Abstract
Dogs develop cognitive decline and a progressive accumulation of oxidative damage. In a previous longitudinal study, we demonstrated that aged dogs treated with either an antioxidant diet or with behavioral enrichment show cognitive improvement. The antioxidant diet included cellular antioxidants (Vitamins E, C, fruits and vegetables) and mitochondrial co-factors (lipoic acid and carnitine). Behavioral enrichment consisted of physical exercise, social enrichment and cognitive training. We hypothesized that the antioxidant treatment improved neuronal function through increased mitochondrial function. Thus, we measured reactive oxygen species (ROS) production and bioenergetics in mitochondria isolated from young, aged and treated aged animals. Aged canine brain mitochondria show significant increases in ROS production and a reduction in NADH-linked respiration. Mitochondrial function (ROS and NADH-linked respiration) was improved selectively in aged dogs treated with an antioxidant diet. In contrast behavioral enrichment had no effect on any mitochondrial parameters. These results suggest that an antioxidant diet improves cognition by maintaining mitochondrial homeostasis, which may be an independent molecular pathway not engaged by behavioral enrichment.
Keywords: beagle, carnitine, carbonyls, cognitive training, environmental enrichment, lipoic acid, vitamin E
INTRODUCTION
Oxidative damage and mitochondrial dysfunction may be critical events leading to neuronal dysfunction and contribute to the development of age-associated neurodegenerative disorders such as Alzheimer disease (AD) (Ames, 1993). In humans, some epidemiological studies have shown a positive effect of antioxidant supplementation on cognition and reduction of risk for developing AD (Engelhart, 2002; Morris, 2002), but other studies have failed to report beneficial effects (Masaki, 2000; Luchsinger, 2003). In some cases, epidemiological studies indicate that combinations of antioxidants are superior to single compound supplementation (Zandi and Group., 2004) and further reduce the risk of developing AD. Moreover, dietary intake has been shown to be superior to supplements in human studies (Morris, 2002). Difficulties in interpreting human studies stem from differences in the amount of supplements taken, the form and source, duration and regularity of use, and the challenges of determining the exact intake of antioxidants. However, recent reviews of the human literature have emphasized that antioxidants may be a promising approach for preventing AD (Rutten, 2002). Studies in animal models have provided strong evidence that oxidative damage contributes to neuronal and behavioral dysfunction. Reducing oxidative damage in aged rodents, by providing antioxidants either in supplements or in fruits and vegetables can improve learning, memory and motor function (e.g.(Socci et al., 1995; Joseph, 1998, 1999; Bickford et al., 2000b)).
Dogs develop cognitive decline in measures of learning and memory as a function of age (Milgram et al., 1994; Tapp et al., 2003b; Christie et al., 2005; Studzinski et al., 2006). In parallel, the aged dog brain progressively accumulates oxidative damage to proteins and lipids (Kiatipattanasakul et al., 1997; Papaioannou et al., 2001; Head et al., 2002; Skoumalova et al., 2003a; Rofina et al., 2004; Rofina et al., 2006). Further, endogenous antioxidant activity decreases with age in dogs (Kiatipattanasakul et al., 1997; Opii et al., 2008; Opii et al., In press). These age-effects suggest a link between oxidative damage and cognitive decline. Oxidative damage may be a consequence of mitochondrial dysfunction and the production of damaging free radicals (Shigenaga, 1994; Zhu et al., 2007). As a test of this hypothesis we treated aged dogs for over 2 years with a diet enriched in a broad spectrum of antioxidants and mitochondrial cofactors in the dose range typically used in human clinical trials. One of the advantages to using canines in diet studies is that absorption of nutrients from food, including antioxidants and mitochondrial co-factors, is similar to that in humans (Roudebush et al., 2005).
We have previously reported that this antioxidant enriched diet significantly improves cognition in aging dogs (Cotman et al., 2002b; Milgram et al., 2002b; Milgram et al., 2002a; Tapp et al., 2003a; Milgram et al., 2005; Siwak et al., 2005). In the same study, we also treated a group of animals with behavioral enrichment (physical exercise, social enrichment and cognitive training) and observed similar improvements in cognition. Interestingly, the combination of the treatments resulted in larger learning and memory improvement than either treatment alone (Milgram et al., 2005). In the current study, we hypothesized that the antioxidant treatment improved cognitive function through increased mitochondrial function.
MATERIALS AND METHODS
Subjects
Twenty-four beagles ranging in age at the start of the study from 8.05-12.35 yrs (Mean = 10.69 yrs SE=.25, 12M/12F) were obtained from the colony at the Lovelace Respiratory Research Institute. The typical median lifespan for animals in this colony is 13.2 years (Lowseth et al., 1990). Animals were born and maintained in the same environment and all had documented dates of birth and comprehensive medical histories. A second group of 5 young beagle dogs (3M,2F) from the same colony were also obtained to serve as untreated controls (Mean = 4.1 yrs SE = 0.19). All studies were conducted in compliance with approved IACUC protocols, consistent with the National Research Council’s Guide for the care and use of laboratory animals.
