Abstract
Exposure to nerve agents and other organophosphorus acetylcholinesterases used in industry and agriculture can cause death, or brain damage, producing long-term cognitive and behavioral deficits. Brain damage is primarily caused by the intense seizure activity induced by these agents. Identifying the brain regions that respond most intensely to nerve agents, in terms of generating and spreading seizure activity, along with knowledge of the physiology and biochemistry of these regions, can facilitate the development of pharmacological treatments that will effectively control seizures even if administered when seizures are well underway. Here, we contrast the pathological (neuronal damage) and pathophysiological (neuronal activity) findings of responses to nerve agents in the amygdala and the hippocampus, the two brain structures that play a central role in the generation and spread of seizures. The evidence so far suggests that the amygdala suffers the most extensive damage by nerve agent exposure, which appears consistent with the tendency of the amygdala to generate prolonged, seizure-like neuronal discharges in vitro in response to the nerve agent soman, at a time when the hippocampus generates only interictal-like activity. In vivo experiments are now required to confirm the primary role that the amygdala seems to play in nerve agent-induced seizure generation.
Nerve agents were first developed by German scientists in the 1930s (López-Muñoz et al., 2008). They are the most toxic of the chemical warfare agents. In today’s global political climate, nerve agents are a major threat, both in military operations and against civilians, because they are relatively simple to produce, transport, and deploy. The most well known nerve agents are soman, sarin, cyclosarin, tabun, and VX (Bajgar, 2005; Barthold and Schier, 2005; Layish et al., 2005). They are organophosphorus compounds, and their primary action is the irreversible inhibition of acetylcholinesterase (AChE), which results in accumulation of acetylcholine at cholinergic synapses; a cholinergic crisis follows due to overstimulation of muscarinic and nicotinic receptors in the central and peripheral nervous system, including the neuromuscular junction (Bajgar, 2005; Barthold and Schier, 2005; Layish et al., 2005, Schecter, 2004). The time-course of nerve agent poisoning is rapid, and death may occur within minutes depending on a number of factors, with the dose and the route of exposure being most important among them (Bajgar, 2005; Barthold and Schier, 2005; Layish et al., 2005). Even when death is prevented pharmacologically, or the dose of exposure is sublethal, long-lasting neurological and behavioral changes may still occur because of damage to the central nervous system (Bajgar, 2004; Brown and Brix, 1998, Joosen et al., 2009; Kassa et al., 2001; McDonough et al., 1986; Morita et al., 1995; Myhrer et al., 2005). Brain damage by nerve agent exposure is primarily due to seizure activity, which can rapidly progress to status epilepticus (Baille et al., 2005; Hayward et al., 1990; Myhrer et al., 2005, Shih et al., 2003).
Brain seizures after nerve agent exposure are induced by overstimulation of cholinergic, primarily muscarinic, receptors (Harrison et al., 2004; McDonough and Shih, 1997). These receptors are widely distributed in the brain, and are located at postsynaptic sites where they mediate excitatory effects of acetylcholine, such as blockade of various potassium conductances (Cole and Nicoll, 1984; Madison et al., 1987; Washburn and Moises, 1992; Womble and Moises, 1992) or activation of a calcium-sensitive nonspecific cation current (Egorov et al., 2006), but also on presynaptic terminals where they modulate the release of glutamate (Yajeya et al., 2000; Fernández de Sevilla and Buño, 2003) and GABA (Fukudome et al., 2004; Salgado et al., 2007). Consequently, inhibition of AChE by nerve agents -and the resulting overstimulation of muscarinic receptors-affects glutamate release (Lallement et al., 1991a, 1991b, 1992; Wade et al., 1987) and GABA release (Grasshoff et al., 2003; Santos et al., 2003), disrupting the balance in the activity of the two major excitatory and inhibitory neurotransmitter systems. The current view, therefore, is that cholinergic hyperactivity initiates nerve agent-induced seizures and triggers glutamatergic hyperactivity, which sustains and reinforces seizures and is eventually responsible for excitotoxic neuronal damage (McDonough and Shih, 1997, Lallement et al, 1992; Solberg and Belkin, 1997). Consistent with this view, central antimuscarinic compounds can reduce or block seizures only when administered within a few minutes after exposure (Lallement et al. 1998, Shih and McDonough, 1999).
