Abstract
AIM: To assess the prevalence of portal hypertension (PH) related colorectal lesions in liver transplant candidates, and to evaluate its association with the severity of PH.
METHODS: Between October 2004 and December 2005, colonoscopy was performed in 92 cirrhotic liver transplant candidates. We described the lesions resulting from colorectal PH and their association with the grade of PH in 77 patients who underwent measurement of hepatic venous pressure gradient (HVPG).
RESULTS: Mean age was 55 years and 80.7% of patients were men. The main etiology of cirrhosis was alcoholism (45.5%). Portal hypertensive colopathy (PHC) was found in 23.9%, colonic varices in 7.6% and polyps in 38% of patients (adenomatous type 65.2%). One asymptomatic patient had a well-differentiated adenocarcinoma. The manifestations of colorectal PH were not associated with the etiology of liver disease or with the Child-Pugh grade. Ninety percent of patients with colopathy presented with gastroesophageal varices (GEV), and 27.5% of patients with GEV presented with colopathy (P = 0.12). A relationship between higher values of HVPG and presence of colopathy was observed (19.9 ± 6.2 mmHg vs 16.8 ± 5.4 mmHg, P = 0.045), but not with the grade of colopathy (P = 0.13). Preneoplastic polyps and neoplasm (P = 0.02) and spontaneous bacterial peritonitis (P = 0.006) were more prevalent in patients with colopathy. We did not observe any association between previous β-blocker therapy and the presence of colorectal portal hypertensive vasculopathy.
CONCLUSION: PHC is common in cirrhotic liver transplant candidates and is associated with higher portal pressure.
Keywords: Liver cirrhosis, Portal hypertension, Polyps, Colopathy, Liver transplantation
INTRODUCTION
Orthotopic liver transplantation (OLT) is an effective therapeutic approach to chronic end-stage and acute liver diseases[1]. Portal hypertension (PH)-related lesions in the gastrointestinal tract of cirrhotic patients are frequent. In the evaluation of liver transplant candidates these lesions must be taken into account so that appropriate treatment can be administered before OLT. Although gastroscopy is a well established technique for the detection of upper PH-related lesions in liver transplant candidates, colonoscopy is not performed routinely in all centers.
In OLT candidates, it is important to identify and prevent the risk of bleeding from gastroesophageal varices (GEV) and portal hypertensive gastropathy. Furthermore, although colorectal lesions are a source of acute and chronic bleeding, they have received little attention in the literature. Colonoscopy allows us to evaluate the presence of PH-related lesions such as portal hypertensive colopathy (PHC), colorectal varices and hemorrhoids[2,3]. The variability of the results of previous studies does not allow us to define with any certainty the prevalence of these lesions. Thus prevalence ranges from 3%[4] to 84%[5] for PHC, and from 3.6%[6] to 89%[7] for colorectal varices and from 22%[8] to 79%[9] for hemorrhoids.
The association between portal hypertensive vasculopathy and the severity of PH has been evaluated elsewhere. Four studies have evaluated this association by measuring indirect parameters of PH[10-13], while four other authors have evaluated it using the gold-standard measurement of PH grade, that is, the hepatic venous pressure gradient (HVPG). Nevertheless the results are contradictory[9,14-16].
On the other hand, recent studies have demonstrated an increase in the risk of adenomatous polyps and neoplasm in liver transplant recipients, so the detection of these lesions prior to OLT is essential[17]. The presence of these lesions has been reported to be as high as 42%. The question is that whether colorectal PH-related lesions influence the development of polyps is unknown.
The objectives of the present study were to define the prevalence of lower gastrointestinal abnormalities detected as a result of an endoscopic screening of cirrhotic patients being evaluated for liver transplantation, and to relate the occurrence of these lesions to the degree of PH measured using the HVPG, and the severity of liver disease.
MATERIALS AND METHODS
Patients
The study was designed and performed according to the principles of the Declaration of Helsinki, and informed consent was obtained from each patient. The protocol was reviewed and approved by the Hospital Ethics Committee.
