG (32.7 mg/dl; nl<5), and opening pressure. Subsequent CSF smears and cultures for bacteria, fungi, and acid fast bacilli were all negative, as was the cryptococcal antigen test. Viral cultures were negative, as were polymerase chain reaction (PCR)-based assays for herpes simplex virus (I and II), varicella zoster virus, cytomegalovirus, JC virus, and enterovirus. Additional negative CSF tests included the venereal disease research laboratory test (VDRL), treponemal antibody test (FTA-ABS), CSF-Lyme enzyme linked immunosorbent assay (ELISA), anti-toxoplasma enzyme immunoassay (EIA), and myelin-basic protein (<2 μg/L). While CSF oligoclonal banding was observed, no serum sample was obtained for comparison. Numerous tests for human immunodeficiency virus (HIV) and hepatitis A, B, and C were all negative. Serum angiotensin converting enzyme and calcium were both normal, and tuberculin skin testing was negative. A repeat lumbar puncture (Day 5) showed persistent hypercellularity but was read as negative for malignant cells by cytology. Cell counts, chemistries, and smears/cultures were otherwise unchanged.
Table 1.
Cerebrospinal Fluid (CSF) Findings.
| Day 1 | Day 5 | Day 48 | Day 120 | |
|---|---|---|---|---|
| RBC (/μl) | 136 | 39 | 488 | 70 |
| Nucleated cells (/μl) | 970 | 454 | 220 | 2 |
| Differential (total # of cells in count) | G92, L5, M3 (100) | G84, L12, M4 (100) | G38, L54, M3 (95) | G1, L3, M1 (5) |
| Protein (mg/dl) | 101 | 79 | 1090 | 5260 |
| Glucose (mg/dl) | 50 | 57 | 47 | 58 |
| Pressure (cm) | 40 | 36 | * | * |
Reference Ranges: RBC (none/μl), Nucleated Cells (<6/μl; G = granulocytes, L = lymphocytes, M = monocytes), Protein (15–45 mg/dl), Glucose (40–70 mg/dl), Pressure (<20 cm).
n.a. = not available.
Upon consultation with the neurosurgical team and consent of the patient, a ventriculoperitoneal (VP) shunt was placed on Day 7, although she declined a surgical biopsy of the area of abnormal brain MRI enhancement. Placement of the shunt was accompanied by significant symptomatic improvement. An MRI of the spine was obtained at discharge (Day 9) to assist with outpatient neurosurgical follow-up, and this imaging showed multiple abnormalities including ill-defined mass lesions along the spinal cord near C2, C5, and C6 levels and coating the superficial surface of the cord near T2 and T7-T10 (Figure 1B).
The patient returned to the emergency department (Day 27) with headache, nausea, photophobia, neck pain, and fevers. Her admission labs were unremarkable, with the exception of an elevated ESR (55 mm/hr; nl = 0–20). Interval worsening was noted on repeat spinal MRI (Day 30), and her symptoms rapidly progressed with diplopia and cranial nerve III palsy. She consented to a C5-T1 cervical laminectomy and tissue biopsy on Day 33. A grossly fibrous, yellow-white tissue with accompanying thick yellow fluid was noted during surgical exploration, and samples were sent for pathological and microbiological analysis.
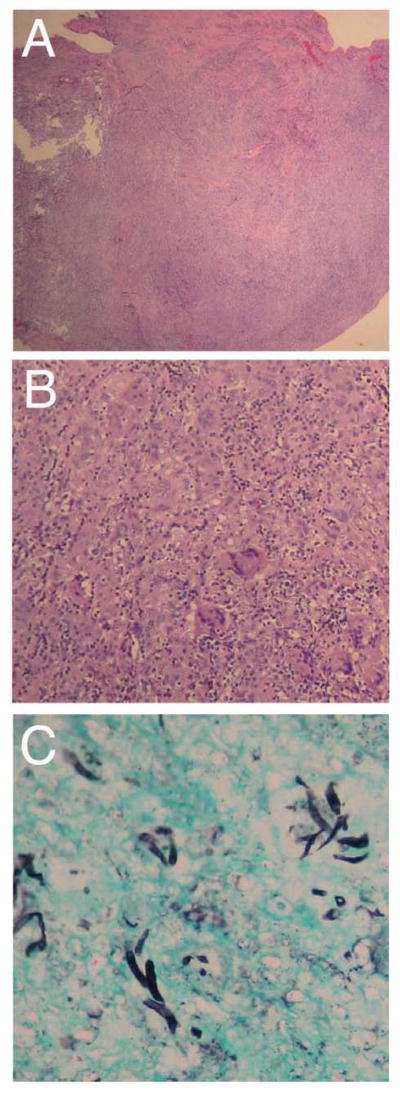
Microscopy revealed extensive tissue necrosis (Figure 2A), which was accompanied by acute and chronic inflammatory infiltration and multinucleate giant cells (Figure 2B). Grocott’s methenamine silver (GMS) staining for fungal elements demonstrated branched septate hyphae (Figure 2C). Additionally, a periodic acid Schiff (PAS) smear was positive for 1+ hyphae (not shown). The patient received one dose of IV amphotericin B (0.5 mg/kg) but was switched to oral voriconazole (200 mg BID) for improved CNS bioavailability.