What are the brain areas that respond most intensely to nerve agents, in terms of generating and spreading seizure activity? This is an important question because identifying these areas (or area), along with knowledge of the physiological and biochemical characteristics of these brain regions, can facilitate the development of pharmacological treatments that can stop nerve agent-induced seizures, even when administered at relatively long latencies after exposure. It is well-known that temporal lobe limbic structures are the most susceptible to seizurogenic insults. Thus, in humans, temporal lobe epilepsy (TLE) is the most common form of epilepsy (Engel, 1989). In TLE patients, the epileptic focus resides either in the hippocampus or the amygdala, or in both regions (Quesney, 1986; Isokawa-Akesson et al., 1987; Dewar et al., 1996; Pitkänen et al., 1998; Morimoto et al., 2004). In addition, either or both the amygdala and the hippocampus display extensive neuropathology (Cendes et al., 1993; Pitkänen et al., 1998; Saukkonen et al., 1994), and removal of either or both of these structures in most cases cures the disease (Feindel and Rasmussen, 1991; Jooma et al., 1995; Wieser 2000). There is also experimental evidence attesting to the high propensity of temporal lobe structures for seizure generation. For example, induction of kindling is accomplished by electrical stimulation of the amygdala, the hippocampus, or other temporal lobe regions (Goddard, 1967; McIntyre and Racine, 1986). It is not surprising, therefore, that temporal lobe structures, particularly the amygdala, hippocampus, and piriform cortex appear to also play a central role in the generation of seizures induced by nerve agents, as suggested by the rapid increases in extracellular glutamate in these brain regions after nerve agent exposure (Lallement et al., 1991a, 1991b, 1992), and the profound damage these brain regions suffer by exposure to nerve agents (Baze, 1993; Hayward et al., 1990; Kadar et al., 1995; 126, Myhrer et al., 2006; Shih et al., 2003).
Although it is the hippocampus that has been studied most extensively in relation to seizure generation and its mechanisms, in response to a variety of experimental manipulations inducing epileptic or epileptiform activity, it is important to note that the amygdala appears to have equal or higher propensity for generating seizures. Thus, kindling develops faster by repeated electrical stimulation of the amygdala than stimulation of the hippocampus (Goddard, 1967, 1969; McIntyre and Racine, 1986), and interictal discharges are initiated in the amygdala and/or piriform cortex regardless of the site of kindling (even when kindling is induced by hippocampal stimulation; Kairiss et al., 1984). In addition, due to its widespread output to cortical and subcortical areas (Sah et al., 2003) the amygdala, and in particular the basolateral nucleus of the amygdala (BLA), has a high capacity for spreading seizures to other temporal and extra-temporal regions (Morimoto et al., 2004). Thus, prolonged electrical stimulation triggers status epilepticus more readily when the stimulation is applied to the BLA than to other amygdala nuclei or extra-amygdalar regions (Mohapel et al., 1996), and activation of the BLA is primarily responsible for the generation of widespread status epilepticus, even when prolonged stimulation is applied to extra-amygdalar regions (White and Price, 1993).
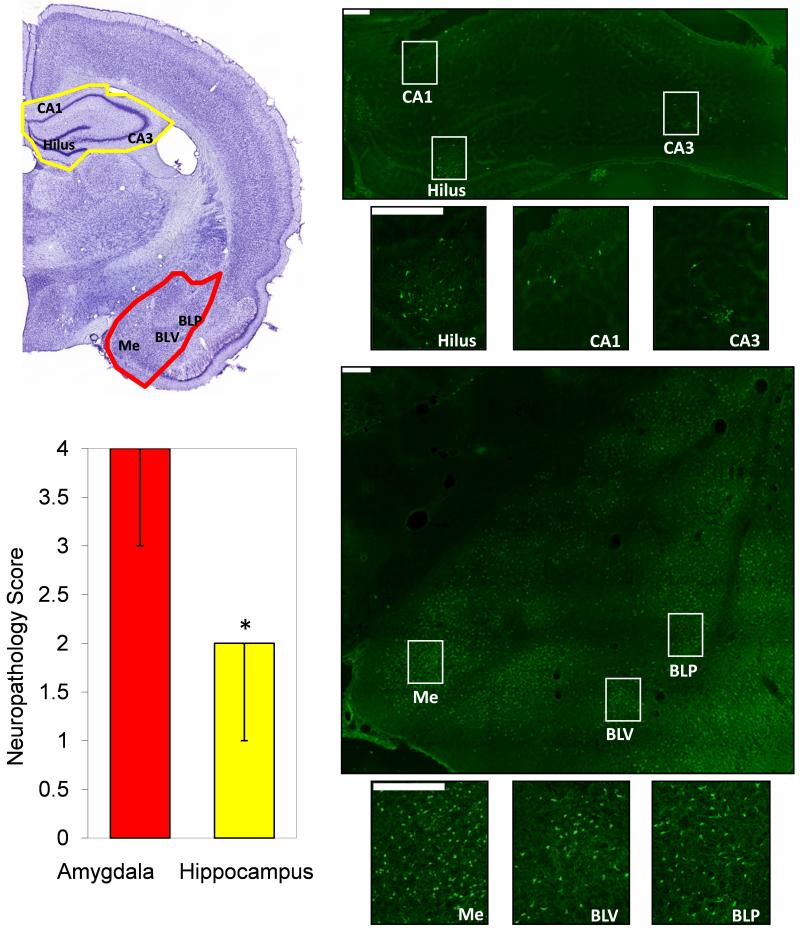
