Abstract
Traditionally, the use of enterococci has been recommended as the fecal indicator bacteria of choice for testing marine recreational water quality, and prior studies have shown that bathers shed large numbers of enterococci into the water. The current study expands upon prior research by evaluating shedding from both toddlers and adults, and by the expansion of measurements to include enterococci shedding via three different methods (membrane filter (MF), chromogenic substrate (CS), and quantitative polymerase chain reaction (qPCR)) and shedding of alternative fecal indicator bacteria (Bacteroidales human markers UCD and HF8 via qPCR). Two sets of experiments were conducted. The first experiment consisted of two groups of 10 adults who bathed together in a large pool. The second study consisted of 14 toddlers who bathed individually in a small pool which allowed for sand recovery. Sand recovery was used to estimate the amount of sand transported on the bodies of toddlers and to estimate the number of fecal indicator bacteria released from this sand. The numbers of estimated enterococci shed per adult ranged from 1.8×104 to 2.8×106 CFU, from 1.9×103 to 4.5×106 MPN, and from 3.8×105 to 5.5×106 GEU based on the MF, CS, and qPCR methods, respectively. The estimated numbers of Bacteroidales human markers ranged from 1.8×104 to 1.3×106 for UCD, and ranged from the below detection limit to 1.6×105 for HF8. The estimated amount of sand transported per toddler (n=14) into the water column after sand exposure was 8±6 g on average. When normalizing the numbers of enterococci shed from toddlers via sand by the 3.9 body-surface area ratio, the differences between toddlers and adults were insignificant. Contributions of sands to the total enterococci (MF) shed per toddler was 3.7 ±4.4% on average. Although shedding via beach sand may contribute a small fraction of the microbial load during initial bathing, it may have a significant role if bathers go to water repetitively after sand exposure.
Keywords: bather shedding, enterococci, Bacteroidales, beach sand
Introduction
Beach advisories are issued when water quality exceeds the microbial standards which in the U.S. are based upon E. coli and enterococci for fresh and marine waters, respectively (U.S. EPA 1986). For many advisories (NRDC 2007), the source of pollution (i.e. humans, animals, and/or environmental) is rarely identified. Humans represent a non-point source of fecal indicator bacteria to recreational waters, and quantifying their bacterial load during bathing can help in developing effective beach management strategies which minimize the number of beach advisories.
Traditionally, measurements of fecal indicator bacteria in recreational waters have relied on common culture-based methods such as membrane filtration (MF). However, more recently measurements have expanded beyond MF to include chromogenic substrate (CS) and quantitative polymerase chain reaction (qPCR) for alternative measurements of fecal indicator bacteria. This expansion was due to the fact that the MF method requires a 24 hour incubation period, and the method does not differentiate between bacteria of animal versus human origin. In this study, CS (another culture based method which has gained considerable use in the regulatory community) and qPCR methods were integrated with the use of the standard MF method for water analysis. Integrating molecular with traditional laboratory techniques could provide data about non-culturable microbes and possible sources (i.e. humans versus animals). While the molecular methodology for the detection of general and human-specific Bacteroidales has not been adopted by the U.S. EPA for routine monitoring, research has shown that it can be potentially used for identifying bacterial contamination from human origin (Gawler et al., 2007; Walters et al., 2007; U.S. EPA 2007); in addition, the U.S. EPA has included Bacteroidales in its recent epidemiologic studies (Wade et al. 2006), suggesting its potential future use for routine monitoring.
The main objectives of the this study were to measure shedding of enterococci and Bacteroidales using traditional and emerging laboratory methods, and to evaluate shedding from toddlers and adults. The field experimental design for the current study was based upon the prior work of Elmir et al. 2007. The added value of the current study was the evaluation of shedding from toddlers (all prior studies used adult volunteers), and the use of additional methods of fecal indicator bacteria analyses (i.e. enterococci by CS and qPCR, and Bacteroidales by qPCR) as no data are available which directly measure fecal indicator bacteria shedding using these alternate methods. The use of the same field study design allowed for the comparison of the MF method results between the Elmir et al. 2007 study and the current study.
Materials and methods
Two distinct sets of experiments (“Large Pool” and “Small Pool”) were conducted as described earlier by Elmir et al. 2007. The study was approved by both the Miami Dade Department of Health Internal Review Board (IRB 1491; DOH IRB Number, H07164) and by the University of Miami Internal Review Board (IRB 20070306). Consistent with IRB approval, consent forms were signed by each volunteer (or parent/guardian) and volunteer identity was kept confidential. The experiments took place at the same sub-tropical non-point source recreational marine beach location as described by Elmir et al. 2007. At the time of the current study the measured salinity was 34 ppt, pH was 7.9, and water temperature was 31 °C.