Figure 2. Histology.
A. Low power image of the tissue biopsy showing a nodular fragment of fibroadipose tissue with chronic lymphohistiocytic infiltrate, necrosis, and fibrosis (hematoxylin and eosin, 20X). B. Higher power view of the same specimen demonstrating a dense granulomatous reaction with multiple foreign body-type giant cells (hematoxylin and eosin, 100x). C. GMS stain shows fungal hyphae with 45° branching (200x).
Over the next six days, fungal culture of the biopsy specimen revealed an initially white, velvet colony which progressed to a cinnamon brown color. Fungal smears demonstrated hyaline, septate hyphae and conidial morphology consistent with A. terreus. Susceptibility testing of the clinical isolate was performed by an outside reference laboratory (Table 2), as was the serum galactomannan assay (negative, 0.475 ratio). A CSF galactomannan assay was not ordered as clinically reportable reference ranges were not available. The patient also had a normal nitroblue tetrazolium test and a normal lymphocyte count and CD4:CD8 ratio.
Table 2.
Susceptibility Testing of the Aspergillus Terreus Isolate.
| MIC (μg/ml) | MLC (μg/ml) | |||
|---|---|---|---|---|
| 24 hrs | 48 hrs | 24 hrs | 48 hrs | |
| Amphotericin | 1 | 2 | 16 | >16 |
| Voriconazole | 0.25 | 0.5 | n.d. | n.d. |
MIC = minimal inhibitory concentration
MLC = minimal lethal concentration
n.d. = not determined
Fungal susceptibility determined according to the M38-A protocol (CLSI, Pennsylvania, 2002).
A repeat lumbar puncture (Day 48) revealed a markedly elevated CSF protein concentration (1,090 mg/dl) while other values remained largely unchanged (Table 1). The patient was discharged from the hospital on Day 64 after symptomatic improvement on a continued course of oral voriconazole (200 mg, BID). She was readmitted on Day 68, however, with worsening right upper quadrant abdominal pain attributed to cholelithiasis. After resolution of symptoms, she was discharged on Day 71. She was readmitted within a week, however, for recurrent abdominal pain, worsening gait, and a fixed and dilated left pupil. Interval worsening was again noted on MRI, and her voriconazole dose was increased to 300 mg BID; IV caspofungin (50 mg QD) was also initiated.
Subsequent lumbar puncture (Day 120) revealed a CSF voriconazole concentration of 1.822 mg/ml (performed by Mayo Medical Laboratories; Rochester, MN). A CSF protein concentration of 5,260 mg/dl was also noted, although CSF cellularity had resolved (Table 1). CSF protein concentration was verified using both benzethonium chloride and biuret reagent-based assays. CSF protein electrophoresis demonstrated a discrete abnormal band measuring 0.63 g/dl present in the gamma region; this was characterized as a monoclonal IgG lambda by immunofixation electrophoresis. An IgG lambda monoclonal component was also found in the serum but at lower concentration.
Brain MRI on Day 132 suggested interval worsening, therefore IV amphotericin-B (60 mg QD) was added to her antibiotic regimen. This was changed to IV amphotericin lipid complex (ABLC; 300 mg QD) six days later. The patient showed symptomatic improvement, although findings from a follow-up MRI (Day 167) were grossly unchanged. The patient was discharged on Day 182 for an additional week of outpatient IV caspofungin and ABLC, then a return to oral voriconazole (300 mg BID) on a compassionate use outpatient protocol. An outpatient MRI (Day 258) showed little overall change.
Aspergillus accounts for a small minority (~5%) of CNS fungal infections (Gottfredsson and Perfect, 2000), although it is associated with extremely high mortality rates approaching 86–99% (Walsh et al., 1985; Denning, 1996; Pongbhaesaj et al., 2004). As an opportunistic infection, disseminated aspergillosis usually begins with pulmonary involvement due to immunosuppression by steroids, antineoplastic agents, or with transplantation (Beal et al., 1982; Walsh et al., 1985; Barrios et al., 1988; Torre-Cisneros et al., 1993; Hori et al., 2002; Kleinschmidt-DeMasters, 2002; Saitoh et al., 2007). CNS aspergillosis has also been seen with diabetes (Torre-Cisneros et al., 1993; Nenoff et al., 2001; Figueiredo et al., 2003) or via contiguous spread from areas of nearby tissue or bone such as the paranasal sinuses (Haran and Chandy, 1993; Botturi et al., 2006; Sundaram et al., 2006). It should be noted that infection can occur in clinically immunocompetent individuals (Haran and Chandy, 1993; Sundaram et al., 2006).
