Abstract
AIM: To establish the roles of lipopolysaccharide (LPS)/CD14/toll-like receptor 4 (TLR4)-mediated inflammation in a rat model of human necrotizing enterocolitis (NEC).
METHODS: Six pairs of intestinal samples from human NEC were collected before and after recovery for histological and molecular analysis of inflammatory cytokines and signaling components. In the rat NEC model, we isolated 10-cm jejunum segments and divided them into six groups (n = 6) for sham operation, treatment with LPS, bowel distension, combined bowel distension and LPS stimulation, and two therapeutic groups. The potential efficacy of a recombinant CD18 peptide and a monoclonal CD14 antibody was evaluated in the latter two groups. The serum and tissue levels of several inflammatory mediators were quantified by real-time polymerase chain reaction, ELISA and immunoblotting.
RESULTS: Human acute phase NEC tissues displayed significant increases (P < 0.05) in levels of TLR4, CD14, myeloid differentiation protein (MD)-2, tumor necrosis factor (TNF)-α and nuclear factor-κB when compared to those after recovery. The histological and inflammatory picture of human NEC was reproduced in rats that were treated with combined bowel distension and LPS, but not in the sham-operated and other control rats. Serum levels of interleukin-6 and TNF-α were also elevated. The NEC pathology was attenuated by treating the NEC rats with a monoclonal CD14 antibody or an LPS-neutralizing peptide.
CONCLUSION: LPS and distension are required to produce the histological and inflammatory features of NEC. A potential treatment option is blocking LPS activation and leukocyte infiltration.
Keywords: CD14 antigen, Lipopolysaccharide, Necrotizing enterocolitis, Pathogenesis, Therapy, Toll-like receptor 4
INTRODUCTION
Necrotizing enterocolitis (NEC) is a common gastrointestinal disorder in newborn and premature infants with a relatively high mortality rate (approximately 30%), and requires emergency medical treatment[1]. While the molecular pathogenesis of neonatal NEC is multifactorial and remains to be elucidated fully, its known risk factors include prematurity and low birth weight[1,2]. Ballance et al[3] have reviewed pathology specimens from a cohort of 84 neonatal NEC patients, and have reported that the most consistent features of the disease are ischemic necrosis, inflammation, and bacterial overgrowth. Since neonatal NEC does not develop in utero in the sterile gut, bacterial contamination and/or microbial involvement have been implicated in its pathogenesis[4]. Furthermore, > 90% of preterm infants who develop NEC have received enteral feeds[5], and there is an increased risk of NEC when these infants are overfed frequently[6-9]. This could result in intestinal injury because of impaired digestion of nutrients, delayed transit time and bacterial overgrowth[10].
Based on these recent findings, we believe that there is a connection between host immunity and pathogenesis of NEC, and the toll-like receptor (TLR)-mediated immunity apparently plays an important role. TLRs are evolutionarily conserved transmembrane molecules that help the immune system to recognize pathogen-associated molecular patterns, and TLR4 sensitizes immune cells to bacterial lipopolysaccharide (LPS). When stimulated by bacterial LPS, many intracellular signaling pathways are activated, and lead to the generation of nuclear factor (NF)-κB, which in turn promotes pro-inflammatory cytokine production and release[11]. Caplan et al[12] have reported upregulation of MD-2, one of the bacterial LPS co-receptors, in patients with NEC.
In overfed or food-intolerant neonates, bowel loops can become distended and filled with milk, and this creates a good nutritive medium for bacterial growth and colonization. We hypothesized that bacterial LPS or endotoxin from cultured bacteria can trigger inflammation in NEC. In order to validate this hypothesis, we studied the TLR4-mediated signaling in the jejunal tissue from a rat model of human NEC, and then used the findings to design novel therapeutic agents for use in neonatal patients with NEC. In this model, we ligated the jejunum, and distended the ligated jejunum with bacterial LPS in order to induce the inflammatory reaction of NEC.