Large pool field study
The Elmir et al. (2007) “Large Pool” field study protocol was used as the basis to evaluate the numbers of enterococci and Bacteroidales released from the bodies of adult bathers. The same source water, and type and size of inflatable pool were used as in the previous work. In this current study, the “Large Pool” protocol differed from Elmir et al. (2007) only in that additional microbial parameters were measured (i.e. addition of enterococci by CS and qPCR and analysis of Bacteroidales), and in that the study was repeated two times on the same day using two groups of adult participants (10 per group) instead of only one group. In addition, 5 L samples were collected (versus 100 ml samples in the original study) to allow for the analysis of additional bacterial indicators. As in the prior “Large Pool” study, each group of 10 adult bathers were subjected to four 15-minute bathing cycles where participants were not exposed to beach sand during the first two cycles, and were exposed to beach sand during the last two cycles. The “Large Pool” field study was conducted on a single day in July 2008, with the first group starting at 9 am and the second group starting at noon; each group participated in the study for a period of approximately 1.5 hours.
Small pool field study
The Elmir et al. (2007) “Small Pool” field study protocol was used to estimate the amount of beach sand transported on the bodies of humans, and to estimate the fecal indicator bacteria released from this sand. The “Small Pool” experimental protocol used in the current study was identical to the Elmir et al. (2007) study, with the exception that the participants were toddlers in regular diapers (instead of adults), and that water samples were split for analysis of enterococci by MF, CS, and qPCR and for Bacteroidales HF-8 and UCD markers. As in the prior study, the sediment was analyzed only by MF because the small sand sample size did not permit for the analysis of additional microbial parameters.
The “Small Pool” field study was conducted during two different dates in July and August of 2008. In brief, during this “Small Pool” study, each toddler wearing a bathing suit over diapers spent 15 to 30 minutes on the beach sand (e.g. playing, sitting, lying, walking, etc). Thereafter, each individual entered a 190-liter tub, while local off-shore marine water was poured gently over their heads and bodies using a sterilized watering can. When necessary the toddlers were held upright in pool by an adult (i.e. parents and staff) with either gloved hands or hands that were sanitized with alcohol. Sanitation of the pool along with sample collection procedures were identical to the earlier study with the exception that larger water samples were collected (5 L) to permit for the analysis of additional bacteria.
Microbial Assays
All samples were analyzed at the Oceans Human Health Centers Laboratory, University of Miami and at the NOAA, Atlantic Oceanographic and Meteorological Laboratory, both of which are located within minutes from the study site. Enterococci in water samples from the large and small pool experiments were quantified based on a standard membrane filter (MF) method (Method 1600, U.S. EPA 2002), chromogenic substrate (CS) method (IDEXX Enterolert™ Westbrook, Maine) (APHA, 1995), and qPCR (Haugland et al. 2005). Bacteroidales human markers Bac-Hum UCD (Kildare et al. 2007) and HF8 (Bernhard and Field 2000) were also analyzed using qPCR. For the qPCR assays, DNA was extracted from the filters into a 100 μl volume solution using the Fast DNA® Spin Kit (Cat# 6540-600, MP Biomedicals). Specifically, 1.0 μl of the sample, 0.125 μl each of forward and reverse primers, 0.100 μl of TaqMan probe, 12.5 μl of two times master mix (Qiagen Quantitect Cat#204343), and 11.25 μl of sterile PCR grade water (from master mix kit) were used giving a total volume of 25 μl. All analyses were run twice in singleplex, requiring different 1 μl aliquots from the 100 μl DNA extract. The average of the duplicate analyses were reported. Enterococcus faecalis cultures from American Type Culture Collection (ATCC) were used as genomic control standards for enterococci and Bacteroides dorei cultures were used as genomic controls for Bacteroidales measurements. All values for qPCR analysis are reported in units of genome equivalence (GE) per 100 ml.
To enumerate enterococci by MF in the sand samples, two preliminary steps were performed. The first step was to measure the water content of sand. Water content was determined by measuring the weight difference of sand before and after drying (110 °C for 24 h). The second step was to extract enterococci from the sand particles to a predefined volume of sterile water. To accomplish this, pre-weighed un-dried sand was aseptically removed from the corresponding sample container and placed into a sterile pre-weighed jar. One hundred and ten milliliters of sterile phosphate buffer saline (PBS) were then added to each jar and the jars were shaken vigorously for 30 seconds. The samples were permitted to settle for 30 seconds, and the supernatant was subsequently used for membrane filtration.