CNS aspergillosis often presents with symptoms of altered mental status, a focal neurological deficit, seizure, persistent headache, or rarely meningeal signs (Gordon et al., 1976; Torre-Cisneros et al., 1993; Figueiredo et al., 2003; Kagawa et al., 2008). Both rapid and slow clinical progressions have been described (Gordon et al., 1976; Kaufman et al., 1976; Moling et al., 2002; Gunaratne et al., 2007; Azarpira et al., 2008).
Most studies demonstrate infection by A. fumigatus, although A. flavus, A. nidulans, A. niger, A. oryzae, A. terreus, and A. versicolor have also been observed (Gordon et al., 1976; Barrios et al., 1988; Gottfredsson and Perfect, 2000; Schwartz and Thiel, 2009). Detection of fungi in the CSF (by smear or culture) is often not successful in patients with fungal meningitis (McGinnis, 1983); indeed, many cases of CNS aspergillosis are only recognized on biopsy or autopsy specimens (Breneman and Colford, 1992; Mori and Ebe, 1992; Torre-Cisneros et al., 1993; Hori et al., 2002; Kleinschmidt-DeMasters, 2002; Sundaram et al., 2006). More rapid, non-culture-based assays for the diagnosis of CNS aspergillosis [using PCR or antigen (galactomannan) based methodologies] are desirable as they may facilitate earlier diagnosis and treatment (Kami et al., 1999; Moling et al., 2002; Viscoli et al., 2002; Klont et al., 2004; Hummel et al., 2006; Aquino et al., 2007). Finally, the value of neuroimaging in the initial workup of CNS aspergillosis cannot be overstated (Oner et al., 2006). In the present report, spinal MRI revealed a site of extensive disease burden much more amenable to biopsy and microscopic examination.
Pathologically, CNS aspergillosis manifests as abscess formation with a propensity for vascular invasion, infarction, hemorrhage, and/or aneurism (Walsh et al., 1985; Torre-Cisneros et al., 1993; Nenoff et al., 2001). Granuloma formation with necrosis is also commonly observed, and is most consistent with our patient’s findings (Kaufman et al., 1976; Kleinschmidt-DeMasters, 2002; Nadkarni and Goel, 2005). Many reports also describe isolated mass lesions, which are suspicious for neoplasm until a yellowish fluid is observed in the surgical field or fungal hyphae are detected with GMS staining (Kaufman et al., 1976; Diengdoh et al., 1983; Azarpira et al., 2008).
Of particular interest to our case, a 2005 outbreak of Aspergillus meningitis occurred in Sri Lanka when contaminated medical supplies were used in spinal anesthesia for cesarean section (Gunaratne et al., 2007). During this well-documented outbreak, five women were affected (mean incubation period of 11.2 days). Three ultimately died, and Aspergillus fumigatus was recovered from four of the cases, with spores identified by cytology in a fifth (Gunaratne et al., 2007; Rodrigo et al., 2007). The outbreak ended after bulk destruction of surgical supplies stored in a damp warehouse used after the 2004 tsunami. CNS aspergillosis has also been observed in patients after multiple epidural steroid injections (Saigal et al., 2004; Kolbe et al., 2007), although the source of Asperigillus (skin versus medical supply contamination) is usually unknown. Given the spinal predominance of infection in our patient, as well as the correlation in time with her onset of headache, it is extremely likely that the disease was a complication of her prior epidural analgesia.
Two prior reports of Aspergillus-associated spinal arachnoiditis present very similar laboratory and pathological findings to the present case. One notes granulomatous leptomeningitis with giant cell formation, spinal compression, CSF pleocytosis, markedly elevated CSF protein (with a peak concentration of 8,000 mg/dl), elevated CSF immunoglobulins, as well as the presence of oligoclonal banding (Van de Wyngaert et al., 1986). The other report showed communicating hydrocephalus, elevated CSF protein (517 mg/dl), and a very similar lesion appearance both on gross observation and by microscopy (Bryan et al., 1980). A definitive source for the Aspergillus infections were not identified in these patients, but the first was correlated in time to a cutaneous infection. Additional reports also show mild to severely elevated CSF protein concentrations in CNS aspergillosis (Gordon et al., 1976; Beal et al., 1982; Morrow et al., 1983; Walsh et al., 1985), although this finding does not seem to be common in all cases. A select literature review of cerebral aspergillosis cases over the past 25 years which presented with meningeal signs and/or CSF involvement is presented in Table 3.
Table 3.