Is there any evidence suggesting that the amygdala may play a more important role than the hippocampus in the generation and spread of seizures after nerve agent exposure? Lallement et al. (1991a) reported that after exposure of rats to toxic levels of soman, the amygdala displays the earliest and most rapid increase in extracellular glutamate (measured by microdialysis), suggesting an early involvement of the amygdala in the development of soman-induced seizures. Shih et al., (2003) found that after exposure of guinea pigs to different nerve agents, with or without subsequent treatment, the amygdala and the cerebral cortex were the most frequently damaged areas, and, the amygdala was the most severely damaged structure, followed by the cerebral cortex, the caudate nucleus, the thalamus and piriform cortex, and lastly the hippocampus. The same study (Shih et al., 2003) supported previous suggestions that there is a strong correlation between the intensity/duration of seizures after nerve agent exposure and the severity of neuropathology (Hayward et al., 1990; McDonough et al., 2000). Therefore, the greater severity of neuropathology observed in the amygdala may imply that this brain structure suffers the strongest seizure activity after exposure to nerve agents. In our hands, after exposure of rats to soman, the amygdala is also the most damaged structure, displaying significantly greater neuronal degeneration than the hippocampus (Fig. 1; the experiments represented in Fig. 1 adhered to the Guide for the Care and Use of Laboratory Animals by the Institute of Laboratory Animal Resources, National Research Council, in accordance with the stipulation mandated for an AAALAC accredited facility, and were approved by the Institutional Animal Care and Use Committee). A note of caution should be added here, however: the ventral hippocampus is, overall, more seizurogenic than the dorsal hippocampus (see for example Akaike et al., 2001), and it appears that in all the published reports so far on hippocampal damage by nerve agents (including our study; Fig. 1) it is the dorsal hippocampus that has been examined, where the amygdala is also present on the same coronal section. It remains to be determined, therefore, if damage of the ventral/posterior region of the hippocampus is also less severe than damage of the amygdala.
Figure 1.
Neuronal degeneration in the amygdala is greater than in the hippocampus, 24 hours after status epilepticus induced by injection of 1.4×LD50 soman (154 μg/kg BW), in rats. Photomicrographs of Cresyl Violet (left) and FluoroJade-C staining (right) from one representative animal. The Cresyl Violet photomicrograph outlines the brain regions (amygdala in red; hippocampus in yellow) and indicates the hippocampal subfields (CA1, CA3 and hilus) and amygdala nuclei (Me, Medial; BLV, basolateral ventral; BLP, basolateral posterior) which are shown in the FluoroJade-C sections (magnification 200×). The bar graph shows the neuropathology score in the amygdala and the hippocampus (Median ± Range; group data from 6 animals). The score assessment was done using a qualitative scale (see McDonough et al., 1995 and Myhrer et al., 2005): 0 = no damage; 1 = 1 to 10% minimal damage; 2 = 11 to 25% mild damage; 3 = 26 to 45% moderate damage; 4 > 45% severe damage. The final score was the average from 5 successive coronal brain sections from each animal. Scale bars are 200 μm. *p<0.05 using Wilcoxon Signed Ranks Test to compare the two brain regions.