The numbers of microbes shed per person were estimated based on the difference in microbial concentrations measured before and after bathing (including the adult participant head emersion) in the pools, and multiplying by the corresponding water volumes. Data analyses, including Pearson Correlations, Student T-Tests, and Sum Rank Tests, were performed using Microsoft Excel 2003 and Sigmaplot 11.
Results and Discussion
Human data
For the “Large Pool” study, the gender ratio of participants in the current study was 1/1, and the demographic characteristics of the 20 participants (10 males and 10 females) included an age range from 19 to 51 years old, and weight range from 50 to 100 kg (See Table S-1 in supplemental text). In the “Small Pool” study, the demographic characteristics of the 14 toddlers (2 males and 12 females) included age ranging from 5 to 47 months, and weight ranging from 6.8 to 16.3 kg (See Table S-2 in supplemental text).
Environmental data
The average concentrations of enterococci in the source water based on the MF, CS, and qPCR were 5 (standard deviation, ±7) CFU, 11 (±12) MPN, and 29 (±49) GEU per 100mL, respectively. The average of Bacteroidales human markers UCD and HF8 based on qPCR were 45 (±183) and 3 (±10) GEU/100mL, respectively. Relatively larger standard deviations of enterococci and Bacteroidales human markers based on qPCR were due to a single sample that showed exceptionally high levels of indicators (e.g. enterococci 238, Bacteroidales UCD 862, and Bacteroidales HF8 44 GEU/100mL), while enterococci in 45 and 68% of samples were lower than detection limits based on the MF and CS methods, respectively. The median enterococci values based on the MF, CS, and qPCR were 2 CFU, 5 MPN, and 14 GEU/100mL and Bacteroidales human markers UCD and HF8 were 4 and 1 GEU/100mL, respectively. Enterococci levels in the source water were significantly larger based on qPCR relative to the results based upon MF and CS methods (p<0.01). Overall, these results suggest that offshore waters were in general characterized by low concentrations of viable indicator microbes, which support earlier studies (Shibata, et al. 2004; Elmir et al. 2007); however, the qPCR assay could identify legacy fecal contamination which was not detected by the culture method.
Large pool study
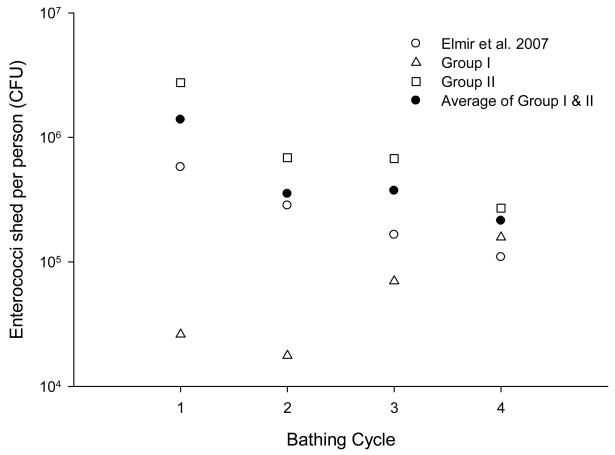
The average estimated numbers of enterococci based on the MF shed per adult (based on 10 bathers) for the first bathing cycle were 2.6×104 CFU in group I and 2.8×106 CFU in group II with an overall average of 2.8×105 CFU, which was similar to the value (5.8×105 CFU reported by Elmir et al. (2007) (Table 1; Figure 1). For the alternative methods of enterococci analysis, the average shed per person was 2.9×104 MPN based on CS and 3.8×105 GEU based on qPCR in group I, and 4.5×106 MPN (CS) and 5.5×106 GEU (qPCR) in group II. The overall average based on the CS and qPCR were 1.4×106 MPN and 2.4×106 GEU, respectively. The estimated average numbers of Bacteroidales human markers UCD and HF8 shed per person in group I were 1.3×106 and below the detection limit, and for group II, 5.0×104 and 1.1×103 GEU, respectively. Comparing shedding between groups, group I was observed to shed more Bacteroidales human marker UCD relative to group II, while group II shed more enterococci and human marker HF8 in comparison to group I.
Table 1.