Between October 2004 and December 2005, one hundred and forty-nine patients were considered for OLT due to acute or chronic decompensated liver disease. One hundred and twenty-one patients had liver cirrhosis. Of these, 92 were evaluated by colonoscopy as a part of pre-OLT screening for colorectal cancer or preneoplastic manifestations. The selection criteria for colonoscopy were age over 50 years and/or clinical symptoms such as abdominal pain or rectal bleeding, or family history of colorectal cancer. Diagnosis of cirrhosis was based on histology assessment or a combination of clinical, laboratory and ultrasonography findings. The severity of cirrhosis was classified according to the Child-Pugh classification. Four patients were excluded due to the presence of an implanted permeable transjugular intrahepatic portosystemic shunt before evaluation for OLT. Therefore the final cohort consisted of 88 patients. Patients with previous abdominal or pelvic surgery, hemorrhoidectomy or a previous history of malignancy (except primary liver cancer) and those who refused to undergo colonoscopic examination were not included in the study. Of the 88 patients evaluated, 50 (56.8%) were receiving non-selective β-blockers as primary or secondary prophylaxis for PH bleeding, and 12 (13.6%) had received previous endoscopic band ligation (EBL).
Information on epidemiological features, etiology of cirrhosis, Child-Pugh grade, blood test results, presence of hepatocellular carcinoma, clinical manifestations of PH [hepatic encephalopathy, ascites, spontaneous bacterial peritonitis (SBP), and variceal bleeding], and upper PH-related endoscopic findings (GEV, portal hypertensive gastropathy and duodenopathy) were analyzed. In addition, the severity of PH was quantified by measuring portal pressure.
Endoscopic examinations
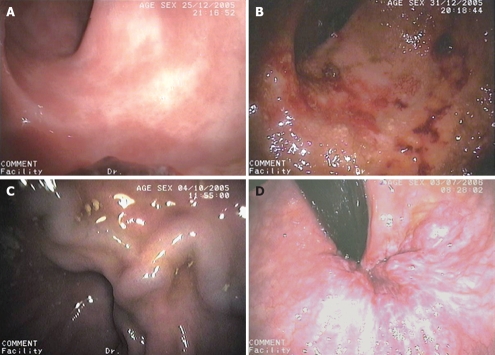
An upper gastrointestinal endoscopy was performed in all patients to evaluate the presence of GEV, portal hypertensive gastropathy and duodenopathy. All colonoscopies were performed by well-experienced endoscopists who were unaware of the diagnosis and the severity of liver disease using a Pentax EC-3840LK model videoscope (Hoya Corporation, Tokyo, Japan). After an overnight fast, patients were administered a polyethylene glycol electrolyte lavage solution (Solución Evacuante Bohm®; Laboratorios Bohm S.A., Madrid, Spain) as a bowel-cleansing regimen. Colorectal portal hypertensive vasculopathy lesions included internal or mixed hemorrhoids, colonic varices and PHC. Hemorrhoids were described as vascular swellings affecting the internal and external arteriovenous vascular plexuses of the anal canal and were classified as external, internal or mixed (Figure 1A). Colonic varices were bluish, dilated, tortuous submucosal veins extending from the anal canal above the level of the hemorrhoids into the colon (Figure 1B). According to previous reports[14], PHC was considered to be present if there were nonspecific inflammatory changes, resembling the lesions found in subjects with inflammatory chronic colitis and defined as granularity, diffuse hyperemia, edema and friability in mild grades (Figure 1C), and lesions such as vascular ectasias, angiodysplasia, arterial spider, and diffuse cherry red spots, in severe grades (Figure 1D). Arterial spider-like lesions are defined as the presence of a central arteriole, which blanches with pressure for a forceps biopsy and from which numerous small vessels radiate. The angiodysplasia-like lesions are defined by an irregular margin with a fern-like pattern and sometimes a pale halo around them, whereas the cherry red spots-like lesions are defined by the presence of a red spot in the colonic mucosa, similar to that seen in the gastric mucosa in patients with portal hypertensive gastropathy. Polyps and neoplasms were also described. Polyps were classified as hyperplastic or adenomatous.