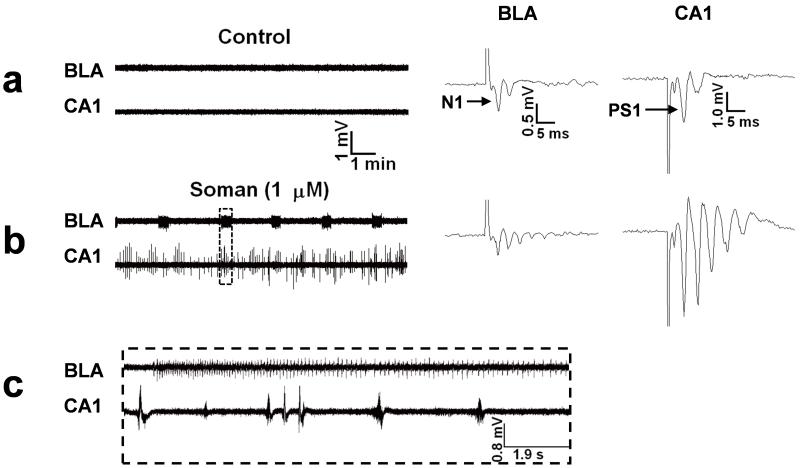
To directly observe the effects of soman on the activity of the amygdalar and hippocampal neuronal networks, we have recorded field potentials simultaneously from the BLA and the CA1 hippocampal area in in vitro coronal slices of rat brain, containing both regions. In the amygdala, soman induced rhythmic, prolonged neuronal discharges resembling brain seizures, while the hippocampus generated only interictal-like activity (Apland et al., 2009; Fig. 2). A similar sharp contrast between the type of epileptiform activity generated by the amygdala (ictal-like) versus the activity in the hippocampus (interictal-like) has also been observed previously in response to 4-aminopyridine, in vitro (Benini et al., 2003). In that study (Benini et al., 2003), the connections between the hippocampus and the amygdala had been preserved in horizontal rodent slices, and it was observed that the amygdala -as well as the entorhinal cortex (Barbarosie and Avoli 1997; Benini et al., 2003)- could freely generate “seizures” only when the connections with the hippocampus were cut. In other words, the interictal-like activity in the hippocampus was inhibiting rather than facilitating the seizure-like epileptiform activity in the amygdala (and entorhinal cortex), an observation that has generated questions in regard to the role of the interictal activity in the occurrence of seizures (Avoli, 2001). Thus, in response to soman application in vitro, the BLA generates seizure-like activity whereas the CA1 hippocampal area generates interictal-like activity, and this difference in the pattern of epileptiform activity produced in the amygdala versus the hippocampus is not associated exclusively with soman and the mechanisms by which soman initiates epileptiform activity. It seems, therefore, that the amygdala may have an inherently higher propensity than the hippocampus to generate ictal neuronal discharges in response to convulsants.
Figure 2. Soman induces ictal-like activity in the amygdala and interictal-like activity in the hippocampus.
Extracellular field recordings, in gap-free mode, were simultaneously obtained in the BLA and the stratum pyramidale of the CA1 hippocampal area, in slices containing both regions. Stimulus pulses were applied every 30 sec to sample the evoked field potentials. Note the amplitude scales of the field potentials; field potentials in the amygdala have substantially smaller amplitude than hippocampal field potentials due to the structure of the amygdala network which does not favor generation of strong dipoles. (a) Field potentials in the BLA, evoked by stimulation of the external capsule, consisted of one major negative component (N1), followed by one or more lower-amplitude, late components. In the CA1 area, field potentials evoked by stimulation of the Schaffer collaterals consisted of a large population spike (PS1), which was often followed by one or two low-amplitude, negative components. No spontaneous activity was present in the BLA or the CA1 area (left panel). (b) Exposure to 1 μM soman for 30 min induced spontaneous, prolonged episodes of synchronous neuronal discharges resembling brain seizures, in the BLA. In the CA1 area, soman exposure produced additional population spikes in the enhanced evoked response, as well as spontaneous, interictal-like bursts. (c) A prolonged ictal-like discharge from the BLA and interictal-like activity from the hippocampus on an expanded time scale (taken from the rectangle-outline shown in (b)). In no case did the hippocampus display seizure-like activity (see Apland et al., 2009). The effects of soman were not reversible; epileptiform activity was maintained after washing out soman and throughout the recording period (for more than 3 hours).