MATERIALS AND METHODS
Human tissues
Necrotic NEC intestinal tissues were collected from six human infants (two girls and four boys) with stage 3 NEC, who underwent curative surgery at Queen Mary Hospital, Hong Kong. The mean gestational age of these NEC patients was 30 wk (range: 25-32 wk), and their mean birth weight was 1.51 kg (range: 0.57-2.51 kg). NEC was diagnosed between 2 and 32 d after birth (mean: 17 d). For comparison, intestinal tissues were collected during the recovery phase at the time of ileostomy closure. After surgical resection, each intestinal tissue sample was divided into two: one was snap-frozen in liquid nitrogen for determination of mRNA/protein levels, and the other was fixed in 10% buffered formalin for histological examination.
Reagents
Bacterial LPS from Salmonella typhimurium was purchased from Sigma (St. Louis, MO, USA), and was used to prepare a 5 μg/mL LPS solution with endotoxin-free saline[13]. A polyclonal antibody for use as an antagonist against the CD14 antigen (M-20) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The LPS-neutralizing peptide was prepared using our established procedures[14,15]. The protein content in each solution was determined using Lowry’s method[16].
Animals
One-month old Sprague-Dawley rats were obtained from the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited Laboratory Animal Unit of the University of Hong Kong (Pokfulam, Hong Kong). The rats were maintained in a pathogen-free facility with a 12-h light/dark cycle at ambient temperature and in a controlled humidity environment. The rats had ad libitum access to sterilized animal chow and water. All experimental procedures were reviewed, approved, and conducted in accordance with the ethical guidelines set forth by the University’s Committee on Using Live Animals for Teaching and Research.
Experimental NEC model
The rat model of human NEC was modified from our published model[17]. Before surgery, rats were fasted overnight, and anesthetized by intraperitoneal injection of sodium pentobarbital (40 mg/kg body weight). Once anesthetized, the abdomen was opened by a midline incision, and a 10-cm segment of the most proximal jejunum was isolated with its vascular pedicle intact. One end of the segment was closed by ligation. An 18-G angiocatheter was inserted and fixed to the other end. The angiocatheter was connected to an infusion set whose height was 100 cm above the operating table.
Each control and experimental group comprised six animals. For the sham-operated control rats, the angiocatheters were closed for 3 h using a plug. For the rats treated with LPS alone, 2 mL of 5 μg/mL LPS solution was injected directly into the jejunal segment. To increase intraluminal bowel pressure, the jejunal segment was distended with sterile saline at a pressure of 100 cm H2O for 3 h in four groups of rats. In two groups, the bowel was distended with sterile saline alone or combined with 5 μg/mL LPS. In the remaining two groups, the bowel was distended in an identical manner, and contained 5 μg/mL bacterial LPS and 0.8 μg/mL CD14 antibody or 0.8 μg/mL CD18 peptide, which has LPS-neutralizing capacity. After 3 h bowel distension, the animals were killed humanely by an overdose of sodium pentobarbital (100 mg/kg). Formalin-fixed and snap-frozen jejunal tissues were prepared for histological examination and determination of mRNA/protein levels, respectively. Blood was also collected from the inferior vena cava of all rats for serum/plasma preparation, and stored at -70°C until use.
Assessment of NEC severity
Sections (4 μm thick) of the harvested jejunal tissues were prepared for hematoxylin and eosin (HE) staining and immunohistochemistry[18,19]. The severity of NEC was scored histologically on the appearance of the intestine, using a modification of the method that was described by Caplan et al[20]: 0, normal intestine; 1, mild hemorrhage; 2, moderate hemorrhage and necrosis; 3, severe hemorrhagic necrotic lesions with or without pneumatosis. The degree of macrophage infiltration was determined following overnight incubation at 4°C with a macrophage staining reagent (Dako, Glostrup, Denmark). The resulting images were reviewed with a Nikon epifluorescent upright microscope E600 (Nikon, Tokyo, Japan), and captured with 3-CCD color camera DC-330 (DAGE-MTI, Michigan City, IN, USA).