Estimated numbers of enterococci and Bacteroidales shed per person based on analysis of water samples from a large pool with 10 adults
| Group I | Group II | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cycle | Enterococci | Bacteroidales Human marker | Enterococci | Bacteroidales Human marker | ||||||
| (MF) | (CS) | (qPCR) | UCD (qPCR) | HF8 (qPCR) | (MF) | (CS) | (qPCR) | UCD (qPCR) | HF8 (qPCR) | |
| 1 | 2.6×104 | 2.9 ×104 | 3.8 ×105 | 1.3 ×106 | B.L. | 2.8 ×106 | 4.5 ×106 | 5.5 ×106 | 5.0 ×104 | 1.1 ×103 |
| 2 | 1.8 ×104 | 1.9 ×103 | 5.7 ×105 | 2.0 ×105 | 6.7 ×104 | 6.9 ×105 | 6.7 ×105 | 1.1 ×106 | 1.8 ×104 | B.L. |
| 3 | 7.0 ×104 | 4.3 ×104 | 3.9 ×105 | 4.3 ×105 | 1.6 ×105 | 6.8 ×105 | 6.3 ×105 | 1.3 ×106 | 4.4 ×104 | B.L. |
| 4 | 1.6 ×105 | 8.1 ×104 | 5.4 ×105 | 2.3 ×105 | 5.2 ×104 | 2.7 ×105 | 2.0 ×105 | 4.8 ×105 | B.L. | B.L. |
| Ave | 6.8 ×104 | 3.9 ×104 | 4.7 ×105 | 5.4 ×105 | 9.2 ×104 | 1.1 ×106 | 1.5 ×106 | 2.1 ×106 | 3.7 ×104 | 1.1 ×103 |
| Stdev | 6.4 ×104 | 3.3 ×104 | 9.7 ×104 | 5.2 ×105 | 5.7×105 | 1.1 ×106 | 2.0 ×106 | 2.3 ×106 | 1.7 ×104 | N/A |
Where B.L. is the background levels and N/A is not applicable. MF = membrane filter, CS = chromogenic substrate, UCD = bacteroidales human markers University of California Davis (Kildare et al. 2007), and HF8 = bacteroidales human fecal marker 8 (Bernhard and Field 2000).
Figure 1.
Numbers of enterococci shed per adult based on three large pool studies. Each group of 10 adult volunteers were exposed to water during 15-minute bathing cycles. During cycles 1 and 2 volunteers were not exposed to sand, whereas during cycles 3 and 4 sand exposure was permitted.
From the first bathing cycle to the second bathing cycle, the estimated average numbers of enterococci (MF) shed per person for group I were reduced by 32% and by 75% for group II, with an overall average reduction of 54% for the two groups. This reduction was consistent with Elmir et al. (2007) with an average 51% reduction. For the alternative methods, enterococci levels based on the CS method were reduced by 94% for group I and 85% for group II with an overall average reduction of 89%; based on the qPCR method levels increased by 50% for group I and were reduced by 80% for group II. For the Bacteroidales human marker UCD, levels were reduced by 85 and 64% in group I and II, respectively with an overall average 74% reduction. In the group I, Bacteroidales human marker HF8 was detected in the second bathing cycle, although it was not detected in the first bathing cycle. In group II, HF8 was reduced 100% in the second bathing cycle, and not detected in the following cycles. On the whole, the numbers of microbes shed were reduced notably in the second bathing cycle, except for enterococci based on qPCR and Bacteroidales human marker HF8.
From the second to the third bathing cycle, the number of enterococci (MF) shed did not decrease by such a large fraction and in some cases increased, presumably because of the exposures to beach sands between these cycles. For group I, the numbers increased by 300% (or 3 times) and for group II were reduced slightly by 2%. The presumed effects from sand were much more noticeable in group I, with an increase in bather shedding due to the low enterococci numbers shed from this group of participants. From the third to the fourth bathing cycle, during which the subjects were again exposed to beach sands, the enterococci (MF) shedding increased by 125% for group I and decreased by 60% for group II. Considering all of the results, the data suggest that the numbers of enterococci shed as measured by MF decreased by repetitive bathing. Although differences in enterococci shedding were apparent between groups I and II, when the results from each of these groups were averaged, the values were similar to that observed by Elmir et al. 2007 (Figure 1).
When comparing enterococci shedding between different methods of analyses, results were very similar, within the same order of magnitude, for shedding numbers as measured by the MF versus CS methods for groups I and II. The average ratios (CS/MF) among four bathing cycles were 0.6±0.4 for group I and 1.1±0.3 for group II. In contrast, shedding by qPCR showed higher enterococci levels by a factor of 14±11 (qPCR/MF) for group I and a factor of 1.8±0.2 for group II. Assuming that qPCR provided total numbers of bacteria shed (viable and non-viable), it could hypothesized that the total numbers of enterococci shed per person in the group I and II were similar, but that many of the enterococci shed by the group I were not viable. Of interest, although shedding of enterococci was lower in group I relative to group II, shedding of Bacteroidales was reversed, with a greater amount of shedding from group I relative to group II. The reason for this reversal in microbe releases as measured by qPCR is uncertain.