Case Reports of CNS Aspergillosis with Evidence of Meningeal and/or CSF Involvement
| Authors | Summary | Clinical Presentation | Predisposing Conditions | Diagnostic Methodology | Antifungal Therapy * | Outcome | Organism |
|---|---|---|---|---|---|---|---|
| Van de Wygaert et al. (1986) | Case report | Skin abscess, headache, and meningeal signs | Healthy | (−) CSF cx (+) laminectomy bx, morphology of gelatinous leptomeninges (+) immunostaining (+) precipitins in CSF but not blood |
AmphoB 5-FC |
Survival (229 day post-onset follow-up) | A. fumigatus (immunostaining) |
| Breneman and Colford (1992) | Case report | Fever, dyspnea, headache, meningeal signs, progressive mental status changes | History of drug abuse, COPD, steroids | (+) sputum cx (+) brain bx and cx (−) CSF cx (+) CSF pleocytosis (4,100/mm3; 95% PMNs) |
AmphoB | Deceased | A. fumigatus |
| Nenoff et al. (2001) | Case report | Exophthalmos, ptosis, diplopia | Diabetes mellitus | (+) morphology and cx from prior extranasal ethmoidectomy and orbitotomy, direct extension into intracranium (+) CSF Aspergillus latex agglutination assay |
AmphoB Itraconazole 5-FC |
Deceased | A. fumigatus |
| Moling et al. (2002) | Case reports (x2) | Fever, headache, mental status changes Fever, mental status changes, nuchal rigidity |
Chronic alcohol abuse Complement 4 deficiency, glomerulonephritis, renal transplant, steroids |
(+) CSF Aspergillus latex agglutination assay (+) CSF cx with multiple prior (−) CSF cxs (+) CSF PCR (+) CSF sediment cx |
AmphoB Voriconazole Itraconazole Fluconazole L-AmphoB IT- L-AmphoB Itraconazole |
Survival (>1 yr follow-up) Survival (>11 mo follow-up) |
A. candidus A. fumigatus |
| Rodrigo et al. (2007); Gunaratne et al. (2007) | Case reports (x5) | Fever, headache, nuchal rigidity; progression to cranial nerve deficits, cerebral infarction, and hemorrhage | Spinal analgesia for cesarian secion | (−) CSF cx (x4) (+) CSF cx (x1) (+) cx post-mortem brain specimens (x3); (+) spores by cytology (x1) |
AmphoB (IV and/or IT) Voriconazole Fluconazole |
Deceased (x3) Survival (x2) (1 yr follow-up) |
A. fumigatus |
| Larson Kolbe et al. (2007) | Case report | Lower extremity weakness, urinary incontinence, low back pain | Discography and multiple epidural steroid injections | (+) cx disc aspiration and abscess | Caspofungin Voriconazole |
Deceased | A. fumigatus |
| Saitoh et al. (2007) | Case report | Fever, headache, nuchal rigidity | AML with granulocytic sarcoma, chemotherapy | (−) CSF cx (+) CSF galactomannan ELISA (+) CSF PCR |
AmphoB Voriconazole |
Survival (>1 yr follow-up) | Not isolated |
| Kagawa et al. (2008) | Case Report | Low grade fever, headache, hydrocephalus | Healthy | (+) morphology in fibrous tissue from VP shunt (−) CSF cultures |
AmphoB Fluconazole Shunt revisions and reconstruction | Survival (15 yrs post onset; 3 yrs post shunt reconstruction) | Aspergillus species |
List of antifungal agents does not imply concurrent therapy. Many patients were also administered prolonged broad spectrum antimicrobial therapy and/or anti-tubercular agents. Please consult the original manuscripts for detailed information regarding individual cases as well as dosing.
ABBREVIATIONS
bx = biopy
cx = culture
mo = month
yr = year
AmphoB = Amphotericin B
5-FC = 5-Fluorocytosine
IT = intrathecal
IV = intravenous
CSF = cerebrospinal fluid
ELISA = enzyme linked immunosorbent assay
PCR = polymerase chain reaction
PMN = polymorphonuclear cell
Voriconazole therapy has been associated with enhanced survival in CNS aspergillosis, likely due to its increased CNS penetration (Schwartz et al., 2005; Redmond et al., 2007; Saitoh et al., 2007; Schwartz and Thiel, 2009). Neurosurgical intervention also appears to have an additional survival benefit in many patients (Coleman et al., 1995). While the CNS concentration of voriconazole (1.822 mg/ml) in our patient exceeded the MIC of our clinical isolate (Table 2), MRI evidence suggested continued disease progression when voriconazole was used as the sole agent. Addition of IV caspofungin also did not appear to halt the course of infection. Adding a third agent, ABLC, did provide symptomatic benefit to our patient and halted the spread of disease, although radiographic evidence of disease persistence clearly remained. Poor CNS bioavailability is likely to limit ABLCs utility in many cases of CNS fungal infection (Schwartz and Thiel, 2009), although in this report it did appear beneficial.
In summary, we present a case of severe CNS Aspergillus terreus infection that developed after prior epidural analgesia. CSF testing proved non-diagnostic, although radiographic images of the spinal cord led to a surgical biopsy and diagnosis. Prolonged hospitalization and antibiotic therapy with three agents was required to halt the spread of disease, although it is likely that persistent infection remains. The patient was discharged on an additional outpatient course of IV ABLC and long-term oral voriconazole therapy.