Certainly, the in vitro data await support from in vivo experiments, where a number of factors can affect the responses of the amygdala and the hippocampus to convulsants like nerve agents, most important among them the integrity of the interconnections of these structures with other brain regions. Nevertheless, the existing data justify inquiry into the physiological and molecular/biochemical parameters that confer to the amygdala a high propensity for generation of rhythmic, prolonged neuronal discharges, as least as observed in the in vitro conditions. It is noteworthy in this regard that the amygdala, and the BLA in particular, is one of the few brain regions that display a markedly high expression of the kainate subtype of glutamate receptors that contain the GluR5 subunit (GluR5KRs) (Bettler et al., 1990; Li et al., 2001; Braga et al., 2003). GluR5KRs have attracted interest for the central role they appear to play in the induction and maintenance of limbic seizures (Smolders et al., 2002), and in the amygdala they have been found to modulate neuronal excitability (Braga et al., 2003; for reviews see Aroniadou-Anderjaska et al., 2007, 2008; Braga et al., 2004). Interestingly, the GluR5KR antagonist UBP302 suppressed the soman-induced epileptiform activity in the amygdala and the hippocampus in vitro, and the effect was more pronounced in the amygdala (Apland et al., 2009). Furthermore, GluR5KR antagonists (LY295338 and UBP302) also display remarkable effectiveness in blocking soman-induced seizures in vivo, even when administered one hour after soman exposure (our group, unpublished). Considering the above, we believe it is important to determine if selective inactivation of the amygdala prevents the generation of seizures after nerve agent exposure, and if application of a nerve agent directly into the amygdala can produce status epilepticus, and compare these results with similar experiments with the hippocampus. Furthermore, whether or not the GluR5KR antagonists act primarily in the amygdala to stop the soman-induced seizures in vivo is also a testable hypothesis.
Acknowledgements
We thank Adriana Souza for assistance in the pathological analysis. This work was supported by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke (award # U01 NS058162-01), and the Defense Threat Reduction Agency-Joint Science and Technology Office, Medical S&T Division grant # 1.E0021_07_US_C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike K, Tanaka S, Hideshi Tojo H, Fukumoto S, Imamura S, Takigawa M. Kainic acid-induced dorsal and ventral hippocampal seizures in rats. Brain Res. 2001;900:65–71. doi: 10.1016/s0006-8993(01)02252-1. [DOI] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Braga MF. Soman induces ictogenesis in the amygdala and interictal activity in the hippocampus that are blocked by a GluR5 kainate receptor antagonist in vitro. Neuroscience. 2009;159:380–9. doi: 10.1016/j.neuroscience.2008.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Qashu F, Braga MF. Mechanisms regulating GABAergic inhibitory transmission in the basolateral amygdala: Implications for epilepsy and anxiety disorders. Amino Acids. 2007;32:305–15. doi: 10.1007/s00726-006-0415-x. [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Fritsch B, Qashu F, Braga MF. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res. 2008;78:102–116. doi: 10.1016/j.eplepsyres.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M. Do interictal discharges promote or control seizures? Experimental evidence from an in vitro model of epileptiform discharge. Epilepsia. 2001;42:2–4. doi: 10.1046/j.1528-1157.2001.042suppl.3002.x. [DOI] [PubMed] [Google Scholar]
- Baille V, Clarke PG, Brochier G, Dorandeu F, Verna JM, Four E, Lallement G, Carpentier P. Soman-induced convulsions: the neuropathology revisited. Toxicology. 2005;215:1–24. doi: 10.1016/j.tox.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Bajgar J, Sevelova L, Krejcova G, Fusek J, Vachek J, Kassa J, Herink J, de Jong LP, Benschop HP. Biochemical and behavioral effects of soman vapors in low concentrations. Inhal Toxicol. 2004;16:497–507. doi: 10.1080/08958370490442430. [DOI] [PubMed] [Google Scholar]