Group Assignments and Study Timeline
All dogs underwent extensive baseline cognitive testing as has been described previously (Milgram et al., 1994). Based on cognitive test scores, animals were ranked in order of cognitive ability and placed into one of four treatment groups such that each group contained animals with equivalent ranges of cognition (e.g. poor to good): C/C - control environment/control diet; E/C - enriched environment/control diet; C/A - control environment/antioxidant diet; E/A - enriched environment/antioxidant diet. One aged animal did not complete the baseline phase of the study and thus, 23 animals were treated for a period of 2.4-2.8 years. Over the duration of the study, 5 additional animals were not available or not included in the current experiments and included 1 C/C, 2 E/C, 2 C/A and 1 E/A animals. Four of these animals including 1 C/C dog (age=13.5 years), 2 E/C dogs (12.5 and 12.2 years), 1 C/A dog (13.8 years) had been euthanized prior to the end of the study for health reasons and one animal (E/A, 13.0 years) was used to pilot the mitochondrial assays (data not included). Thus at the end of the study and for the mitochondrial studies described in the current experiments there were a total of 18 animals available: N=5 (Mean age 14.05 years, SD=0.79) C/C animals, n=4 (Mean age 13.58 years, SD = 0.60) in the E/C group, N=4 (Mean age 12.75, SD=1.37) in the C/A group and N=5 (Mean age = 12.61 SD=1.72) in the E/A group. Age differences at the end of the study were not statistically significantly different (F(3,17)=1.46 p=0.27).
Environmental Enrichment
The environmental enrichment protocol consisted of housing animals in pairs (social enrichment), providing 2-20 minute outdoor walks per week (physical exercise) and continuous cognitive testing (cognitive enrichment). The cognitive enrichment consisted of a landmark discrimination task (Milgram et al., 2002b), an oddity discrimination task (Cotman et al., 2002b), and a size discrimination learning and reversal task (Head et al., 1998; Milgram et al., 2005).
Diet
The two foods were formulated to meet the nutrient profile for the American Association of Feed Control Officials recommendations for adult dogs (AAFCO 1999). Control and test diets were identical in composition, other than inclusion of a broad-based antioxidant and mitochondrial cofactor supplementation to the test diet. The control and enriched foods had the following differences in formulation on an as fed basis respectively: dl-alpha-tocopherol acetate, (120 ppm vs 1050 ppm), l-carnitine (< 20 ppm vs 260 ppm), dl-alpha-lipoic acid (<20 ppm vs 128 ppm), ascorbic acid as Stay-C (< 30 ppm vs 80 ppm), and 1% inclusions of each of the following (1 to 1 exchange for corn): spinach flakes, tomato pomace, grape pomace, carrot granules and citrus pulp.
Cortical Biopsy Procedure
Prior to euthanasia, 18aged dogs and all 5 young dogs underwent a cortical biopsy procedure. Animals were sedated with 0.2 mg/kg acepromazine given subcutaneously 20 min before induction of general anesthesia. General anesthesia was induced with 5% isoflurane by inhalation. An endotracheal tube was then placed in each dog and surgical level of anesthesia maintained with 2-3% isoflurane in oxygen. An incision was made just to the right of the sagittal crest over the parietal and frontal bones to the external frontal crest and then down on each end to form a flap. The muscles were then dissected away from the bones covering right parietal cortex region. A bone saw was used to cut a small 3 by 3 cm piece of the parietal bone and this was removed to expose the underlying meninges and brain. Cautery was used to cut the meninges and to provide hemostasis in the sample site. A sterile metal “scoop” was used to dissect out a 2 × 1× 1 cm block of parietal cortex tissue. Dogs were maintained under anesthesia until the final euthanasia procedure.
Mitochondrial Isolation
Isolated brain mitochondria were prepared as previously described with slight modifications (Sullivan et al., 1999; Sullivan et al., 2003). The fresh biopsied tissue was dissected and minced in ice-cold homogenization buffer (215 mM mannitol, 75 mM sucrose, 0.1% BSA, 1 mM EGTA, 20 mM HEPES, pH 7.2). All subsequent steps were conducted at 4°C. The tissue was rinsed with 10 ml homogenization buffer to remove residual blood and processed (8 strokes) using a hand-held tissue homogenizer (Thomas Scientific). The resulting homogenate was centrifuged for 5 min at 1300 x g, the supernatant was removed and centrifuged at 13,000 x g for 10 min and the pellet resuspended in 1 ml of isolation buffer. Synaptic mitochondria were then released by nitrogen cell disruption (1000 PSI, 10 min) as previously described (Sullivan et al., 2003). Following nitrogen decompression the sample was centrifuged at 13,000 x g and the pellet resuspended in ETGA-free isolation buffer and centrifuged at 10,000 x g and the final pellet collected for all subsequent experiments. Mitochondrial protein concentration was determined using a Pierce BCA kit. Purity of the preparations was assessed by Western blot analysis (data not shown) of the mitochondrial protein cytochrome oxidase subunit IV (Molecular Probes) and the synaptic protein postsynaptic density (PSD) 95 (Transduction Laboratories).