Acknowledgments
Diagnostic testing was performed at the Yale University School of Medicine/Yale-New Haven Hospital, in the Departments of Laboratory Medicine and Pathology unless otherwise indicated. Particular gratitude is extended to the infectious disease service, as well as the clinical chemistry, immunology, microbiology, and virology laboratories for extensive testing during this prolonged hospitalization. Dr. Igor Latic provided assistance with radiographic images. JRG is supported by a grant from the National Institute of Health 2T32HL007974-05.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aquino VR, Goldani LZ, Pasqualotto AC. Update on the contribution of galactomannan for the diagnosis of invasive aspergillosis. Mycopathologia. 2007;163:191–202. doi: 10.1007/s11046-007-9010-2. [DOI] [PubMed] [Google Scholar]
- Azarpira N, Esfandiari M, Bagheri MH, Rakei S, Salari S. Cerebral aspergillosis presenting as a mass lesion. Braz J Infect Dis. 2008;12:349–351. doi: 10.1590/s1413-86702008000400019. [DOI] [PubMed] [Google Scholar]
- Barrios N, Tebbi CK, Rotstein C, Siddiqui S, Humbert JR. Brainstem invasion by Aspergillus fumigatus in a child with leukemia. N Y State J Med. 1988;88:656–658. [PubMed] [Google Scholar]
- Beal MF, O’Carroll CP, Kleinman GM, Grossman RI. Aspergillosis of the nervous system. Neurology. 1982;32:473–479. doi: 10.1212/wnl.32.5.473. [DOI] [PubMed] [Google Scholar]
- Botturi A, Salmaggi A, Pollo B, Lamperti E, Erbetta A, Boiardi A. Meningitis following relapsing painful ophthalmoplegia in aspergillus sphenoidal sinusitis: a case report. Neurol Sci. 2006;27:284–287. doi: 10.1007/s10072-006-0686-8. [DOI] [PubMed] [Google Scholar]
- Breneman E, Colford JM., Jr Aspergillosis of the CNS presenting as aseptic meningitis. Clin Infect Dis. 1992;15:737–738. doi: 10.1093/clind/15.4.737. [DOI] [PubMed] [Google Scholar]
- Bryan CS, DiSalvo AF, Huffman LJ, Kaplan W, Kaufman L. Communicating hydrocephalus caused by Aspergillus flavus. South Med J. 1980;73:1641–1644. doi: 10.1097/00007611-198012000-00032. [DOI] [PubMed] [Google Scholar]
- Coleman JM, Hogg GG, Rosenfeld JV, Waters KD. Invasive central nervous system aspergillosis: cure with liposomal amphotericin B, itraconazole, and radical surgery--case report and review of the literature. Neurosurgery. 1995;36:858–863. doi: 10.1227/00006123-199504000-00032. [DOI] [PubMed] [Google Scholar]
- Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- Diengdoh JV, Barnard RO, Thomas DG. Aspergillosis of the nervous system. Report of two cases. Neuropathol Appl Neurobiol. 1983;9:477–484. doi: 10.1111/j.1365-2990.1983.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Figueiredo EG, Fonoff E, Gomes M, Macedo E, Marino Junior R. Tumoral form of aspergillosis in central nervous system (cerebral aspergilloma): case report. Sao Paulo Med J. 2003;121:251–253. doi: 10.1590/S1516-31802003000600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MA, Holzman RS, Senter H, Lapa EW, Kupersmith MJ. Aspergillus oryzae meningitis. Jama. 1976;235:2122–2123. [PubMed] [Google Scholar]
- Gottfredsson M, Perfect JR. Fungal meningitis. Semin Neurol. 2000;20:307–322. doi: 10.1055/s-2000-9394. [DOI] [PubMed] [Google Scholar]
- Gunaratne PS, Wijeyaratne CN, Seneviratne HR. Aspergillus meningitis in Sri Lanka--a post-tsunami effect? N Engl J Med. 2007;356:754–756. doi: 10.1056/NEJMc062547. [DOI] [PubMed] [Google Scholar]
- Haran RP, Chandy MJ. Intracranial aspergillus granuloma. Br J Neurosurg. 1993;7:383–388. doi: 10.3109/02688699309103492. [DOI] [PubMed] [Google Scholar]
- Hori A, Kami M, Kishi Y, Machida U, Matsumura T, Kashima T. Clinical significance of extra-pulmonary involvement of invasive aspergillosis: a retrospective autopsy-based study of 107 patients. J Hosp Infect. 2002;50:175–182. doi: 10.1053/jhin.2001.1170. [DOI] [PubMed] [Google Scholar]
- Hummel M, Spiess B, Kentouche K, Niggemann S, Bohm C, Reuter S, Kiehl M, Morz H, Hehlmann R, Buchheidt D. Detection of Aspergillus DNA in cerebrospinal fluid from patients with cerebral aspergillosis by a nested PCR assay. J Clin Microbiol. 2006;44:3989–3993. doi: 10.1128/JCM.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa R, Okada Y, Moritake K. Fungal meningitic hydrocephalus with repeated shunt malfunction. Neurol Med Chir (Tokyo) 2008;48:43–46. doi: 10.2176/nmc.48.43. [DOI] [PubMed] [Google Scholar]