- Bajgar J. Complex view on poisoning with nerve agents and organophosphates. Acta Medica (Hradec Kralove) 2005;48:3–21. [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci. 1997;17:9308–14. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold CL, Schier JG. Organic phosphorus compounds--nerve agents. Crit Care Clin. 2005;21:673–89. doi: 10.1016/j.ccc.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Baze WB. Soman-induced morphological changes: an overview in the nonhuman primate. J Appl Toxicol. 1993;13:173–177. doi: 10.1002/jat.2550130306. [DOI] [PubMed] [Google Scholar]
- Benini R, D’Antuono M, Pralong E, Avoli M. Involvement of amygdala networks in epileptiform synchronization in vitro. Neuroscience. 2003;120:75–84. doi: 10.1016/s0306-4522(03)00262-8. [DOI] [PubMed] [Google Scholar]
- Bettler B, Boulter J, Hermans-Borgmeyer I, O’Shea-Greenfield A, Deneris ES, Moll C, Borgmeyer U, Hollmann M, Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990;5:583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci. 2003;23:442–452. doi: 10.1523/JNEUROSCI.23-02-00442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Li H. The physiological role of kainate receptors in the amygdala. Mol Neurobiol. 2004;30:127–141. doi: 10.1385/MN:30:2:127. [DOI] [PubMed] [Google Scholar]
- Brown MA, Brix KA. Review of health consequences from high, intermediate, and low-level exposure to organophosphorus nerve agents. J Appl Toxicol. 1998;6:393–408. doi: 10.1002/(sici)1099-1263(199811/12)18:6<393::aid-jat528>3.0.co;2-0. 1998. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Gloor P, Evans A, Jones-Gotman M, Watson C, Melanson D, Olivier A, Peters T, Lopes-Cendes I. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–725. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. Characterization of a slow cholinergic postsynaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol (Lond) 1984;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar S, Passaro E, Fried I, Engel J., Jr. Intracranial electrode monitoring for seizure localization: indications, methods and the prevention of complications. J Neurosci Nurs. 1996;28:280–289. doi: 10.1097/01376517-199610000-00002. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Unsicker K, von Bohlen und Halbach O. Muscarinic control of graded persistent activity in lateral amygdala neurons. Eur J Neurosci. 2006;24:3183–94. doi: 10.1111/j.1460-9568.2006.05200.x. [DOI] [PubMed] [Google Scholar]
- Engel J. Seizures and Epilepsy. F.A. Davis; Philadelphia: 1989. [Google Scholar]
- Feindel W, Rasmussen T. Temporal lobectomy with amygdalectomy and minimal hippocampal resection: review of 100 cases. Can. J Neurol Sci. 1991;18:603–605. doi: 10.1017/s0317167100032807. [DOI] [PubMed] [Google Scholar]
- Fernández de Sevilla D, Buño W. Presynaptic inhibition of Schaffer collateral synapses by stimulation of hippocampal cholinergic afferent fibres. Eur J Neurosci. 2003;17:555–558. doi: 10.1046/j.1460-9568.2003.02490.x. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci. 2004;19:2682–92. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- Goddard GV. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214:1020–21. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Grasshoff C, Gillessen T, Thiermann H, Wagner E, Szinicz L. The effect of acetylcholinesterase-inhibition on depolarization-induced GABA release from rat striatal slices. Toxicology. 2003;184:149–56. doi: 10.1016/s0300-483x(02)00571-1. [DOI] [PubMed] [Google Scholar]
- Harrison PK, Sheridan RD, Green AC, Scott IR, Tattersall JE. A guinea pig hippocampal slice model of organophosphate-induced seizure activity. J Pharmacol Exp Ther. 2004;310:678–686. doi: 10.1124/jpet.104.065433. [DOI] [PubMed] [Google Scholar]
- Hayward IJ, Wall HG, Jaax NK, Wade JV, Marlow DD, Nold JB. Decreased brain pathology in organophosphate-exposed rhesus monkeys following benzodiazepine therapy. Neurol Sci. 1990;98:99–106. doi: 10.1016/0022-510x(90)90185-p. [DOI] [PubMed] [Google Scholar]
- Isokawa-Akesson M, Wilson CL, Babb TL. Structurally stable burst and synchronized firing in human amygdala neurons: auto- and cross-correlation analyses in temporal lobe epilepsy. Epilepsy Res. 1987;1:17–34. doi: 10.1016/0920-1211(87)90047-7. [DOI] [PubMed] [Google Scholar]
- Jooma R, Yeh HS, Privitera MD, Rigrish D, Gartner M. Seizure control and extent of mesial temporal resection. Acta Neurochir. (Wien) 1995;133:44–49. doi: 10.1007/BF01404946. [DOI] [PubMed] [Google Scholar]