Mitochondrial Respiration
Respiration activity of the isolated mitochondria was measured using a Clark-type oxygen electrode (Hansatech Instruments, Norfolk, UK), and approximately 100-150 μg of isolated striatal or nigral mitochondrial protein were suspended and constantly stirred in a sealed and thermostatically controlled chamber at 37°C in respiration buffer (215 mmol/L mannitol, 75 mmol/L sucrose, 0.1% BSA, 20 mmol/L HEPES, 2 mmol/L MgCl, 2.5 mmol/L KH2PO4 at pH 7.2). The rate of oxygen consumption was determined based on the response slope of the isolated mitochondria to oxidative substrates as previously described (Sullivan et al., 2000; Sullivan et al., 2003). State II respiration was initiated by the addition of 5 mmol/L pyruvate and 2.5 mmol/L malate. State III respiration was initiated by the addition of 150 μmol/L ADP followed by the addition of oligomycin (1 μg/mL) to inhibit the ATP synthase and induce state IV respiration. The mitochondrial uncoupler carbonyl cyanide 4-trifluoromethoxy phenylhydrazone (1 μmol/L) was added to the chamber to induce maximum NADH-linked state V respiration (complex I driven). Rotenone (1 μmol/L) was added to the chamber to inhibit complex I of the electron transport system. Then, FADH maximum respiration (complex II driven) was assessed by the addition of succinate (10 mmol/L) to the chamber. Three runs were performed for each sample and the average was used for statistical analysis. All rates are expressed as nmoles of O2 consumed per minute per milligram of protein (O2/min/mg).
Mitochondrial ROS production
Mitochondrial ROS production was measured under state II conditions using the indicator dichlorodihydrofluorescein diacetate (H2DCFDA, Molecular Probes) as previously described (Sullivan et al., 1999; Sullivan et al., 2003). Briefly, 100-150 μg of isolated mitochondrial protein was incubated in a total volume of 200 μl respiration buffer including 5 mmol/L pyruvate and 2.5 mmol/L malate as substrate at 37°C for 15 min in the presence of 10 μM H2DCFDA, which was made fresh before each use. Negative controls in which mitochondrial samples were omitted were used for non-specific sources of ROS were background subtracted from all experimental samples. The relative amount of mitochondrial H2O2 and free radical production was measured using a CytoFluor 4000 fluorometric plate reader (excitation 490 nm, emission 526 nm). Wells containing known amounts of H2O2 were used as positive controls and for linear calibration of each plate.
Data Analysis
To measure age effects on mitochondrial function, untreated young (n=5) and aged (n=5) were compared. A repeated measures analysis of variance was used to detect age effects in ROS production and mitochondrial respiration (ETC). A repeated measures analysis of variance (ROS-5,10,15 min) with two independent factors - diet (aox, ctl) and behavioral enrichment (enr, ctl) was used to test for treatment effects on ROS production and on ETC components. All analyses were conducted using SPSS 14.0 for Windows and an alpha level of 0.05.
RESULTS
Mitochondrial ROS production increased as a function of time (5,10,15 min) for both young and aged animals (F(2,16)=90.9 p<.0005)(Figure 1A). Overall, mitochondria isolated from aged canine brain produced higher levels of ROS relative to young animals (F(1,8)=17.6 p=0.003)(Figure 1A). Note that young animals show minimal individual variability and error bars in the graph cannot be seen with the scaling of the y-axis. Further, aged animals produced higher levels of mitochondrial ROS at a more rapid rate than young animals (F(2,16)=14.9 p<.0005) (Figure 1A) and thus the slopes of the two curves are significantly different (Young slope = 31 OD/min and aged = 55 OD/min when fitted to a straight line). To detect age effects a repeated measures ANOVA was used to compare mitochondrial respiration rates for components of the ETC between young (n=5) and aged untreated control animals (n=5). There was a significant main effect of age (F(1,8)=37.3 p<.001) and an age by ETC component interaction (F(4,32)=37.2 p<.001)(Figure 1B). NADH-linked, complex I-driven respiration rate was higher in young relative to old animals (t(8)=6.1 p<.0005)(Figure 1B). No significant differences were detected in complex-II driven respiration as a function of age.
Figure 1.