- Kami M, Ogawa S, Kanda Y, Tanaka Y, Machida U, Matsumura T, Sakamaki J, Hirai H. Early diagnosis of central nervous system aspergillosis using polymerase chain reaction, latex agglutination test, and enzyme-linked immunosorbent assay. Br J Haematol. 1999;106:536–537. doi: 10.1046/j.1365-2141.1999.01542.x. [DOI] [PubMed] [Google Scholar]
- Kaufman DM, Thal LJ, Farmer PM. Central nervous system aspergillosis in two young adults. Neurology. 1976;26:484–488. doi: 10.1212/wnl.26.5.484. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK. Central nervous system aspergillosis: a 20-year retrospective series. Hum Pathol. 2002;33:116–124. doi: 10.1053/hupa.2002.30186. [DOI] [PubMed] [Google Scholar]
- Klont RR, Mennink-Kersten MA, Verweij PE. Utility of Aspergillus antigen detection in specimens other than serum specimens. Clin Infect Dis. 2004;39:1467–1474. doi: 10.1086/425317. [DOI] [PubMed] [Google Scholar]
- Kolbe AB, McKinney AM, Kendi AT, Misselt D. Aspergillus meningitis and discitis from low-back procedures in an immunocompetent patient. Acta Radiol. 2007;48:687–689. doi: 10.1080/02841850701342153. [DOI] [PubMed] [Google Scholar]
- McGinnis MR. Detection of fungi in cerebrospinal fluid. Am J Med. 1983;75:129–138. doi: 10.1016/0002-9343(83)90084-0. [DOI] [PubMed] [Google Scholar]
- Moling O, Lass-Floerl C, Verweij PE, Porte M, Boiron P, Prugger M, Gebert U, Corradini R, Vedovelli C, Rimenti G, Mian P. Case Reports. Chronic and acute Aspergillus meningitis. Mycoses. 2002;45:504–511. doi: 10.1046/j.1439-0507.2002.00789.x. [DOI] [PubMed] [Google Scholar]
- Mori T, Ebe T. Analysis of cases of central nervous system fungal infections reported in Japan between January 1979 and June 1989. Intern Med. 1992;31:174–179. doi: 10.2169/internalmedicine.31.174. [DOI] [PubMed] [Google Scholar]
- Morrow R, Wong B, Finkelstein WE, Sternberg SS, Armstrong D. Aspergillosis of the cerebral ventricles in a heroin abuser. Case report and review of the literature. Arch Intern Med. 1983;143:161–164. [PubMed] [Google Scholar]
- Nadkarni T, Goel A. Aspergilloma of the brain: an overview. J Postgrad Med. 2005;51(Suppl 1):S37–41. [PubMed] [Google Scholar]
- Nenoff P, Kellermann S, Horn LC, Keiner S, Bootz F, Schneider S, Haustein UF. Case report. Mycotic arteritis due to Aspergillus fumigatus in a diabetic with retrobulbar aspergillosis and mycotic meningitis. Mycoses. 2001;44:407–414. doi: 10.1046/j.1439-0507.2001.00687.x. [DOI] [PubMed] [Google Scholar]
- Oner AY, Celik H, Akpek S, Tokgoz N. Central nervous system aspergillosis: magnetic resonance imaging, diffusion-weighted imaging, and magnetic resonance spectroscopy features. Acta Radiol. 2006;47:408–412. doi: 10.1080/02841850600580325. [DOI] [PubMed] [Google Scholar]
- Pongbhaesaj P, Dejthevaporn C, Tunlayadechanont S, Witoonpanich R, Sungkanuparph S, Vibhagool A. Aspergillosis of the central nervous system: a catastrophic opportunistic infection. Southeast Asian J Trop Med Public Health. 2004;35:119–125. [PubMed] [Google Scholar]
- Redmond A, Dancer C, Woods ML. Fungal infections of the central nervous system: A review of fungal pathogens and treatment. Neurol India. 2007;55:251–259. doi: 10.4103/0028-3886.35686. [DOI] [PubMed] [Google Scholar]
- Rodrigo N, Perera KN, Ranwala R, Jayasinghe S, Warnakulasuriya A, Hapuarachchi S. Aspergillus meningitis following spinal anaesthesia for caesarean section in Colombo, Sri Lanka. Int J Obstet Anesth. 2007;16:256–260. doi: 10.1016/j.ijoa.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Saigal G, Donovan Post M, Kozic D. Thoracic intradural aspergillus abscess formation following epidural steroid injection. Am J Neuroradiol. 2004:25. [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Matsushima T, Shimizu H, Yokohama A, Irisawa H, Handa H, Tsukamoto N, Karasawa M, Nojima Y, Murakami H. Successful treatment with voriconazole of Aspergillus meningitis in a patient with acute myeloid leukemia. Ann Hematol. 2007;86:697–698. doi: 10.1007/s00277-007-0292-8. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Thiel E. Cerebral aspergillosis: tissue penetration is the key. Med Mycol. 2009:1–7. doi: 10.1080/13693780802537953. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Ruhnke M, Ribaud P, Corey L, Driscoll T, Cornely OA, Schuler U, Lutsar I, Troke P, Thiel E. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood. 2005;106:2641–2645. doi: 10.1182/blood-2005-02-0733. [DOI] [PubMed] [Google Scholar]