Figure 1.
Endoscopic portal hypertension-related lesions. A: Mild portal hypertensive colopathy; B: Severe portal hypertensive colopathy; C: Colonic varix; D: Hemorrhoids.
Hemodynamic measurements
A splanchnic hemodynamic study with portal pressure measurement was performed in 77 of 88 patients. The time between the colonoscopy and the hemodynamic study was no more than 1 mo in all cases. The hemodynamic study was not performed in 11 patients because of previous clinical, laboratory or imaging findings that contraindicated the OLT. In all cases if the patient had significant ascites, a total paracentesis was performed before the study to avoid interference in the measurement of HVPG, to diminish complications, and to make catheterization easier. After an overnight fast, the patient was prepared for the study in the supine position. Under local anesthesia, a vascular introducer sheath (Medikit Co. Ltd., Tokyo, Japan) was inserted into the right internal jugular vein. Then, under fluoroscopy, a 7F balloon catheter (Cordis Corporation, Miami, Florida, USA) was placed in the right hepatic vein to measure free and wedged hepatic venous pressure (FHVP and WHVP) as described previously[18]. The wedged position was confirmed by the absence of reflux after injection of 2 mL of contrast medium, and FHVP was measured in the hepatic vein with the tip of the catheter just beyond the junction with the inferior cava vein. The HVPG was calculated as WHVP minus FHVP. PH was defined as the presence of an HVPG value > 5 mmHg. All hemodynamic measurements were performed using a previously calibrated strain-gauge transducer and recorded at least in duplicate. Two independent investigators who were unaware of the diagnosis, evaluated the tracings from the hemodynamic studies.
Statistical analysis
Quantitative variables were expressed as the mean ± SD or median (range) if parametric or non-parametric, respectively, and qualitative variables were expressed as frequencies. Categorical and continuous variables were compared using the χ2 and the Student’s t test or the Mann-Whitney U test respectively, when appropriate. One-way analysis of variance (ANOVA) with polynomial contrasts was applied. A two-tailed 0.05 significance level was used in all statistical tests. Statistical analysis was performed using the Statistical Program for the Social Sciences version 13.0 (SPSS® 13.0; SPSS Inc, Chicago, Illinois, USA).
RESULTS
Clinical characteristics and endoscopic findings
The clinical characteristics and hemodynamic data of the study population are shown in Table 1. Men comprised 80.7% of the sample and the median age was 55 (29-69) years. The main etiologies of cirrhosis were alcohol consumption (45.5%) and hepatitis C virus infection (31.8%). Most patients were Child-Pugh grade B-C (80.7%) and the HVPG tended to be high in relation to the severity of cirrhosis (Child-Pugh A 15.3 ± 6.6 mmHg vs Child-Pugh B-C 18.2 ± 5.4 mmHg, P = 0.07).
Table 1.
Clinical characteristics and hemodynamic data (mean ± SD, n = 88) n (%)
| Sex (M/F) | 71 (80.7)/17 (19.3) |
| Etiology | |
| Excessive alcohol consumption | 40 (45.5) |
| HCV | 20 (22.7) |
| HBV | 9 (10.2) |
| HCV and excessive alcohol consumption | 8 (9.1) |
| Other | 11 (12.5) |
| Child-Pugh grade | |
| A | 17 (19.3) |
| B and C | 71 (80.7) |
| Hepatocellular carcinoma | 30 (34.1) |
| Ascites | 63 (71.6) |
| SBP | 22 (23.9) |
| Hepatic encephalopathy | 30 (34.1) |
| Previous variceal bleeding | 16 (18.2) |
| Age (yr) | 55 ± 7 |
| Hemoglobin (g/dL) | 11.8 ± 2.4 |
| Platelets (cells/μL) | 87 443 ± 51 720 |
| Leukocytes (cells/μL) | 6286 ± 8932 |
| INR | 1.58 ± 0.53 |
| Total bilirubin (mg/dL) | 4.2 ± 4.4 |
| Serum albumin (g/dL) | 3.2 ± 0.6 |
| α-fetoprotein (ng/mL) | 65.1 ± 299.3 |
| CEA (ng/mL) | 5.4 ± 10.6 |
| HVPG (mmHg, n = 77) | 17.5 ± 5.8 |
HCV: Hepatitis C virus; HBV: Hepatitis B virus; SBP: Spontaneous bacterial peritonitis; CEA: Carcino-embryonic antigen; HVPG: Hepatic venous pressure gradient.