- Joosen MJ, Jousma E, van den Boom TM, Kuijpers WC, Smit AB, Lucassen PJ, van Helden HPM. Long-term cognitive deficits accompanied by reduced neurogenesis after soman poisoning. NeuroToxicology. 2009;30:72–80. doi: 10.1016/j.neuro.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Kadar T, Shapira S, Cohen G, Sahar R, Alkalay D, Raveh l. Sarin-induced neuropathology in rats. Hum Exp Toxicol. 1995;14:252–259. doi: 10.1177/096032719501400304. [DOI] [PubMed] [Google Scholar]
- Kairiss EW, Racine RJ, Smith GK. The development of the interictal spike during kindling in the rat. Brain Res. 1984;322:101–110. doi: 10.1016/0006-8993(84)91185-5. [DOI] [PubMed] [Google Scholar]
- Kassa J, Koupilova M, Herink J, Vachek J. The long-term influence of low-level sarin exposure on behavioral and neurophysiological functions in rats. Acta Medica (Hradec Kralove) 2001;44:21–7. [PubMed] [Google Scholar]
- Lallement G, Carpentier P, Collet A, Pernot-Marino I, Baubichon D, Sentenac-Roumanou H, Blanchet G. Involvement of glutamatergic system of amygdala in generalized seizures induced by soman: comparison with the hippocampus. CR Acad Sci III. 1991a;313:421–426. [PubMed] [Google Scholar]
- Lallement G, Carpentier P, Collet A, Pernot-Marino I, Baubichon D, Blanchet G. Effects of soman-induced seizures on different extracellular amino acid levels and on glutamate uptake in rat hippocampus. Brain Res. 1991b;563:234–40. doi: 10.1016/0006-8993(91)91539-d. [DOI] [PubMed] [Google Scholar]
- Lallement G, Denoyer M, Collet A, Pernot-Marino I, Baubichon D, Monmaur P, Blanchet G. Changes in hippocampal acetylcholine and glutamate extracellular levels during soman-induced seizures: influence of septal cholinoceptive cells. Neurosci Lett. 1992;139:104–7. doi: 10.1016/0304-3940(92)90868-8. [DOI] [PubMed] [Google Scholar]
- Lallement G, Dorandeu F, Filliat P, Carpentier P, Baille V, Blanchet G. Medical management of organophosphate-induced seizures. J Physiol (Paris) 1998;92:369–73. doi: 10.1016/S0928-4257(99)80007-2. [DOI] [PubMed] [Google Scholar]
- Layish I, Krivoy A, Rotman E, Finkelstein A, Tashma Z, Yehezkelli Y. Pharmacologic prophylaxis against nerve agent poisoning. Isr Med Assoc J. 2005;7:182–7. [PubMed] [Google Scholar]
- Li H, Chen A, Xing G, Wei ML, Rogawski MA. Kainate receptor-mediated heterosynaptic facilitation in the amygdala. Nat Neurosci. 2001;4:612–620. doi: 10.1038/88432. [DOI] [PubMed] [Google Scholar]
- López-Muñoz F, Alamo C, Guerra JA, García-García P. The development of neurotoxic agents as chemical weapons during the National Socialist period in Germany. Rev Neurol. 2008;47:99–106. [PubMed] [Google Scholar]
- Madison DV, Lancaster B, Nicoll RA. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987;7:733–41. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough JH, Jr, Smith RF, Smith CD. Behavioral correlates of soman-induced neuropathology: deficits in DRL acquisition. Toxicol Teratol. 1986;8:179–87. [PubMed] [Google Scholar]
- McDonough JH, Jr, Dochterman W, Smith CD, Shih T-M. Protection against nerve agent-induced neuropathology, but not cardiac pathology, is associated with the anticonvulsant action of drug treatment. Neurotoxicology. 1995;15:123–32. [PubMed] [Google Scholar]
- McDonough JH, Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–79. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Zoeffel LD, McMonagle J, Copeland TL, Smith CD, Shih TM. Anticonvulsant treatment of nerve agent seizures: anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Res. 2000;38:1–14. doi: 10.1016/s0920-1211(99)00060-1. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Racine RJ. Kindling mechanisms: current progress on an experimental epilepsy model. Prog Neurobiol. 1986;27:1–12. doi: 10.1016/0301-0082(86)90010-9. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Dufresne C, Kelly ME, McIntyre DC. Differential sensitivity of various temporal lobe structures in the rat to kindling and status epilepticus induction. Epilepsy Res. 1996;23:179–187. doi: 10.1016/0920-1211(95)00084-4. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Morita H, Yanagisawa N, Nakajima T, Shimizu M, Hirabayashi H, Okudera H, Nohara M, Midorikawa Y, Mimura S. Sarin poisoning in Matsumoto, Japan. Lancet. 1995;346:260–261. doi: 10.1016/s0140-6736(95)92170-2. [DOI] [PubMed] [Google Scholar]