- Sundaram C, Umabala P, Laxmi V, Purohit AK, Prasad VS, Panigrahi M, Sahu BP, Sarathi MV, Kaul S, Borghain R, Meena AK, Jayalakshmi SS, Suvarna A, Mohandas S, Murthy JM. Pathology of fungal infections of the central nervous system: 17 years’ experience from Southern India. Histopathology. 2006;49:396–405. doi: 10.1111/j.1365-2559.2006.02515.x. [DOI] [PubMed] [Google Scholar]
- Torre-Cisneros J, Lopez OL, Kusne S, Martinez AJ, Starzl TE, Simmons RL, Martin M. CNS aspergillosis in organ transplantation: a clinicopathological study. J Neurol Neurosurg Psychiatry. 1993;56:188–193. doi: 10.1136/jnnp.56.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Wyngaert FA, Sindic CJ, Rousseau JJ, Fernandes Xavier FG, Brucher JM, Laterre EC. Spinal arachnoiditis due to aspergillus meningitis in a previously healthy patient. J Neurol. 1986;233:41–43. doi: 10.1007/BF00313990. [DOI] [PubMed] [Google Scholar]
- Viscoli C, Machetti M, Gazzola P, De Maria A, Paola D, Van Lint MT, Gualandi F, Truini M, Bacigalupo A. Aspergillus galactomannan antigen in the cerebrospinal fluid of bone marrow transplant recipients with probable cerebral aspergillosis. J Clin Microbiol. 2002;40:1496–1499. doi: 10.1128/JCM.40.4.1496-1499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TJ, Hier DB, Caplan LR. Aspergillosis of the central nervous system: clinicopathological analysis of 17 patients. Ann Neurol. 1985;18:574–582. doi: 10.1002/ana.410180511. [DOI] [PubMed] [Google Scholar]
- Adunsky A, Rubinstein E, Goldsmith A. Aspergillus flavus meningitis and pontine hemorrhage in an older patient. J Am Geriatr Soc. 1996;44:739–740. doi: 10.1111/j.1532-5415.1996.tb01852.x. [DOI] [PubMed] [Google Scholar]
- Beal MF, O’Carroll CP, Kleinman GM, Grossman RI. Aspergillosis of the nervous system. Neurology. 1982;32:473–479. doi: 10.1212/wnl.32.5.473. [DOI] [PubMed] [Google Scholar]
- Breneman E, Colford JM., Jr Aspergillosis of the CNS presenting as aseptic meningitis. Clin Infect Dis. 1992;15:737–738. doi: 10.1093/clind/15.4.737. [DOI] [PubMed] [Google Scholar]
- Bryan CS, DiSalvo AF, Huffman LJ, Kaplan W, Kaufman L. Communicating hydrocephalus caused by Aspergillus flavus. South Med J. 1980;73:1641–1644. doi: 10.1097/00007611-198012000-00032. [DOI] [PubMed] [Google Scholar]
- Carrazana EJ, Rossitch E, Jr, Morris J. Isolated central nervous system aspergillosis in the acquired immunodeficiency syndrome. Clin Neurol Neurosurg. 1991;93:227–230. doi: 10.1016/s0303-8467(05)80008-3. [DOI] [PubMed] [Google Scholar]
- Diengdoh JV, Barnard RO, Thomas DG. Aspergillosis of the nervous system. Report of two cases. Neuropathol Appl Neurobiol. 1983;9:477–484. doi: 10.1111/j.1365-2990.1983.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Feely M, Steinberg M. Aspergillus infection complicating trassphenoidal yttrium-90 pituitary implant. Report of two cases. J Neurosurg. 1977;46:530–532. doi: 10.3171/jns.1977.46.4.0530. [DOI] [PubMed] [Google Scholar]
- Gordon MA, Holzman RS, Senter H, Lapa EW, Kupersmith MJ. Aspergillus oryzae meningitis. Jama. 1976;235:2122–2123. [PubMed] [Google Scholar]
- Gunaratne PS, Wijeyaratne CN, Seneviratne HR. Aspergillus meningitis in Sri Lanka--a post-tsunami effect? N Engl J Med. 2007;356:754–756. doi: 10.1056/NEJMc062547. [DOI] [PubMed] [Google Scholar]
- Kagawa R, Okada Y, Moritake K. Fungal meningitic hydrocephalus with repeated shunt malfunction. Neurol Med Chir (Tokyo) 2008;48:43–46. doi: 10.2176/nmc.48.43. [DOI] [PubMed] [Google Scholar]
- Kaufman DM, Thal LJ, Farmer PM. Central nervous system aspergillosis in two young adults. Neurology. 1976;26:484–488. doi: 10.1212/wnl.26.5.484. [DOI] [PubMed] [Google Scholar]