Real-time polymerase chain reaction (PCR)
Real-time PCR was employed to determine the relative expression levels of CD14, TLR4 and MD-2 mRNA in human and rat jejunal tissues. First-strand cDNA was synthesized from extracted RNA from jejunal tissue[20], and amplifications were done using the Power SYBR Green PCR Master Mix (Invitrogen, Carlsbad, CA, USA) in an ABI PRISM 7700 sequence detector system (Applied Biosystems, Foster City, CA, USA)[21]. Normalization and comparison of mRNA transcript levels between the groups were then performed, as described previously[22].
Immunoblotting of NF-κB p65 and tumor necrosis factor (TNF)-α in the intestine of NEC patients
The nuclear levels of NF-κB p65 and TNF-α in the jejunal tissue of NEC patients were detected by antibody against NF-κB p65 (Invitrogen) and TNF-α (Santa Cruz Biotechnology), as described previously[15].
Determination of serum pro-inflammatory cytokine levels
Serum TNF-α and interleukin (IL)-6 levels in the control, sham-operated, and treated rats were determined using commercially available ELISA kits that were purchased from Dakewe (Shenzhen, China) and eBioscience (San Diego, CA, USA), respectively, in accordance with the manufacturer’s instructions.
Statistical analysis
Statistical analyses of the experimental data were performed using GraphPad Prism (version 4.0, GraphPad Software Inc, San Diego, CA, USA). Statistical significance was set at 5%, and data are presented as mean ± SD.
RESULTS
Human NEC intestinal histology and inflammatory pathology
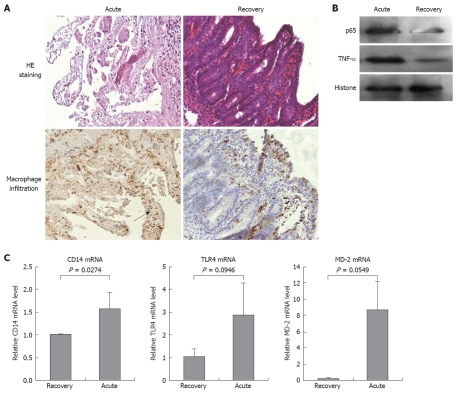
Serial samples of intestinal tissue from the acute and recovery phases of NEC were collected, prepared, and scored. Intestinal tissue from the acute-phase in which severe coagulative necrosis was observed histologically was given a score of 3 (Figure 1A). The appearance of the intestinal tissues from the recovery phase was normal, and given a score of 0. The extent of macrophage infiltration in acute-phase intestinal tissues was greater than that in the recovery-phase tissues. To determine the potential causal immunological factors, we studied the expression of key signaling components that are mediated by LPS activation. The protein levels of NF-κB p65 and TNF-α (Figure 1B) in acute-phase intestinal tissues were significantly higher than those in recovery-phase tissues. Furthermore, the gene transcript levels of CD14 and MD-2 (Figure 1C) were significantly increased in the acute-phase tissues, when compared to those in recovery-phase tissues. The TLR4 mRNA level was also higher in the acute-phase tissues than in the recovery-phase tissues, but this increase was not significant statistically.
Figure 1.
Pathological features of human necrotizing enterocolitis (NEC). A: Representative micrographs that show the microarchitecture of (upper panel), and the extent of macrophage infiltration (as marked by arrow) (lower panel) in jejunal tissues from NEC patients, which were collected in the acute phase and during recovery. Magnification × 200; B: Immunoblotting showed overexpression of TNF-α and upregulated nuclear content of the p65 subunit of NF-κB in jejunal tissues from NEC patients in the acute phase. Nuclear histone level was used as the loading control; C: Real-time PCR detected the mRNA transcript levels of CD14, TLR4 and MD-2 in jejunal tissues from NEC patients in the acute phase and during recovery. Statistical significance was determined by Student's t test, and all data are presented as the mean ± SD of triplicate samples from two independent experiments.