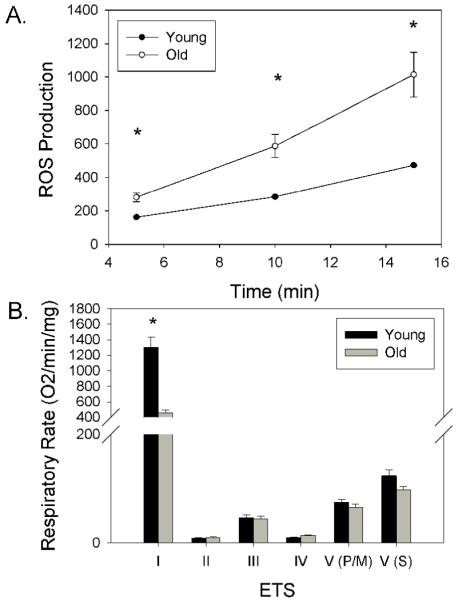
Mitochondrial dysfunction occurs with age in dogs. A. Reactive oxygen species production was higher overall in aged dogs and was further increased over time. B. Age effects were seen selectively as a decrease in NADH-linked, complex I-driven respiration rate but not in complex II, III, IV, or in complex V in the presence of the mitochondrial uncoupler FCCP and pyruvate/malate (V(P/M)) or following the addition of rotenone and succinate treatment (V(S)). Bars represent group means, error bars represent standard error of the mean. * p<.05 between young and old dogs at each time point measured. A. Note that young animals showed minimal individual variability and error bars in the graph cannot be seen with the scaling of the y-axis but the standard errors were as follows: ROS at 5 min = 0.87, at 10 min = 1.7 and at 15 min = 2.73.
To test the hypothesis that mitochondrial ROS production decreases in aged treated animals we used a repeated measures 2-way ANOVA. As with the age analysis, a significant rise in mitochondrial ROS was observed across time (F(2,28)=99.67 p<.0001)(Figure 2A). A significant main effect of diet (F(1,14)=5.0 p=0.042) but not behavioral enrichment (F(1,14)=0.17 p=0.69) nor a two way interaction between the treatment conditions was observed in terms of mitochondrial ROS production (F(1,14)=1.35 p=0.265) (Figure 2A). Further, a significant interaction between the rate of mitochondrial ROS production and diet treatment indicates that those fed an antioxidant diet produce less ROS (F(2,28)=5.6 p=0.009) (Figure 2B). A repeated measures ANOVA was used to compare different treatment conditions within the aged group on measures of mitochondrial bioenergetics. A significant main effect of diet (F(1,14)=4.9 p=0.044) but not of behavioral enrichment (F(1,14)=0.16 p=0.695) was observed (Figure 2C). However, the antioxidant diet selectively increased NADH-linked, complex I-driven respiration (F(4,56)=5.47 p=0.001)(Figure 2C). In comparison, aged treated dogs showed an increase in the maximum NADH-linked respiration, assessed using the uncoupler FCCP, relative to untreated aged animals but was still lower than younger animals (Figure 2D) (F(2,22)=41.9 p<.0005).
Figure 2.
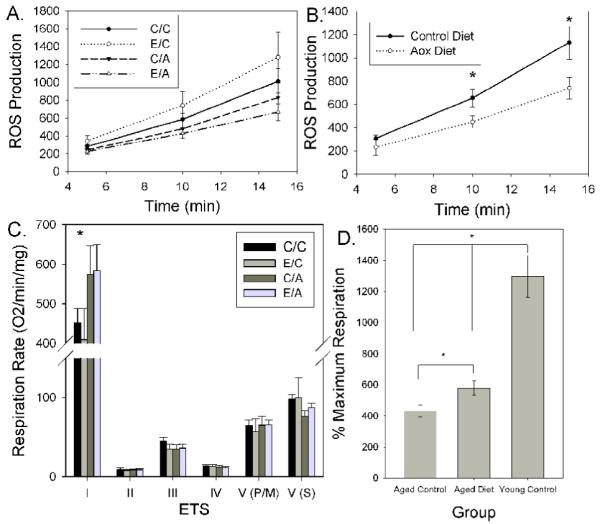
Mitochondrial dysfunction in aged dogs can be reduced with an antioxidant enriched diet. A. ROS production by mitochondria of aged dogs was reduced with treatment with either an antioxidant diet alone or with a combination of an antioxidant diet with behavioral enrichment. B. Reduced ROS production was selectively due to aged animals fed an antioxidant enriched diet. C. Respiration rates were significantly improved in the two treatment groups that received the antioxidant enriched diet in contrast to little change in complex II, III, IV and complex V respiration in the presence of the mitochondrial uncoupler FCCP and pyruvate/malate (V(P/M)) or following the addition of rotenone and succinate treatment (V(S)). * p<.05. D. For comparison, the % maximum respiration (state V/state IV) associated with NADH substrates shows that treated aged animals were significantly improved relative to untreated controls (p<.05) but not to the extent of untreated young dog levels. Bars represent group means, error bars represent standard error of the mean. In D, lines represent group differences. C/C - control environment/control diet, E/C - enriched environment/control diet; C/A - control environment/antioxidant diet; E/A - enriched environment/antioxidant diet.