Small Pool Study
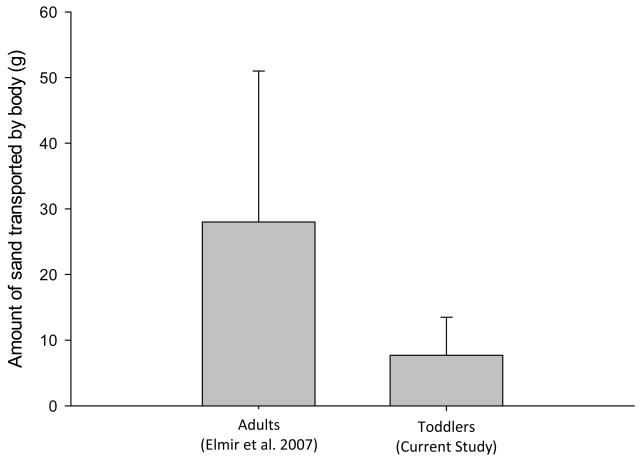
Total shedding of bacteria from the small pool study was assumed to be the sum of the bacteria observed in the sand component and in the water component. It was also assumed that the amount shed from the sand component was dependent on the amount of sand carried by the bathers. The amount of sand transported per toddler ranged from less than 0.1 to 24 g with an average of 8±6 g; these levels were significantly different from those measured by Elmir et al. 2007 with individual adults releasing a quantity of sand ranging from 7 to 70 g with an average of 28±24 g (p<0.05) (Figure 2). Such a difference (e.g. 3.5 times on the average) can be explained by the fact that estimated adult body surface areas based on height and weight (U.S. EPA 1997) in Elmir et al. 2007 were approximately 3.9 times larger than the toddlers in this study.
Figure 2.
Average amount of beach sand transferred into the water by adults and toddlers based on two small pool studies. Vertical bars correspond to the standard deviation of results. The values for adults correspond to the Elmir et al. 2007 study. The values for toddlers correspond to values measured during the current study. The values shown correspond to total sand quantities which were not normalized to account for the difference in body surface area between adults and children.
Based on the sand analysis using the MF method, the estimated numbers of enterococci transported per toddler via sand into the water column ranged from 37 to 920 CFU with an average of 330±250 CFU. This estimate was significantly different from the individual adults as measured by Elmir et al. (2007) who found 42 to 3.8×105 CFU with an average of 9.6×103±1.3×104 CFU(p<0.05). When normalizing the numbers of enterococci shed from adults via sand by the 3.9 body-surface area ratio, the differences between adults and toddlers were insignificant.
The estimated numbers of enterococci shed per toddlers based on the water analysis ranged from 620 to 5.6×105 CFU with an average of 8.2×104±1.6×105. This estimate was not statistically significantly different from the individual adults as measured by Elmir et al. 2007 who found 2.6×103 to 1.2×105 CFU with an average of 4.7×104±4.7×104 CFU. The lack of statistical difference was observed regardless of whether or not the numbers were adjusted for the difference in body surface area between adults and toddlers. This lack of difference due to the large range between adults and toddlers and due to the small number of subjects participating.
Assuming that the total numbers of enterococci introduced into water body could be estimated based on a sum of the water and sand analyses, the total numbers of enterococci shed per toddler ranged from 700 to 5.6×105 CFU with an average of 8.2×104±1.6×105. This estimate was not statistically different from the individual adult values measured by Elmir et al. 2007 who found 2.6×103 to 1.2×105 CFU with an average of4.8×104± 4.7×104. Again, as for the water component, the lack of statistical difference for the total amount shed (sum of water plus sand component) was observed regardless of whether or not the numbers were adjusted for the difference in body surface area between adults and toddlers. The range of shedding between individuals within a particular adult or toddler group (3 orders of magnitude within the toddler group and 1.5 orders of magnitude within the adult group) masked the ability to observe any differences in shedding between the two groups.