- Myhrer T, Andersen JM, Nguyen NH, Aas P. Soman-induced convulsions in rats terminated with pharmacological agents after 45 min: neuropathology and cognitive performance. Neurotoxicology. 2005;26:39–48. doi: 10.1016/j.neuro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Myhrer T, Enger S, Aas P. Efficacy of immediate and subsequent therapies against soman-induced seizures and lethality in rats. Basic Clin Pharmacol Toxicol. 2006;98:184–91. doi: 10.1111/j.1742-7843.2006.pto_268.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Tuunanen J, Kalviainen R, Partanen K, Salmenpera T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32:233–253. doi: 10.1016/s0920-1211(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Quesney LF. Clinical and EEG features of complex partial seizures of temporal lobe origin. Epilepsia. 1986;27:S27–45. doi: 10.1111/j.1528-1157.1986.tb05738.x. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–34. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Salgado H, Bellay T, Nichols JA, Bose M, Martinolich L, Perrotti L, Atzori M. Muscarinic M2 and M1 receptors reduce GABA release by Ca2+ channel modulation through activation of PI3K/Ca2+ -independent and PLC/Ca2+ -dependent PKC. J Neurophysiol. 2007;98:952–965. doi: 10.1152/jn.00060.2007. [DOI] [PubMed] [Google Scholar]
- Santos MD, Pereira EF, Aracava Y, Castro NG, Fawcett WP, Randall WR, Albuquerque EX. Low concentrations of pyridostigmine prevent soman-induced inhibition of GABAergic transmission in the central nervous system: involvement of muscarinic receptors. J Pharmacol Exp Ther. 2003;304:254–65. doi: 10.1124/jpet.102.043109. [DOI] [PubMed] [Google Scholar]
- Saukkonen A, Kalviainen R, Partanen K, Vainio P, Riekkinen P, Pitkänen A. Do seizures cause neuronal damage? An MRI study in newly diagnosed and chronic epilepsy. Neuroreport. 1994;6:219–223. doi: 10.1097/00001756-199412300-00055. [DOI] [PubMed] [Google Scholar]
- Schecter WP. Cholinergic symptoms due to nerve agent attack: a strategy for management. Anesthesiol Clin North America. 2004;22:579–90. doi: 10.1016/j.atc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH., Jr. Organophosphorus nerve agents-induced seizures and efficacy of atropine sulfate as anticonvulsant treatment. Pharmacol Biochem Behav. 1999;64:147–53. doi: 10.1016/s0091-3057(99)00114-8. [DOI] [PubMed] [Google Scholar]
- Shih T, Duniho SM, McDonough JH. Control of nerve agent induced seizures is critical for neuroprotection and survival. Tox and Applied Pharm. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- Smolders I, Bortolotto ZA, Clarke VR, Warre R, Khan GM, O’Neill MJ, Ornstein PL, Bleakman D, Ogden A, Weiss B, Stables JP, Ho KH, Ebinger G, Collingridge GL, Lodge D, Michotte Y. Antagonists of GLU(K5)-containing kainate receptors prevent pilocarpine-induced limbic seizures. Nat Neurosci. 2002;5:796–804. doi: 10.1038/nn880. [DOI] [PubMed] [Google Scholar]
- Solberg Y, Belkin M. The role of excitotoxicity in organophosphorous nerve agents central poisoning. TiPS. 1997;18:183–185. doi: 10.1016/s0165-6147(97)89540-5. [DOI] [PubMed] [Google Scholar]
- Wade JV, Samson FE, Nelson SR, Pazdernik TL. Changes in extracellular amino acids during soman- and kainic acid-induced seizures. J Neurochem. 1987;49:645–50. doi: 10.1111/j.1471-4159.1987.tb02912.x. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Muscarinic responses of rat basolateral amygdaloid neurons recorded in vitro. J Physiol. 1992;449:121–154. doi: 10.1113/jphysiol.1992.sp019078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LE, Price JL. The functional anatomy of limbic status epilepticus in the rat. II. The effects of focal deactivation. J Neurosci. 1993;13:4810–4830. doi: 10.1523/JNEUROSCI.13-11-04810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser HG. Mesial temporal lobe epilepsy versus amygdalar epilepsy: late seizure recurrence after initially successful amygdalotomy and regained seizure control following hippocampectomy. Epileptic Disord. 2000;2:141–152. [PubMed] [Google Scholar]
- Womble MD, Moises HC. Muscarinic inhibition of M-current and a potassium leak conductance in neurones of the rat basolateral amygdala. J Physiol. 1992;457:93–114. doi: 10.1113/jphysiol.1992.sp019366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajeya J, De La Fuente A, Criado JM, Bajo V, Sánchez-Riolobos A, Heredia M. Muscarinic agonist carbachol depresses excitatory synaptic transmission in the rat basolateral amygdala in vitro. Synapse. 2000;38:151–160. doi: 10.1002/1098-2396(200011)38:2<151::AID-SYN6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]