The colonoscopy revealed no lesions in 20.7% of the patients. The endoscopic PH-related lesions of the 88 patients evaluated are shown in Table 2. PHC was discovered in 23.9% of patients, colonic varices in 8% and internal or mixed hemorrhoids in 52.3%. No differences were observed between the colorectal manifestations of PH and the etiology of liver cirrhosis, Child-Pugh grade, history of ascites, hepatic encephalopathy, hepatocellular carcinoma or levels of serum albumin, total bilirubin, alanine aminotransferase, carcinoembryonic antigen, international normalized ratio or platelet count. Instead, we found a significant association between a history of SBP and PHC (45.5% vs 16.7%, P = 0.006).
Table 2.
Portal hypertension-related endoscopic variables
| n (%) | |
| Gastroesophageal varices | 69 (78.4) |
| Portal hypertensive gastropathy | 40 (48.2) |
| Portal hypertensive duodenopathy | 11 (13.3) |
| Portal hypertensive colopathy | 21 (23.9) |
| Mild | 13 (61.9) |
| Severe | 8 (38.1) |
| Colonic varices | 7 (8) |
| Internal or mixed hemorrhoids | 46 (52.3) |
Association between upper and lower gastrointestinal PH-related lesions
Table 3 compares clinical and endoscopic findings between the PHC and non-PHC groups. As for the lower and upper gastrointestinal tract, we found no association between PHC and portal hypertensive gastropathy or duodenopathy. Of the patients with PHC, 90.5% had GEV. On the other hand, colonoscopy revealed that 27.5% of patients with GEV had PHC compared with 10.5% of patients without GEV (P = 0.12). We did not find an association between colonic varices and GEV.
Table 3.
Comparison of clinical and endoscopic findings between PHC and non-PHC groups (mean ± SD) n (%)
| PHC group (n = 21) | Non-PHC group (n = 67) | |
| Age (yr) | 54.6 ± 7.4 | 55.2 ± 7 |
| Hemoglobin (g/dL) | 11.8 ± 2.6 | 11.8 ± 2.4 |
| HVPG (mmHg, n = 77) | 19.9 ± 6.3 | 16.8 ± 5.5a |
| Sex | ||
| Male | 18 (85.7) | 53 (79.1) |
| Female | 3 (14.3) | 14 (20.9) |
| Child-Pugh grade | ||
| A | 4 (19) | 13 (19.4) |
| B and C | 17 (81) | 54 (80.6) |
| Excessive alcohol consumption | 13 (61.9) | 35 (52.2) |
| SBP | 10 (47.6) | 12 (17.9)a |
| Gastroesophageal varices | 19 (90.5) | 50 (74.6) |
| PH gastropathy | 11 (55.0) | 29 (46.0) |
| PH duodenopathy | 2 (10.0) | 9 (14.3) |
| Adenoma/adenocarcinoma | 13 (61.9) | 21 (31.3)a |
P < 0.05 (ANOVA) vs PHC group. PHC: Portal hypertensive colopathy; PH: Portal hypertension.
Clinical implication of the presence of gastrointestinal PH-related lesions
Anemia is one of the most important consequences of chronic bleeding from PH-related lesions. We did not observe differences in hemoglobin values between patients with and without PHC (11.8 ± 2.6 g/dL vs 11.8 ± 2.4 g/dL, P = 0.93). There were also no differences in PHC grade (11.8 ± 2.6 g/dL vs 11.7 ± 2.7 g/dL, P = 0.94), although an association was found between the presence of anemia and portal hypertensive gastropathy (11.2 ± 2.4 g/dL vs 12.4 ± 2.3 g/dL, P = 0.022).