When evaluating the significance of the sand relative to the total amount shed, results show that the sand contributions for the first and only “Small Pool” bathing cycle ranged from 0.1 to 13% with an average of 3.7 ±4.4%, which were statistically not significantly different from the findings reported in Elmir et al. 2007 who measured sand contributions in adults ranging from 0.4 to 3.5% with an average of 1.9±3.5%. It must be noted that beach sand at the study site, where bathers spent some time, were not collected for enterococci analysis, and that the sands introduced in the pool could have transferred enterococci to the water column during the bathing process before sand collection and assay. Thus, the enterococci levels in the sand recovered from the pool could be underestimated. The measured enterococci concentrations in the sand, which were recovered from the bottom of the small pool in the current study, ranged from 9 to 136 CFU/dry g with an average of 45±38 CFU/dry g, which were comparatively lower than values observed in dry sand at the same study beach on prior occasions (Wright et al. personal communication). These low levels observed in the sand portion could have contributed to the relatively small contribution of sand relative to that observed in the water column.
When evaluating the alternative laboratory methods used to analyze water for enterococci in the “Small Pool” study, the numbers of enterococci based on the CS and qPCR were not statistically different from the MF method. The CS method was significantly correlated with the MF (r = 0.91; p<0.01). The correlation was also significant for the log transformed data (r = 0.84, p <0.01) while insignificant correlation was observed between qPCR and MF (r = 0.36; p=0.31). Poor correlations between the culture and molecular methods suggested that at least some of the enterococci detected by qPCR were associated with non-culturable DNA.
Bacteroidales human marker UCD was detected in 9 out of 14 toddlers (64%). Among the positive samples, the estimated amount shed per toddler ranged from 50 to 5.7×105 GEU with an average of 7.1×104±1.9×105 GEU. The numbers of Bacteroidales human marker UCD based on qPCR were significantly correlated with enterococci based on the MF and qPCR (r = 0.96; p<0.01) while insignificant correlation was observed with the CS (r = 0.37; p= 0.36). For the log transformed data, no significant correlations were observed between Bacteroidales human marker UCD and any of the enterococci measurements. Bacteroidales human marker HF8 was detected from only two out of 14 toddlers (36 and 485 GEU).
Summary and Conclusions
Based on the above discussion points, one can conclude that human bathers have the potential to release significant amounts of fecal indicator bacteria into the water column via direct shedding off their body and via sand transported by their skin. Direct shedding from the body can include releases from fecally contaminated body areas and skin, and releases from fecally contaminated diapers. In thie study, the quantity of enterococci released was a function of bathing cycle, sand exposure, beach sand contamination levels, and microbial flora variations between swimmers. Overall, the quantities of enterococci released during the first bathing cycle was on the order of 300,000 to 600,000 CFU (as measured by the MF method), with average results from the current study consistent with earlier studies by Elmir et al. 2007 and others (Smith and Dufour 1993; Gerba 2000). Similar or higher values can be anticipated through qPCR measurements relative to measurements via MF and CS, with results from MF and CS methods found to be statistically the same. In order to evaluate the significance of enterococci shedding in the context of regulatory limits, results should be combined with a hydrodynamic and water quality model of the beach area to evaluate the net contribution of human bathing to enterococci levels within the beach water.
Bacteroidales releases were variable, with the human UCD marker more frequently detected among the individual toddlers (57%) and generally observed at higher levels within the adults relative to the human HF8 marker (15% within the 14 individual toddlers and generally lower levels from the adults). This observation supports the hypothesis that the BacHum-UCD marker is found more commonly within human populations, and may be potentially useful to track fecal contributions from individual people.
On average, direct body shedding was the most significant contribution of enterococci (by MF) during the first bathing cycle (representing over 95% of the contribution with sand representing less than 5%). These results thus affirm the universal recommendation that bathers should shower before they enter recreational waters for beaches as well as swimming pools in order to reduce the microbial load in the water. However in subsequent bathing cycles, where the direct body contribution tended to decline, the microbial contribution from sand can become large relative to the direct body contribution. The contribution from sand could be further underestimated in this study considering that the amount of sand adhering to skin is likely to be greater once the skin is wet, and, as such, the sand contribution may be significant for individuals who bath repeatedly with sand exposure between bathing events. The quality of sand which adheres to skin and subsequently transported to the water column during bathing can also have impacts on water quality, especially for repeated bathing cycles; therefore, beach management efforts would benefit by maintaining sand with relatively low indicator counts.
Significant differences were not observed when comparing total enterococci shedding (via MF method) between adults and toddlers. This lack of difference can be attributed, in part, to the large variability in the results observed between individuals within each group. Thus, when estimating bacteria releases from toddlers, the results of this study support that the “adult” values can be used to estimate “toddler” shedding when evaluating the total number of bacteria shed. However, when evaluating specifically the component from sand, differences in body surface area, which impact the amount of sand adhered to skin, can result in measureable differences in the sand microbe contribution between toddlers and adults. The significance of this difference should be considered in the context of the relatively small contribution of sand to the total bacteria shed during the first bathing cycle and the observation that the significance of the sand component increases in subsequent bathing cycles after the initial wash-off of microbes from the body.