DISCUSSSION
The current study tested the hypothesis that mitochondrial dysfunction increases with age and can be reduced with appropriate antioxidant dietary intervention using a dog model of human brain aging. The function of isolated mitochondria from 5 young and 20 aged beagles that were provided with either or both dietary and behavioral modifications for a period of over 2.5 years was examined. Our results show an age-dependent decrease in mitochondrial function and an increase in ROS production from aged dogs that could be reduced selectively with an antioxidant enriched diet but not through behavioral enrichment.
In dog brain, the accumulation of carbonyl groups, which is a measure of oxidative damage to proteins, increases with age (Head et al., 2002; Skoumalova et al., 2003b) and is associated with reduced endogenous antioxidant enzyme activity or protein levels such as in glutamine synthetase and superoxide dismutase (SOD) (Kiatipattanasakul et al., 1997; Head et al., 2002; Hwang et al., 2008; Opii et al., 2008). In several studies, a relation between age and increased oxidative damage has been inferred by measuring the amount of end products of lipid peroxidation (oxidative damage to lipids) including the extent of 4-hydroxynonenal (4HNE) (Papaioannou, 2001; Rofina et al., 2004; Rofina et al., 2006; Hwang et al., 2008), lipofuscin (LF) (Rofina et al., 2006), lipofuscin-like pigments (LFP) (Papaioannou, 2001; Rofina et al., 2004), or malondialdehyde (Head et al., 2002). Last, evidence of increased oxidative damage to DNA or RNA (8OHdG) in aged dog brain has been reported (Rofina et al., 2006). The results of the current study suggest that mitochondrial ROS production may be a contributor to increased oxidative damage in the dog brain. More specifically, our studies with isolated mitochondria now also show ROS production is significantly higher in aged animals relative to young controls suggesting impaired mitochondrial function.
Mitochondrial dysfunction can be partially reversed by providing aged animals with a diet rich in antioxidants and mitochondrial co-factors (Liu et al., 2002b; Liu and Ames, 2005) thereby reducing cognitive dysfunction (Cotman et al., 2002a). Fruits and vegetables are rich in flavonoids and carotenoids and other antioxidants (Joseph et al., 1998; Bickford et al., 2000a). Vitamin E is lipid soluble and acts to protect cell membranes from oxidative damage and vitamin C is essential in maintaining oxidative protection for the soluble phase of cells as well as preventing vitamin E from propagating free radical production (Butterfield et al., 2002). Alpha-lipoic acid is a cofactor for the mitochondrial respiratory chain enzymes, pyruvate and alpha-ketoglutarate dehydrogenases, as well as an antioxidant capable of redox recycling other antioxidants and raising intracellular glutathione levels (Packer et al., 1997a; Pocernich and Butterfield, 2003). In addition, l-carnitine is a precursor to acetyl-l-carnitine and is involved in mitochondrial lipid metabolism and maintaining efficient function (Rebouche, 1992; Calabrese et al., 2006).
As we hypothesized, aged animals treated with the antioxidant diet showed significant decreases in ROS levels compared to the control group implying the involvement of oxidative stress in mitochondrial dysfunction. It is also possible that age-related ROS increases that are subsequently decreased with treatment are generated by mitochondria themselves and implies that ROS are a byproduct of mitochondrial failure. In contrast, animals receiving the behavioral enrichment alone, levels of ROS production were comparable to untreated aged animals. One interpretation of these results is that cognition benefited from treatment independently of improved mitochondrial function. However, it is also possible, and likely, that multiple different pathways could be modified in dogs provided with either or both behavioral enrichment and the antioxidant diet leading to improved cognition. Thus, an alternative explanation is that reduced mitochondrial ROS production that is selective to animals fed the anti-oxidant enriched diet may also lead to cognitive improvements.
It is proposed the mitochondrial enzymes are particularly susceptible to oxidative damage and lose their enzymatic activities with age that may be countered by high doses of anti-oxidants and/or substrates and co-factors. Complex I or NADH-ubiquinone oxidoreductase of the ETC is the major acceptor of electrons from NADH and transfers them to coenzyme Q (ubiquinone), accompanied by pumping of protons from matrix into the inter-membrane space. Components of Complex I are also a major site for free-radical generation within the ETC. NADH driven respiration was found to be significantly higher in young compared to old animals. In the current study only NADH-linked, complex-I driven respiration was demonstrated to be reduced in the aged animals indicated by the absence of any respiratory decline after circumventing complex I and driving respiration via complex II of the ETC. However the current paradigm cannot distinguish between specific complex I enzyme dysfunction or damage upstream in components of the Kreb cycle. Regardless, NADH-linked respiration was improved in aged animals provided with a diet rich in a broad spectrum of cellular antioxidants as well as mitochondrial co-factors. Thus, the addition of mitochondrial co-factors either alone or in combination with the cellular antioxidants may have resulted in improved mitochondrial function by increasing NADH-linked respiration. However, it is not clear in the present study if the increase in mitochondrial NADH-linked, complex I driven respiration itself was the cause or effect of the decrease in ROS production.