Relation between HVPG and colorectal PH-related lesions
In the whole cohort, a greater mean HVPG was found in patients with PHC than in those without (19.9 ± 6.3 mmHg vs 16.8 ± 5.5 mmHg, respectively, P = 0.045) (Figure 2), but it was not associated with PHC grade (mild PHC 19.8 ± 7.3 mmHg vs severe PHC 20.2 ± 4.1 mmHg, P = 0.91). When we analyzed only the patients who had not previously been treated with non-selective β-blockers we found a higher association between the presence of PHC and HVPG (22.9 ± 6.7 mmHg vs 16.0 ± 5.2 mmHg, P = 0.007).
Figure 2.
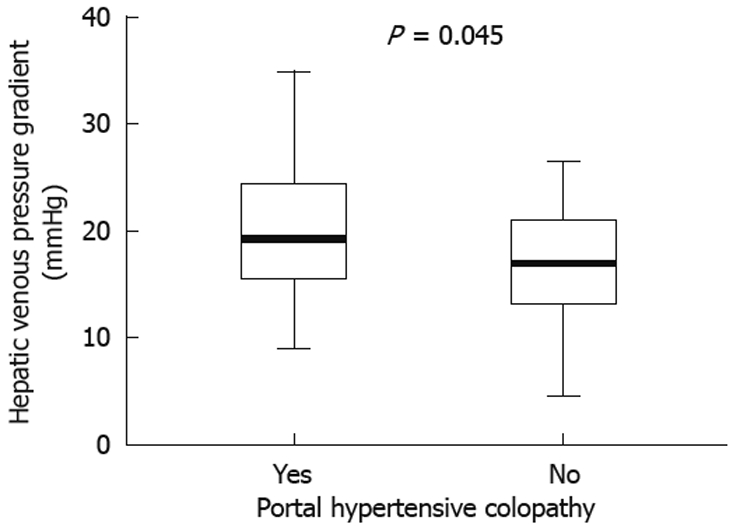
Comparison of HVPG between PHC and non-PHC groups.
HVPG did not differ significantly between patients with or without colonic varices (17.8 ± 3.2 mmHg vs 17.5 ± 5.9 mmHg, respectively, P = 0.91) or with or without hemorrhoids (17.9 ± 5.7 mmHg vs 17.1 ± 5.8 mmHg, respectively, P = 0.54).
Influence of treatment of PH in the development of colorectal PH-related lesions
We did not find a significant association between treatment with non-selective β-blockers and the presence of PHC (24% vs 23.7%, P = 0.97) or colonic varices (10% vs 5.3%, P = 0.69). We could not demonstrate an influence of previous EBL in the development of these lesions.
Relation between PHC and colonic polyps and neoplasia
The prevalence of colonic polyps in our patients was 39.7% (35/88). Sixty-five percent of patients with polyps had adenomas and 30.4% had hyperplastic polyps. Six patients (40%) with adenomatous polyps presented with mild dysplasia. One asymptomatic patient (1.1%) had a well-differentiated adenocarcinoma. When we analyzed the association between PHC and polyps and/or adenocarcinoma we found that 42.9% of PHC patients had preneoplastic lesions or neoplasms compared with 10.4% of non-PHC patients (P = 0.02) (Table 3).
DISCUSSION
In the present study we suggest that colorectal endoscopic evaluation of OLT candidates can reveal findings that are directly related to the PH grade. It is reasonable to think that PH causes hemodynamic changes not only in the upper gastrointestinal tract but also in other areas, especially in the colon, a condition that is now receiving increasing attention in the literature. In our study, the prevalence of PHC was 23.9%, similar to that found previously[6], although the prevalence is highly variable. These discrepancies may be due to the lack of consensus on the endoscopic appearance of PHC, unlike portal hypertensive gastropathy, in which there does appear to be a certain degree of consensus. In our study we have chosen a simple classification of PHC: mild and severe grades. The reason for these categories is the higher risk of chronic or acute bleeding for severe grade lesions and their importance in clinical management and therapeutic options.