Supplementary Material
Table 2.
Estimated numbers of enterococci and Bacteroidales shed per person based on the analysis of water and sediment samples from a small pool with individual toddlers
| Enterococci | Bacteroidales | ||||||
|---|---|---|---|---|---|---|---|
| Subject ID | Sand | Water | Water | ||||
| (g) | (MF) | (MF) | (CS) | (qPCR) | UCD (qPCR) | HF8 (qPCR) | |
| T1 | <0.1 | N.D. | 2.6 ×104 | 2.8 ×104 | B.L. | B.L. | B.L. |
| T2 | 7 | 9.2 ×102 | 6.4 ×103 | 7.2 ×104 | 1.2 ×105 | 1.5 ×103 | B.L. |
| T3 | 10 | 2.5 ×102 | 3.2 ×105 | 1.1 ×105 | 4.6 ×105 | 5.7 ×105 | B.L. |
| T4 | 13 | 2.2 ×102 | 3.1 ×103 | 1.1 ×104 | B.L. | B.L. | B.L. |
| T5 | 4 | 4.2 ×101 | 2.6 ×104 | 5.7 ×103 | 2.6 ×103 | 6.6 ×104 | 4.8 ×102 |
| T6 | 24 | 4.7 ×102 | 2.3 ×104 | 2.7 ×104 | 1.7E+02 | B.L. | B.L. |
| T7 | 4 | 2.0 ×102 | 9.0 ×103 | 1.9 ×104 | 6.8 ×103 | 4.8 ×101 | B.L. |
| T8 | 4 | 4.5 ×102 | 2.5 ×104 | 2.7 ×104 | B.L. | B.L. | B.L. |
| T9 | 6 | 3.1 ×102 | 4.0 ×103 | 9.0 ×103 | 8.9 ×102 | 5.4×102 | B.L. |
| T10 | 9 | 8.0 ×101 | 6.2 ×102 | 2.8 ×103 | 2.3 ×103 | 2.4 ×103 | B.L. |
| T11 | 4 | 3.7 ×101 | 6.0 ×104 | 1.3 ×105 | 1.6 ×104 | 4.2 ×102 | B.L. |
| T12 | 6 | 3.6 ×102 | 8.3 ×104 | 9.1 ×104 | 2.1 ×104 | 5.9 ×102 | B.L. |
| T13 | 10 | 6.7 ×102 | 5.6 ×105 | 5.9 ×105 | 5.2 ×103 | B.L. | B.L. |
| T14 | 8 | 2.4 ×102 | 6.8 ×103 | 1.1 ×104 | 6.4 ×103 | 5.1 ×102 | 3.6 ×101 |
| Ave | 7.7 | 3.3 ×102 | 8.2 ×104 | 8.0 ×104 | 5.8 ×104 | 7.1 ×104 | 2.6 ×102 |
| Stdev | 5.8 | 2.5 ×102 | 1.6 ×105 | 1.5 ×105 | 1.4 ×105 | 1.9 ×105 | 3.2 ×102 |
Where B.L. is the background level and N.D. is no data available due to limited sand recovery. MF = membrane filter, CS = chromogenic substrate, UCD = Bacteroidales human markers University of California Davis (Kildare et al. 2007), and HF8 = Bacteroidales human fecal marker 8 (Bernhard and Field 2000).