Significant improvements in mitochondrial function in aged treated animals may be due to the addition of mitochondrial co-factors into the antioxidant diet. Targeting mitochondria specifically may be another way in which to reduce the production of reactive oxygen species and consequently, reduce oxidative damage. Thus, antioxidant strategies reported over the last several years also have been directed toward improving mitochondrial function (Liu et al., 2002c; Liu et al., 2002d; Liu et al., 2002a). While antioxidants such as vitamin E, the spin-trapping agent alpha-phenyl-N-tert-butylnitrone, and blueberry extracts (containing powerful antioxidants in polyphenolics) all have positive effects on behavior and brain pathology, these compounds are generally not specifically directed at the mitochondria but to cytoplasmic targets. Mitochondrial cofactors, particularly alpha-lipoic acid, carnitine and coenzyme Q, all have been shown to be effective in targeting mitochondria and reducing ROS production both in tissue culture and rodent models (Packer et al., 1997b; Hagen, 1998; Hagen et al., 1999; McGahon, 1999; Liu et al., 2002c; Liu et al., 2002d; Liu et al., 2002a). However, rodents do not naturally develop some forms of neuropathology observed in human brain aging, suggesting the need for extension (Kawarabayashi, 2001; Oddo et al., 2003) higher mammalian model systems (Price, 1985; Head, 2001) such as the canine (Cummings et al., 1996; Head, 2001).
When the results of the current study are taken in combination with our previous reports of improved cognition in aged antioxidant-treated dogs, we may now suggest that one of the neurobiological mechanisms underlying improved cognition, in antioxidant and mitochondrial co-factor fed animals is through improved mitochondrial function. These mechanisms may converge to improve neuronal, cortical and cognitive function.
Acknowledgements
This study was supported by funding from the NIH/NIA AG12694 and NS 048191 to PGS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford PC, Gould T, Briederick L, Chadman K, Pollock A, Young D, Shukitt-Hale B, Joseph J. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000a;866:211–217. doi: 10.1016/s0006-8993(00)02280-0. [DOI] [PubMed] [Google Scholar]
- Bickford PC, Gould T, Briederick L, Chadman K, Pollock A, Young D, Shukitt-Hale B, Joseph J. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000b;866:211–217. doi: 10.1016/s0006-8993(00)02280-0. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Drake J, Scapagnini G, Calabrese V. Vitamin E and neurodegenerative disorders associated with oxidative stress. Nutr Neurosci. 2002;5:229–239. doi: 10.1080/10284150290028954. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Giuffrida Stella AM, Calvani M, Butterfield DA. Acetylcarnitine and cellular stress response: roles in nutritional redox homeostasis and regulation of longevity genes. J Nutr Biochem. 2006;17:73–88. doi: 10.1016/j.jnutbio.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Christie LA, Studzinski CM, Araujo JA, Leung CS, Ikeda-Douglas CJ, Head E, Cotman CW, Milgram NW. A comparison of egocentric and allocentric age-dependent spatial learning in the beagle dog. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:361–369. doi: 10.1016/j.pnpbp.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Head E, Muggenburg BA, Zicker S, Milgram NW. Brain aging in the canine: a diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol Aging. 2002a;23:809–818. doi: 10.1016/s0197-4580(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Head E, Muggenburg BA, Zicker S, Milgram NW. Brain Aging in the Canine: A Diet Enriched in Antioxidants Reduces Cognitive Dysfunction. Neurobiology of Aging. 2002b;23:809–818. doi: 10.1016/s0197-4580(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Head E, Ruehl WW, Milgram NW, Cotman CW. The canine as an animal model of human aging and dementia. Neurobiology of Aging. 1996;17:259–268. doi: 10.1016/0197-4580(95)02060-8. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- Hagen TM, Ingersoll RT, Lykkesfeldt J, Liu J, Wehr CM, Vinarsky V, Bartholomew JC, Ames AB. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB. 1999;13:411–418. doi: 10.1096/fasebj.13.2.411. [DOI] [PubMed] [Google Scholar]
- Hagen TM, Ingersoll RT, Wehr CM, Lykkesfeldt J, Vinarsky V, Bartholomew JC, Song M-H, Ames BN. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc Natl Acad Sci USA. 1998;95:9562–9566. doi: 10.1073/pnas.95.16.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiol Aging. 1998;19:415–425. doi: 10.1016/s0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Head E, Liu J, Hagen TM, Muggenburg BA, Milgram NW, Ames BN, Cotman CW. Oxidative Damage Increases with Age in a Canine Model of Human Brain Aging. Journal of Neurochemistry. 2002;82:375–381. doi: 10.1046/j.1471-4159.2002.00969.x. [DOI] [PubMed] [Google Scholar]