Several studies have demonstrated the relationship between PHC and indirect measurements of PH, for example low platelet counts[10], the presence of portal hypertensive gastropathy[11,13] or large esophageal varices[11,12]. In our study we found an association between PHC and GEV, although it was not statistically significant.
Our main objective was to assess the association between PH vasculopathy and the severity of PH. We found a clear association between PHC and higher values of HVPG, with a higher PH grade in the PHC group. This difference was maintained in patients who were not treated with non-selective β-blockers, avoiding possible bias from treatment influence. Few studies have compared this association with a hemodynamic measurement of PH. Sugano et al[15] and Yamakado et al[16] also demonstrated, as we did, an association between PHC and higher values of HVPG. Unlike Sugano et al[15], we found that the presence of higher HVPG values was related to the existence of PHC but not to PHC grade. Chen et al[14] also studied the relationship between colorectal portal hypertensive vasculopathy and PH severity, although they did not find a relationship between them.
We found no association between colorectal manifestations of PH and etiology of liver disease, Child-Pugh grade or previous history of hepatic decompensation, results which are consistent with those of some authors[9,15]. Furthermore, like other authors, we were unable to demonstrate a relationship between HVPG and colonic varices[14,15]. However, we did find a statistical association between a history of previous SBP and the presence of PHC. In this sense, the main pathogenic mechanism of SBP, that is bacterial translocation, may be favoured by increased intestinal permeability at this site due to mucosal damage and vasodilatation in PHC.
The clinical implications of the presence of PH lesions and the therapeutic approach to chronic or acute bleeding have not been fully evaluated. Although the cross-sectional design of our study does not allow us to analyze adequately the effect of β-blockers on the outcome of these lesions, we observed no relationship between these drugs and PHC or colonic varices. In this sense, only two studies have evaluated the influence of β-blockers and nitroglycerin on the outcome of PHC, demonstrating that PHC improves after treatment with these drugs[11,15]. Although there are no evidence at the moment that these treatments improve anemia or recurrent bleeding from PHC, the lesser presence and grade of these lesions should improve the clinical status of the patients, so the classification of PHC used in our study is adequate, which is consistent with overall thinking in daily clinical practice.
A remarkable association was observed between preneoplastic lesions, neoplasms and PHC. In our study, the prevalence of polyps was 38%. Many of these were adenomatous (65.2%) and one adenocarcinoma was discovered. This association has not been previously described, suggesting that involvement of the colonic mucosal microvasculature in PHC could stimulate the proliferation of polyps, although the pathologic mechanisms are unknown and any explanation at this time would be highly speculative.
Although the best indicator of PH grade is the direct measurement of portal pressure by portal vein catheterisation, the difficulty and the morbidity of the procedure impede the habitual performance of this technique, particularly in patients with cirrhosis with altered coagulation factors and risk of complication. It is well documented that WHVP correlates well with direct portal pressure measurement and the agreement is sufficiently good to use it as a surrogate measurement of PH[19], and it has been demonstrated in patients with hepatitis C[20] and hepatitis B-related cirrhosis[21]. In our study all the patients were cirrhotic and nearly 90% had viral or alcoholic etiology. In spite of this, the HVPG is less accurate in liver diseases with a presinusoidal component and in our study this situation may occur only in a few patients.
Finally, our sample size was probably too small to obtain sufficient statistical power to extrapolate the results, although we do report the largest number of patients to date. Likewise, the same team of gastroenterologists were responsible for the endoscopic procedures and reporting and for reviewing the photographs taken during the explorations, thus ensuring the reliability of our data. However, the cross-sectional design of the study prevents us from evaluating the influence of β-blockers on the outcome of the lesions. Future prospective studies must take this into account if they are to improve the quality of life of these patients.