Molecular pathogenesis of experimental NEC
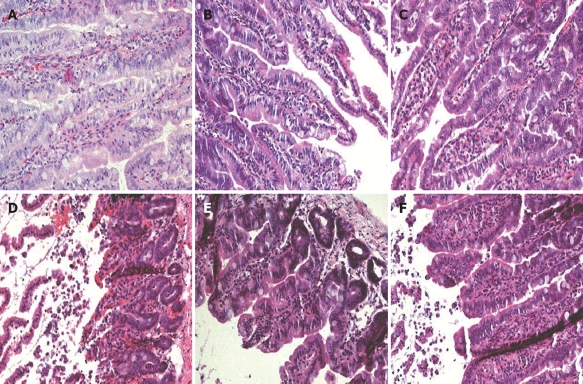
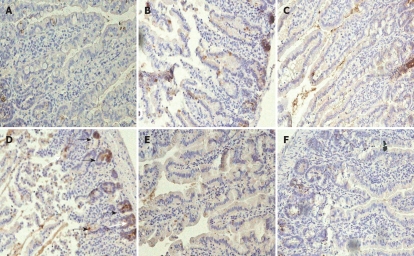
Rat jejunal histology: Compared to the sham-operated control specimens (Figure 2A), there was no apparent histological damage (score 0) in jejunal loops that were treated with LPS alone (Figure 2B) or distended with normal saline (Figure 2C). We observed histological changes (Figure 2D) (score 2), such as sloughing of the villi and vascular congestion in jejunal specimens from rats that were treated with a combination of saline distension and bacterial LPS. Although the changes were less severe, they did resemble those that were observed in acute-phase intestinal specimens from NEC patients, and were associated with substantial macrophage infiltration (Figure 3A-D).
Figure 2.
Histological features of rat jejunum. The severity of damage in the jejunum of rats from the different treatment groups was assessed histologically after HE staining. A: Sham-operated controls; B: LPS jejunal injection; C: Saline jejunal distension; D: Saline jejunal distension and LPS jejunal injection; E: Saline jejunal distension and LPS jejunal injection, and CD14 antibody treatment; F: Saline jejunal distension and LPS jejunal injection, and LPS-neutralizing peptide treatment. Only in (D) did the jejunal tissue show histological changes with a score of 2 for NEC (sloughing of villi and vascular congestion). Magnification × 200.
Figure 3.
Macrophage infiltration into the rat jejunum. The extent of macrophage infiltration into the jejunum of rats from the different treatment groups was evaluated by immunohistochemistry using a macrophage staining reagent. Stained macrophages are marked by arrows. A: Sham-operated control; B: LPS jejunal injection; C: Saline jejunal distension; D: Saline jejunal distension and LPS jejunal injection; E: Saline jejunal distension and LPS jejunal injection, and CD14 antibody treatment; F: Saline jejunal distension and LPS jejunal injection, and LPS-neutralizing peptide treatment. Magnification × 200.
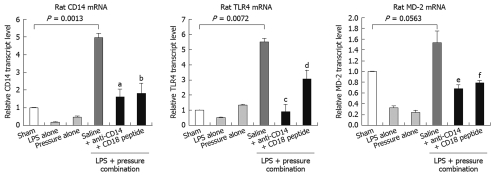
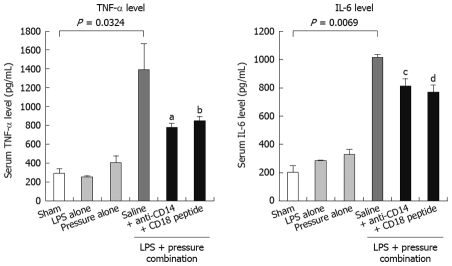
Jejunal distension requires bacterial LPS to trigger inflammatory reactions: To determine whether the inflammatory responses could be induced in the experimental NEC model, we assessed the key LPS-mediated signaling components or reporters (CD14, TLR4, MD-2, TNF-α and IL-6) at the transcript or protein level in the jejunal segments and plasma from each experimental group. The results were consistent with the histological findings. Combination treatment of intestinal distension and bacterial LPS jejunal injection significantly raised the level of CD14, TLR4 and MD-2 mRNA (P < 0.05) when compared to those in saline-distended jejunal tissues, those following jejunal distension alone and LPS jejunal injection alone, or those from the sham-operated controls (Figure 4). We then measured the systemic cytokine responses in the experimental NEC animals by ELISA. The serum TNF-α and IL-6 levels in the combined distension + LPS group were 3-4 times higher than those of the other single treatment groups (distension or LPS jejunal injection alone) and the sham-operated control rats (Figure 5A and B).