- Head E, Milgram NW, Cotman CW. Neurobiological Models of Aging in the Dog and Other Vertebrate Species. In: Hof P, Mobbs C, editors. Functional Neurobiology of Aging. Academic Press; San Diego: 2001. pp. 457–468. [Google Scholar]
- Hwang IK, Yoon YS, Yoo KY, Li H, Choi JH, Kim DW, Yi SS, Seong JK, Lee IS, Won MH. Differences in Lipid Peroxidation and Cu,Zn-Superoxide Dismutase in the Hippocampal CA1 Region Between Adult and Aged Dogs. J Vet Med Sci. 2008;70:273–277. doi: 10.1292/jvms.70.273. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Prior RL, Cao G, Martin A, Taglialatela G, Bickford PC. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J Neurosci. 1998;18:8047–8055. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. The Journal of Neuroscience. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Shukkitt-Hale B, Denisova NA, Prior RL, Cao G, Martin A, Taglialatela G, Bickford PC. Long-term dietary strawberry, spinach or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J Neurosci. 1998;18:8047–8055. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiatipattanasakul W, Nakamura S, Kuroki K, Nakayama H, Doi K. Immunohistochemical detection of anti-oxidative stress enzymes in the dog brain. Neuropathology. 1997;17:307–312. [Google Scholar]
- Liu J, Ames BN. Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer’s disease, and Parkinson’s disease. Nutr Neurosci. 2005;8:67–89. doi: 10.1080/10284150500047161. [DOI] [PubMed] [Google Scholar]
- Liu J, Killilea DW, Ames BN. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L- carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002a;99:1876–1881. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Atamna H, Kuratsune H, Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci. 2002b;959:133–166. doi: 10.1111/j.1749-6632.2002.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Atamna H, Kuratsune H, Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci. 2002c;959:133–166. doi: 10.1111/j.1749-6632.2002.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: Partial reversal by feeding acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci U S A. 2002d;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowseth LA, Gillett NA, Gerlach RF, Muggenburg BA. The effects of aging on hematology and serum chemistry values in the beagle dog. Vet Clin Path. 1990;19:13–19. doi: 10.1111/j.1939-165x.1990.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60:203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- Masaki KH, Losonczy KG, Izmirlian G, Foley DJ, Ross GW, Petrovitch H, Havlik R, White LR. Association of vitamin E and C supplement use with cogntive function and dementia in elderly men. Neurology. 2000;54:1265–1272. doi: 10.1212/wnl.54.6.1265. [DOI] [PubMed] [Google Scholar]
- McGahon BM, Martin DSS, Horrobin DF, Lynch MA. Age-related changes in LTP and antioxidant defenses are reversed by an α-lipoic acid-enriched diet. Neurobiology of Aging. 1999;20:655–664. doi: 10.1016/s0197-4580(99)00050-0. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Weiner E, Thomas E. Cognitive functions and aging in the dog: Acquisition of nonspatial visual tasks. Behav Neurosci. 1994;108:57–68. doi: 10.1037//0735-7044.108.1.57. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Zicker SC, Head E, Muggenburg BA, Murphey H, Ikeda-Douglas C, Cotman CW. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiology of Aging. 2002a;23:737–745. doi: 10.1016/s0197-4580(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Muggenburg BA, Holowachuk D, Murphey H, Estrada J, Ikeda-Douglas CJ, Zicker SC, Cotman CW. Landmark discrimination learning in the dog: effects of age, an antioxidant fortified diet, and cognitive strategy. Neuroscience and Biobehavioral Reviews. 2002b;26:679–695. doi: 10.1016/s0149-7634(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Zicker SC, Ikeda-Douglas CJ, Murphey H, Muggenburg B, Siwak C, Tapp D, Cotman CW. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: a two-year longitudinal study. Neurobiol Aging. 2005;26:77–90. doi: 10.1016/j.neurobiolaging.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol. 2002;59:1125–1132. doi: 10.1001/archneur.59.7.1125. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Pierce WM, Cotman CW, Butterfield DA. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer’s disease. Neurobiol Aging. 2008;29:51–70. doi: 10.1016/j.neurobiolaging.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Pierce WM, Cotman CW, Butterfield DA. Proteomic Identification of Brain Proteins in the Canine Model of Human Aging Following a Long-Term Treatment with Antioxidants and a Program of Behavioral Enrichment: Relevance to Alzheimer’s Disease. Neurbiol Aging. doi: 10.1016/j.neurobiolaging.2006.09.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med. 1997a;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radical Biology and Medicine. 1997b;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- Papaioannou N, Tooten PC, van Ederen AM, Bohl JR, Rofina J, Tsangaris T, Gruys E. Immunohistochemical investigation of the brain of aged dogs. I. Detection of neurofibrillary tangles and of 4-hydroxynonenal protein, an oxidative damage product, in senile plaques. Amyloid. 2001;8:11–21. doi: 10.3109/13506120108993810. [DOI] [PubMed] [Google Scholar]