- Kolbe AB, McKinney AM, Kendi AT, Misselt D. Aspergillus meningitis and discitis from low-back procedures in an immunocompetent patient. Acta Radiol. 2007;48:687–689. doi: 10.1080/02841850701342153. [DOI] [PubMed] [Google Scholar]
- Kowacs PA, Monteiro de Almeida S, Pinheiro RL, Fameli H, Piovesan EJ, Correia A, Werneck LC. Central nervous system Aspergillus fumigatus infection after near drowning. J Clin Pathol. 2004;57:202–204. doi: 10.1136/jcp.2003.010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammens M, Robberecht W, Waer M, Carton H, Dom R. Purulent meningitis due to aspergillosis in a patient with systemic lupus erythematosus. Clin Neurol Neurosurg. 1992;94:39–43. doi: 10.1016/0303-8467(92)90117-l. [DOI] [PubMed] [Google Scholar]
- Mariushi WM, Arruda WO, Tsubouchi MH, Ramina R. Chronic Aspergillus sp. meningitis successfully treated with fluconazole. Case report. Arq Neuropsiquiatr. 1999;57:288–291. doi: 10.1590/s0004-282x1999000200020. [DOI] [PubMed] [Google Scholar]
- Mikolich DJ, Kinsella LJ, Skowron G, Friedman J, Sugar AM. Aspergillus meningitis in an immunocompetent adult successfully treated with itraconazole. Clin Infect Dis. 1996;23:1318–1319. doi: 10.1093/clinids/23.6.1318. [DOI] [PubMed] [Google Scholar]
- Moling O, Lass-Floerl C, Verweij PE, Porte M, Boiron P, Prugger M, Gebert U, Corradini R, Vedovelli C, Rimenti G, Mian P. Case Reports. Chronic and acute Aspergillus meningitis. Mycoses. 2002;45:504–511. doi: 10.1046/j.1439-0507.2002.00789.x. [DOI] [PubMed] [Google Scholar]
- Morrow R, Wong B, Finkelstein WE, Sternberg SS, Armstrong D. Aspergillosis of the cerebral ventricles in a heroin abuser. Case report and review of the literature. Arch Intern Med. 1983;143:161–164. [PubMed] [Google Scholar]
- Murai H, Kira J, Kobayashi T, Goto I, Inoue H, Hasuo K. Hypertrophic cranial pachymeningitis due to Aspergillus flavus. Clin Neurol Neurosurg. 1992;94:247–250. doi: 10.1016/0303-8467(92)90097-m. [DOI] [PubMed] [Google Scholar]
- Nenoff P, Kellermann S, Horn LC, Keiner S, Bootz F, Schneider S, Haustein UF. Case report. Mycotic arteritis due to Aspergillus fumigatus in a diabetic with retrobulbar aspergillosis and mycotic meningitis. Mycoses. 2001;44:407–414. doi: 10.1046/j.1439-0507.2001.00687.x. [DOI] [PubMed] [Google Scholar]
- Pagliano P, Attanasio V, Fusco U, Rossi M, Scarano F, Faella FS. Pulmonary aspergillosis with possible cerebral involvement in a previously healthy pregnant woman. J Chemother. 2004;16:604–607. doi: 10.1179/joc.2004.16.6.604. [DOI] [PubMed] [Google Scholar]
- Rodrigo N, Perera KN, Ranwala R, Jayasinghe S, Warnakulasuriya A, Hapuarachchi S. Aspergillus meningitis following spinal anaesthesia for caesarean section in Colombo, Sri Lanka. Int J Obstet Anesth. 2007;16:256–260. doi: 10.1016/j.ijoa.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Matsushima T, Shimizu H, Yokohama A, Irisawa H, Handa H, Tsukamoto N, Karasawa M, Nojima Y, Murakami H. Successful treatment with voriconazole of Aspergillus meningitis in a patient with acute myeloid leukemia. Ann Hematol. 2007;86:697–698. doi: 10.1007/s00277-007-0292-8. [DOI] [PubMed] [Google Scholar]
- van de Beek D, Patel R, Campeau NG, Badley A, Parisi JE, Rabinstein AA, Manno EM, Wijdicks EF. Insidious sinusitis leading to catastrophic cerebral aspergillosis in transplant recipients. Neurology. 2008;70:2411–2413. doi: 10.1212/01.wnl.0000314690.18731.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Wyngaert FA, Sindic CJ, Rousseau JJ, Fernandes Xavier FG, Brucher JM, Laterre EC. Spinal arachnoiditis due to aspergillus meningitis in a previously healthy patient. J Neurol. 1986;233:41–43. doi: 10.1007/BF00313990. [DOI] [PubMed] [Google Scholar]
- van Landeghem FK, Stiller B, Lehmann TN, Sarioglu N, Sander B, Lange PE, Stoltenburg-Didinger G. Aqueductal stenosis and hydrocephalus in an infant due to aspergillus infection. Clin Neuropathol. 2000;19:26–29. [PubMed] [Google Scholar]
- Verweij PE, Brinkman K, Kremer HP, Kullberg BJ, Meis JF. Aspergillus meningitis: diagnosis by non-culture-based microbiological methods and management. J Clin Microbiol. 1999;37:1186–1189. doi: 10.1128/jcm.37.4.1186-1189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]