Figure 4.
CD14, TLR4 and MD-2 mRNA expression in rat jejunum. Real-time PCR detected mRNA transcript levels for CD14, TLR4 and MD-2 in the jejunum of rats from the different treatment groups. Distension of jejunum with LPS-containing saline upregulated the expression of all studied transcripts, and these upregulated levels were attenuated by CD14 antibody and LPS-neutralizing peptide treatments. There were six rats in each experimental group. Data are displayed as the mean ± SD, and analyzed statistically by one-way ANOVA. aP = 0.00126; bP = 0.00498; cP = 0.00838; dP = 0.0127; eP = 0.0151; fP = 0.0187.
Figure 5.
Serum TNF-α and IL-6 levels in NEC rats. Serum TNF-α and IL-6 levels in all NEC rats were measured by commercial ELISA kits. Distension of jejunum with LPS-containing increased significantly serum TNF-α and IL-6 levels, and these increases were suppressed when CD14 antibody and LPS-neutralizing peptide were added to the saline. There were six rats in each experimental group. Data are displayed as the mean ± SD, and analyzed statistically by one-way ANOVA. aP = 0.0237; bP = 0.0259; cP = 0.0819; dP = 0.0477.
Therapeutic potential of CD14/CD18 blockage in NEC inflammatory response
We tested the therapeutic efficacy of CD14 or CD18 blockade in the established rat NEC model by using a CD14-neutralizing monoclonal antibody (M-20) as an antagonist, and a novel LPS-neutralizing peptide, CD18 βA peptide, which have been shown to neutralize the biological activity of endotoxin. Treatment with M-20 (Figure 2E) or LPS-neutralizing peptide (Figure 2F) restored the jejunal microarchitecture to near normal (score 0), and attenuated the increased extent of macrophage infiltration in those loops that was distended with LPS-containing saline, and contained either an antibody against CD 14 (Figure 3E) or LPS-neutralizing peptide (Figure 3F). This was a sharp contrast to the jejunal distension that was caused by LPS-containing saline (Figures 2D and 3D), which caused marked histological changes such as villous sloughing and destruction. These histological changes were accompanied by concomitant changes in the expression levels of CD14, MD-2 and TLR4 mRNA (Figure 4), and the serum levels of TNF-α and IL-6 (Figure 5).
DISCUSSION
We investigated the role of innate immunity in the pathogenesis of NEC by examining the LPS/CD14/TLR4-mediated inflammatory response in jejunal tissues from premature neonates with NEC. Using our recently established rat NEC model, we demonstrated that disease development requires intestinal bowel distension and bacterial LPS stimulation. In the human NEC tissues, increases in NF-κB p65 and TNF-α and mRNA for CD14, TLR4 and MD-2 were found. All these signaling-cascade increases were also noted in the intestine of the rat NEC model, with a parallel increase in serum TNFα and IL-6. Histologically, changes in the LPS-distended rat bowel and human NEC tissue were similar. Furthermore, we showed in this model that the consequent pro-inflammatory reactions could be suppressed or modulated by blocking endotoxin activity using a monoclonal CD14 antibody, and a CD18 βA peptide that neutralizes LPS binding and inhibits leukocyte infiltration into inflamed tissues. From these results, we concluded that each molecule may have therapeutic potential for treating infants with NEC.