- Papaioannou N, Tooten PCJ, van Ederen AM, Bohl JRE, Rofina J, Tsangaris T, Gruys E. Immunohistochemical investigation of the brain of aged dogs. I. Detection of neurofibrillary tangles and of 4-hydroxynonenal protein, an oxidative damage product, in senile plaques. Amyloid: J Protein Folding Disord. 2001;8:11–21. doi: 10.3109/13506120108993810. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Butterfield DA. Acrolein inhibits NADH-linked mitochondrial enzyme activity: implications for Alzheimer’s disease. Neurotox Res. 2003;5:515–520. doi: 10.1007/BF03033161. [DOI] [PubMed] [Google Scholar]
- Price DL, Cork LC, Struble RG, Kitt CA, Price DL, Jr., Lehmann J, Hedreen JC. Neuropathological, neurochemical, and behavioral studies of the aging nonhuman primate. In: Davis RT, Leathers CW, editors. Behavior and Pathology of Aging in the Rhesus Monkey. Alan R. Liss, Inc.; New York: 1985. pp. 113–135. [Google Scholar]
- Rebouche CJ. Carnitine function and requirements during the life cycle. Faseb J. 1992;6:3379–3386. [PubMed] [Google Scholar]
- Rofina JE, Singh K, Skoumalova-Vesela A, van Ederen AM, van Asten AJ, Wilhelm J, Gruys E. Histochemical accumulation of oxidative damage products is associated with Alzheimer-like pathology in the canine. Amyloid. 2004;11:90–100. doi: 10.1080/13506120412331285779. [DOI] [PubMed] [Google Scholar]
- Rofina JE, van Ederen AM, Toussaint MJ, Secreve M, van der Spek A, van der Meer I, Van Eerdenburg FJ, Gruys E. Cognitive disturbances in old dogs suffering from the canine counterpart of Alzheimer’s disease. Brain Res. 2006;1069:216–226. doi: 10.1016/j.brainres.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Roudebush P, Zicker SC, Cotman CW, Milgram NW, Muggenburg BA, Head E. Nutritional management of brain aging in dogs. J Am Vet Med Assoc. 2005;227:722–728. doi: 10.2460/javma.2005.227.722. [DOI] [PubMed] [Google Scholar]
- Rutten BP, Steinbusch HW, Korr H, Schmitz C. Antioxidants and Alzheimer’s disease: from bench to bedside (and back again) Curr Opin Clin Nutr Metab Care. 2002;5:645–651. doi: 10.1097/00075197-200211000-00006. [DOI] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwak CT, Tapp PD, Head E, Zicker SC, Murphey HL, Muggenburg BA, Ikeda-Douglas CJ, Cotman CW, Milgram NW. Chronic antioxidant and mitochondrial cofactor administration improves discrimination learning in aged but not young dogs. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:461–469. doi: 10.1016/j.pnpbp.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Skoumalova A, Rofina J, Schwippelova Z, Gruys E, Wilhelm J. The role of free radicals in canine counterpart of senile dementia of the Alzheimer type. Exp Gerontol. 2003a;38:711–719. doi: 10.1016/s0531-5565(03)00071-8. [DOI] [PubMed] [Google Scholar]
- Skoumalova A, Rofina J, Schwippelova Z, Gruys E, Wilhelm J. The role of free radicals in canine counterpart of senile dementia of the Alzheimer type. Exp Gerontol. 2003b;38:711–719. doi: 10.1016/s0531-5565(03)00071-8. [DOI] [PubMed] [Google Scholar]
- Socci DJ, Crandall BM, Arendash GW. Chronic antioxidant treatment improves the cognitive performance of aged rats. Brain Research. 1995;693:88–94. doi: 10.1016/0006-8993(95)00707-w. [DOI] [PubMed] [Google Scholar]
- Studzinski CM, Christie LA, Araujo JA, Burnham WM, Head E, Cotman CW, Milgram NW. Visuospatial function in the beagle dog: An early marker of cognitive decline in a model of human aging and dementia. Neurobiol Learn Mem. 2006 doi: 10.1016/j.nlm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Sullivan P, Dube C, Dorenbos K, Steward O, Baram T. Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann Neurol. 2003;53:711–717. doi: 10.1002/ana.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Thompson MB, SW S. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp Neurol. 1999;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Ann Neurol. 2000;48:723–729. [PubMed] [Google Scholar]
- Tapp D, Siwak CT, Zicker SC, Head E, Muggenburg BA, Cotman CW, Murphey HL, Ikeda-Douglas CJ, Milgram NW. An Antioxidant Enriched Diet Improves Concept Learning in Aged Dogs. Society for Neuroscience Abstracts Abstract 836.12. 2003a [Google Scholar]
- Tapp PD, Siwak CT, Estrada J, Muggenburg BA, Head E, Cotman CW, Milgram NW. Size and Reversal Learning in the Beagle Dog as a Measure of Executive Function and Inhibitory Control in Aging. Learning and Memory. 2003b;10:64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, Norton C, Welsh-Bohmer KA, Breitner JC, Group CCS. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- Zhu X, Su B, Wang X, Smith MA, Perry G. Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci. 2007;64:2202–2210. doi: 10.1007/s00018-007-7218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