In conclusion, we demonstrated a high prevalence of colorectal hypertensive vasculopathy lesions in this cohort of patients, and a higher severity of PH with increased HVPG values in patients with PHC, although we were unable to show an effect on PHC grade. Previous treatment with β-blockers does not appear to affect the outcome of PHC.
COMMENTS
Background
Preoperative liver transplantation evaluation is performed to exclude contraindications and end-stage liver disease must be completely studied. Colonoscopy allows researchers to evaluate the presence of portal hypertension (PH)-related lesions such as portal hypertensive colopathy (PHC), colorectal varices and hemorrhoids. PHC is an area of increasing interest but little is known about its relation with the severity of PH. The measurement of the hepatic venous pressure gradient (HVPG) is the gold standard of PH grade.
Research frontiers
The relation between PHC and the severity of PH is not well established. In this study, the authors demonstrate that a higher value of HVPG is associated with the presence of PHC but not with other manifestations of PH-related colorectal lesions.
Innovations and breakthroughs
Previous reports remark on the association between PHC and the severity of PH with indirect measurements, and some studies with the gold standard of the HVPG, with contradictory results. The present study is the biggest cohort of patients in which HVPG measurement is performed to demonstrate this association.
Applications
Their study demonstrates that this association reflects a broad spectrum of lesions covering the whole gastrointestinal tract related with PH, so this supposes an advance in the knowledge of these lesions. Treatment of PH-related colorectal lesions is not well established and it must be elucidated.
Peer review
The authors exposed cirrhotic candidates for liver transplantation in which colonoscopy was performed, searching the association between PH-related colorectal lesions and the severity of PH measured by HVPG. The article is well written and the contents are credible.
Acknowledgments
We are indebted to Mr. Tom O’Boyle for his expert management of the manuscript.
Footnotes
Peer reviewer: Mercedes Susan Mandell, MD, PhD, Department of Anesthesiology, University of Colorado Health Sciences Ctr., 12401 E. 17th Ave, B113 Aurora, CO 80045, United States
S- Editor Li LF L- Editor O’Neill M E- Editor Zheng XM
References
- 1.Carithers RL Jr. Liver transplantation. American Association for the Study of Liver Diseases. Liver Transpl. 2000;6:122–135. doi: 10.1002/lt.500060122. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs DM, Bubrick MP, Onstad GR, Hitchcock CR. The relationship of hemorrhoids to portal hypertension. Dis Colon Rectum. 1980;23:567–569. doi: 10.1007/BF02988998. [DOI] [PubMed] [Google Scholar]
- 3.Hosking SW, Smart HL, Johnson AG, Triger DR. Anorectal varices, haemorrhoids, and portal hypertension. Lancet. 1989;1:349–352. doi: 10.1016/s0140-6736(89)91724-8. [DOI] [PubMed] [Google Scholar]
- 4.Zaman A, Hapke R, Flora K, Rosen H, Benner K. Prevalence of upper and lower gastrointestinal tract findings in liver transplant candidates undergoing screening endoscopic evaluation. Am J Gastroenterol. 1999;94:895–899. doi: 10.1111/j.1572-0241.1999.984_g.x. [DOI] [PubMed] [Google Scholar]
- 5.Tam TN, NG WW, Lee SD. Colonic mucosal changes in patients with liver cirrhosis. Gastrointest Endosc. 1995;42:408–412. doi: 10.1016/s0016-5107(95)70040-4. [DOI] [PubMed] [Google Scholar]