The essential role of the innate TLR4 immunity has been reported in a recent study in which TLR4-mutant C3H/HeJ mice were protected from developing NEC[23]. The findings in our study support this essential role. We demonstrated that the activity of the TLR4 signaling pathway was upregulated in intestinal tissues from premature neonates with NEC. Given that TLR4 is the major receptor for bacterial LPS on immune and some epithelial cells, as well as endothelial cells, we examined whether activation of the TLR4 signaling pathway occurred in the intestine of rats with experimentally induced NEC. In this model, we injected or infused bacterial LPS into a jejunal segment because the jejunum, unlike the ileum and colon, is less likely to be contaminated by resident bacteria. By choosing this segment, we assumed that we would have better control over the bacterial LPS concentration in our investigation. We studied also the effect of bowel distension on the pathogenesis of NEC, because intestinal distension as a result of overfeeding is one of the many risk factors for NEC.
One-month-old rats were chosen for the study because, in our previous study[17], the typical NEC histological pictures in the intestine could be seen when the correct distension pressure and bacterial concentration were used. Further, these rats are more stable and can avoid changes caused by operative risks such as hypotension and hypothermia. NEC also occurs in full-term and older infants, and these infants have been found to have higher congenital heart diseases that also can cause hypoxia[24].
Our data showed that bowel distension with saline or LPS injection alone could not induce significant inflammatory responses in rat jejunal segments. In contrast, bowel distension with saline that contained bacterial LPS induced substantial release of TNF-α and IL-6 into the serum, and increased the expressions of the LPS receptor and co-receptor mRNAs, and the extent of leukocyte infiltration into the jejunal mucosa. These findings suggest that bowel distension in overfed premature neonates is capable of potentiating LPS action on innate TLR4 immunity. The underlying mechanism by which bowel distension potentiates the action of LPS was not studied here. However, we believe that bowel distension injures the intestinal mucosa, and exposes the enterocytes to bacterial LPS. The hypoxic environment that is induced by bowel distension might also participate in this process by upregulating TNF-α production from inflammatory macrophages[24].
Perhaps more importantly, our results identified a monoclonal CD14 neutralizing antibody and an LPS-neutralizing peptide as potential therapeutic agents for use in NEC. We showed that both molecules could suppress the inflammatory response that was induced by bowel distension and bacterial LPS. This provides additional evidence for the critical role of innate TLR4 immunity in the pathogenesis of NEC. Moreover, our findings suggest that antagonism against members of the LPS signaling pathway could be beneficial therapeutically in the treatment of NEC. Given that the current management of premature neonates with NEC is still challenging, our novel molecules for treating NEC are of potential therapeutic importance.
In conclusion, our results suggest that bowel distension from overfeeding in premature neonates damages the intestinal mucosa. This resultant injury potentiates the action of bacterial LPS on enterocytes and resident immune cells, and triggers an immunological cascade that results in the development of NEC. In addition, we have shown that antagonistic molecules that target key members of this cascade can be used as potential therapeutic agents for treating infants with NEC.
COMMENTS
Background
Neonatal necrotizing enterocolitis (NEC) is a disease that affects mainly premature babies and its mortality and morbidity remain high. The pathogenesis of the disease is still not known and preventive measures have not been agreed upon. Its treatment is still difficult.
Research frontiers
Bacteria are required for the development of NEC. Since there are no bacteria in utero, there is no NEC in fetuses. At present, there is no single bacterium that has been identified to cause NEC. Many clinical studies have shown that premature babies with overfeeding or feed intolerance have a higher incidence of NEC. However, the mechanism by which feeding problems cause NEC is still unknown.