Acknowledgments
This study was funded in part from the following sources: the National Center for Environmental Health (NCEH), Centers for Disease Control and Prevention (CDC); Florida Dept of Health (FL DOH) through monies from the Florida Dept of Environmental Protection (FL DEP); the Environmental Protection Agency (EPA) Internship Program; the National Science Foundation (NSF) and the National Institute of Environmental Health Sciences (NIEHS) Oceans and Human Health Center at the University of Miami Rosenstiel School (NSF 0CE0432368/0911373; NIEHS 1 P50 ES12736) and NSF REU in Oceans and Human Health, and the NSF SGER (NSF SGER 0743987) in Oceans and Human Health. We would also like to thank IDEXX Corporation for their support of our project through the provision of supplies needed for the chromogenic substrate analysis of enterococci. The research team gratefully acknowledges all organizations and their staff who collaborated, provided support, and/or participated in all various aspects of this research effort including: University of Miami, Florida International University, University of Florida, Miami Dade County Public Works, Miami Dade County Health Department Environmental Health, Florida Department of Health Bureau of Laboratory Services Miami Branch, US Department of Commerce National Oceanic and Atmospheric Administration, and U.S. Department of Health Human Services (DHHS). Finally, the researchers would like to thank Ms Kathy Vergara (Director), the Staff and the families of the Debbie School of the University of Miami for their support of and participation in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Public Health Association. Standard methods for the examination of water and wastewater. 19. American Public Health Association, Inc; Washington, D.C: 1995. [Google Scholar]
- Bernhard AE, Field KG. A PCR Assay To Discriminate Human and Ruminant Feces on the Basis of Host Differences in Bacteroides-Prevotella Genes Encoding 16S rRNA. Applied and Environmental Microbiology. 2000;66:4571–4574. doi: 10.1128/aem.66.10.4571-4574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmir SM, Wright ME, Solo-Gabriele HM, Abdelzaher A, Fleming LE, Miller G, Rybolowik M, Shih M-TP, Pillai SP, Cooper JA, Quaye EA. Quantitative Evaluation of Bacteria Released by Bathers in a Marine Water. Water Research. 2007;41:3–10. doi: 10.1016/j.watres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawler AH, Beecher JE, Brandao J, Carroll NM, Falcao L, Gourmelon M, Masterson B, Nunes B, Porter J, Rince A, Rodrigues R, Thorp M, Walters JM, Meijer WG. Validation of host-specific Bacteroidales 16S rRNA genes as markers to determine the origin of faecal pollution in Atlantic Rim countries of the European Union. Water Research. 2007;41(16):3780–3784. doi: 10.1016/j.watres.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Gerba CP. Assessment of enteric pathogen shedding by bathers during recreational activities and its impact on water quality. Quant Microbiol. 2000;2:55–68. [Google Scholar]
- Haugland RA, Siefring SC, Wymer LJ, Brenner KP, Dufour AP. Comparison of Enterococcus measurements in freshwater at two recreational beaches by quantitative polymerase chain reaction and membrane filter culture analysis. Water Research. 2005;39:559–568. doi: 10.1016/j.watres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kildare BJ, Leutenegger CM, McSwain BS, Bambic DG, Rajal VB, Wuertz S. 16S rRNA-based assays for quantitative detection of universal, human-, cow-, and dog-specific Bacteroidales: A Bayesian approach. Water Research. 2007;41:3701–3715. doi: 10.1016/j.watres.2007.06.037. [DOI] [PubMed] [Google Scholar]
- National Resources Defense Council (NRDC) A Guide to Water Quality at Vacation Beaches. Washington, D.C: 2007. Testing the Waters 2005. [Google Scholar]
- Shibata T, Solo-Gabriele HM, Fleming LE, Elmir SM. Monitoring Marine Recreational Water Quality Using Multiple Microbial Indicators in an Urban Tropical Environment. Water Research. 2004;38:3119–3131. doi: 10.1016/j.watres.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BG, Dufour AP. Effects of the microbiological quality of recreational waters: a simulation study. American Society for Microbiology 93rd General Meeting; May 16–20, 1993; Atlanta, Georgia. 1993. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Ambient Water Quality Criteria for Bacteria - 1986. Washington, DC: US EPA; 1986. EPA440/5–84–002. [Google Scholar]
- U.S. Environmental Protection Agency. Exposure Factor Handbook US EPA. National Center for Environmental Assessment; Washington, D.C: 1997. [Google Scholar]
- U.S. Environmental Protection Agency. Method 1600: membrane filter test method for enterococci in water. U.S. Environmental Protection Agency; Washington, D.C: 2002. EPA-821-R-02–022. [Google Scholar]
- U.S. Environmental Protection Agency. Report of the Experts Scientific Workshop on Critical Research Needs for the Development of New or R Recreational Water Quality Criteria. 2007 EPA 823-R-07–006. [Google Scholar]
- Wade TJ, Calderon RL, Sams E, Beach M, Brenner KP, Williams AH, Dufour AP. Rapidly Measured Indicators of Recreational Water Quality Are Predictive of Swimming-Associated Gastrointestinal Illness. Environmental Health Perspectives. 2006 January 2006;114(1) doi: 10.1289/ehp.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters SP, Gannon VPJ, Field KG. Detection of Bacteroidales fecal indicators and the zoonotic pathogens E. coli O157:H7, Salmonella, and Campylobacter in river water. Environmental Science and Technology. 2007;41(6):1856–1862. doi: 10.1021/es0620989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.