- 6.Rabinovitz M, Schade RR, Dindzans VJ, Belle SH, Van Thiel DH, Gavaler JS. Colonic disease in cirrhosis. An endoscopic evaluation in 412 patients. Gastroenterology. 1990;99:195–199. doi: 10.1016/0016-5085(90)91248-5. [DOI] [PubMed] [Google Scholar]
- 7.Goenka MK, Kochhar R, Nagi B, Mehta SK. Rectosigmoid varices and other mucosal changes in patients with portal hypertension. Am J Gastroenterol. 1991;86:1185–1189. [PubMed] [Google Scholar]
- 8.Ghoshal UC, Biswas PK, Roy G, Pal BB, Dhar K, Banerjee PK. Colonic mucosal changes in portal hypertension. Trop Gastroenterol. 2001;22:25–27. [PubMed] [Google Scholar]
- 9.Wang TF, Lee FY, Tsai YT, Lee SD, Wang SS, Hsia HC, Lin WJ, Lin HC, Lai KH, Chan CY. Relationship of portal pressure, anorectal varices and hemorrhoids in cirrhotic patients. J Hepatol. 1992;15:170–173. doi: 10.1016/0168-8278(92)90031-j. [DOI] [PubMed] [Google Scholar]
- 10.Ito K, Shiraki K, Sakai T, Yoshimura H, Nakano T. Portal hypertensive colopathy in patients with liver cirrhosis. World J Gastroenterol. 2005;11:3127–3130. doi: 10.3748/wjg.v11.i20.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bini EJ, Lascarides CE, Micale PL, Weinshel EH. Mucosal abnormalities of the colon in patients with portal hypertension: an endoscopic study. Gastrointest Endosc. 2000;52:511–516. doi: 10.1067/mge.2000.108478. [DOI] [PubMed] [Google Scholar]
- 12.Misra SP, Dwivedi M, Misra V. Prevalence and factors influencing hemorrhoids, anorectal varices, and colopathy in patients with portal hypertension. Endoscopy. 1996;28:340–345. doi: 10.1055/s-2007-1005477. [DOI] [PubMed] [Google Scholar]
- 13.Ganguly S, Sarin SK, Bhatia V, Lahoti D. The prevalence and spectrum of colonic lesions in patients with cirrhotic and noncirrhotic portal hypertension. Hepatology. 1995;21:1226–1231. [PubMed] [Google Scholar]
- 14.Chen LS, Lin HC, Lee FY, Hou MC, Lee SD. Portal hypertensive colopathy in patients with cirrhosis. Scand J Gastroenterol. 1996;31:490–494. doi: 10.3109/00365529609006770. [DOI] [PubMed] [Google Scholar]
- 15.Sugano S, Nishio M, Makino H, Suzuki T. Relationship of portal pressure and colorectal vasculopathy in patients with cirrhosis. Dig Dis Sci. 1999;44:149–154. doi: 10.1023/a:1026670604551. [DOI] [PubMed] [Google Scholar]
- 16.Yamakado S, Kanazawa H, Kobayashi M. Portal hypertensive colopathy: endoscopic findings and the relation to portal pressure. Intern Med. 1995;34:153–157. doi: 10.2169/internalmedicine.34.153. [DOI] [PubMed] [Google Scholar]
- 17.Atassi T, Thuluvath PJ. Risk of colorectal adenoma in liver transplant recipients compared to immunocompetent control population undergoing routine screening colonoscopy. J Clin Gastroenterol. 2003;37:72–73. doi: 10.1097/00004836-200307000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280–282. doi: 10.1002/hep.20062. [DOI] [PubMed] [Google Scholar]
- 19.Thalheimer U, Leandro G, Samonakis DN, Triantos CK, Patch D, Burroughs AK. Assessment of the agreement between wedge hepatic vein pressure and portal vein pressure in cirrhotic patients. Dig Liver Dis. 2005;37:601–608. doi: 10.1016/j.dld.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Perelló A, Escorsell A, Bru C, Gilabert R, Moitinho E, García-Pagán JC, Bosch J. Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus-related cirrhosis. Hepatology. 1999;30:1393–1397. doi: 10.1002/hep.510300628. [DOI] [PubMed] [Google Scholar]
- 21.Lin HC, Tsai YT, Lee FY, Chang TT, Wang SS, Lay CS, Lee SD, Lo KJ. Comparison between portal vein pressure and wedged hepatic vein pressure in hepatitis B-related cirrhosis. J Hepatol. 1989;9:326–330. doi: 10.1016/0168-8278(89)90141-4. [DOI] [PubMed] [Google Scholar]