Innovations and breakthroughs
In many other studies, it has been shown that, in NEC tissue and patient serum, there is a marked increase in the pro-inflammatory cytokine profile, although again, the cause is unknown. In the present study, human NEC tissue showed increased levels of nuclear factor (NF)-κB p65, tumor necrosis factor (TNF)-α, and CD14, toll-like receptor 4 (TLR4) and myeloid differentiation protein (MD)-2 mRNA. Bowel distension is common in overfed and feed-intolerant babies. In the present rat model used to mimic this condition, the intestine distended with lipopolysaccharide (LPS) showed increased mRNA for CD14, TLR4 and MD-2. Also, the rat serum showed raised TNF-α and IL6. Furthermore, the intestinal tissue in the rat after distension with LPS showed the typical features of NEC with villous sloughing and vascular congestion. These changes could be suppressed by blocking the endotoxin activity using a monoclonal CD14 antibody or a CD18 peptide that neutralized LPS binding and thus inhibited leukocyte infiltration into the inflamed tissue.
Applications
This study provides evidence that NEC can be caused by bowel distension with bacterial LPS, which is common in overfeeding and feed intolerance. The process can be prevented by applying a carefully controlled feeding regimen to premature babies. Early detection and stoppage of feeding in the feed-intolerant babies can prevent the activation of the cytokine cascade pathway of the LPS and TLR4. In babies who have developed NEC, anti-CD14 antibody and CD18 neutralizing peptide can be a potential strategy to block the LPS activation pathway and prevention of leukocyte infiltration.
Terminology
TLRs are transmembrane molecules that help the immune system to recognize pathogens, and TLR4 is among the one that sensitizes immune cells to bacterial LPS. When stimulated by bacterial LPS, many intracellular signaling pathways are activated and lead to the generation of a cascade of pro-inflammatory cytokine production and release.
Peer review
It should be emphasized that this rat model of NEC resembles the phenotype of human NEC. Therefore, further studies may be needed to prove whether there is a correlation between the pathogenesis and possible treatment in the rat and human models.
Acknowledgments
The authors acknowledge the technical assistance of Mr. Jensen To and Ms. Jana Wo of the Experimental Surgical Unit of The Hong Kong University, and Dr. Arieh Bomzon for his critical review and professional editing of the manuscript.
Footnotes
Supported by The Research Grants Council of Hong Kong to Luk JM
Peer reviewers: Didier Merlin, PhD, Associate Professor, Department of Medicine Division of Digestive Diseases, Emory University, 615 Michael Street, Atlanta, GA 30322, United States; Frank Hoentjen, MD, PhD, Department of Gastroenterology, VU Medical Center, Sumatrastraat 16, 2022XL Haarlem, The Netherlands
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
References
- 1.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 2.Neu J. Neonatal necrotizing enterocolitis: an update. Acta Paediatr Suppl. 2005;94:100–105. doi: 10.1111/j.1651-2227.2005.tb02163.x. [DOI] [PubMed] [Google Scholar]
- 3.Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr. 1990;117:S6–S13. doi: 10.1016/s0022-3476(05)81124-2. [DOI] [PubMed] [Google Scholar]
- 4.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32:70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Henderson G, Craig S, Brocklehurst P, McGuire W. Enteral feeding regimens and necrotising enterocolitis in preterm infants: a multicentre case-control study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F120–F123. doi: 10.1136/adc.2007.119560. [DOI] [PubMed] [Google Scholar]
- 6.Patole SK, de Klerk N. Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: a systematic review and meta-analysis of observational studies. Arch Dis Child Fetal Neonatal Ed. 2005;90:F147–F151. doi: 10.1136/adc.2004.059741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patole S. Strategies for prevention of feed intolerance in preterm neonates: a systematic review. J Matern Fetal Neonatal Med. 2005;18:67–76. doi: 10.1080/14767050500127724. [DOI] [PubMed] [Google Scholar]
- 8.Kamitsuka MD, Horton MK, Williams MA. The incidence of necrotizing enterocolitis after introducing standardized feeding schedules for infants between 1250 and 2500 grams and less than 35 weeks of gestation. Pediatrics. 2000;105:379–384. doi: 10.1542/peds.105.2.379. [DOI] [PubMed] [Google Scholar]
- 9.Berseth CL, Bisquera JA, Paje VU. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2003;111:529–534. doi: 10.1542/peds.111.3.529. [DOI] [PubMed] [Google Scholar]
- 10.Schnabl KL, Van Aerde JE, Thomson AB, Clandinin MT. Necrotizing enterocolitis: a multifactorial disease with no cure. World J Gastroenterol. 2008;14:2142–2161. doi: 10.3748/wjg.14.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Caplan MS, Simon D, Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:145–151. doi: 10.1053/j.sempedsurg.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Luk JM, Kumar A, Tsang R, Staunton D. Biotinylated lipopolysaccharide binds to endotoxin receptor in endothelial and monocytic cells. Anal Biochem. 1995;232:217–224. doi: 10.1006/abio.1995.0010. [DOI] [PubMed] [Google Scholar]
- 14.Lee NP, Tsang S, Cheng RH, Luk JM. Increased solubility of integrin betaA domain using maltose-binding protein as a fusion tag. Protein Pept Lett. 2006;13:431–435. doi: 10.2174/092986606776819493. [DOI] [PubMed] [Google Scholar]
- 15.Wong KF, Luk JM, Cheng RH, Klickstein LB, Fan ST. Characterization of two novel LPS-binding sites in leukocyte integrin betaA domain. FASEB J. 2007;21:3231–3239. doi: 10.1096/fj.06-7579com. [DOI] [PubMed] [Google Scholar]
- 16.Luk JM, Lam CT, Siu AF, Lam BY, Ng IO, Hu MY, Che CM, Fan ST. Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values. Proteomics. 2006;6:1049–1057. doi: 10.1002/pmic.200500306. [DOI] [PubMed] [Google Scholar]
- 17.Chan KL, Ng SP, Chan KW, Wo YH, Tam PK. Pathogenesis of neonatal necrotizing enterocolitis: a study of the role of intraluminal pressure, age and bacterial concentration. Pediatr Surg Int. 2003;19:573–577. doi: 10.1007/s00383-003-0979-4. [DOI] [PubMed] [Google Scholar]
- 18.Luk JM, Su YC, Lam SC, Lee CK, Hu MY, He QY, Lau GK, Wong FW, Fan ST. Proteomic identification of Ku70/Ku80 autoantigen recognized by monoclonal antibody against hepatocellular carcinoma. Proteomics. 2005;5:1980–1986. doi: 10.1002/pmic.200401084. [DOI] [PubMed] [Google Scholar]
- 19.Mok BW, Yeung WS, Luk JM. Differential expression of gap-junction gene connexin 31 in seminiferous epithelium of rat testes. FEBS Lett. 1999;453:243–248. doi: 10.1016/s0014-5793(99)00714-0. [DOI] [PubMed] [Google Scholar]
- 20.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, Xiao Y, Thomson R Jr. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 21.Luk JM, Mok BW, Shum CK, Yeung WS, Tam PC, Tse JY, Chow JF, Woo J, Kam K, Lee KF. Identification of novel genes expressed during spermatogenesis in stage-synchronized rat testes by differential display. Biochem Biophys Res Commun. 2003;307:782–790. doi: 10.1016/s0006-291x(03)01250-6. [DOI] [PubMed] [Google Scholar]
- 22.Lo CY, Lam KY, Leung PP, Luk JM. High prevalence of cyclooxygenase 2 expression in papillary thyroid carcinoma. Eur J Endocrinol. 2005;152:545–550. doi: 10.1530/eje.1.01883. [DOI] [PubMed] [Google Scholar]
- 23.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 24.Liu FQ, Liu Y, Lui VC, Lamb JR, Tam PK, Chen Y. Hypoxia modulates lipopolysaccharide induced TNF-alpha expression in murine macrophages. Exp Cell Res. 2008;314:1327–1336. doi: 10.1016/j.yexcr.2008.01.007. [DOI] [PubMed] [Google Scholar]