Abstract
AIM: To evaluate the protective effect of diallyl sulfide (DAS) against N-nitrosodiethylamine (NDEA)-induced liver carcinogenesis.
METHODS: Male Wistar rats received either NDEA or NDEA together with DAS as protection. Liver energy metabolism was assessed in terms of lactate, pyruvate, lactate/pyruvate, ATP levels, lactate dehydrogenase (LDH) and glucose-6-phosphate dehydrogenase (G6PD) activities. In addition, membrane disintegration of the liver cells was evaluated by measuring lipid-peroxidation products, measured as malondialdehyde (MDA); nitric oxide (NO) levels; glucose-6-phosphatase (G6Pase), catalase (CAT) and superoxide dismutase (SOD) activities. Liver DNA level, glutathione-S-transferase (GST) and cytochrome c oxidase activities were used as DNA fragmentation indices. Aldose reductase (AR) activity was measured as an index for cancer cells resistant to chemotherapy and histopathological examination was performed on liver sections from different groups.
RESULTS: NDEA significantly disturbed liver functions and most of the aforementioned indices. Treatment with DAS significantly restored liver functions and hepatocellular integrity; improved parameters of energy metabolism and suppressed free-radical generation.
CONCLUSION: We provide evidence that DAS exerts a protective role on liver functions and tissue integrity in face of enhanced tumorigenesis caused by NDEA, as well as improving cancer-cell sensitivity to chemotherapy. This is mediated through combating oxidative stress of free radicals, improving the energy metabolic state of the cell, and enhancing the activity of G6Pase, GST and AR enzymes.
Keywords: N-nitrosodiethylamine, Diallyl sulfide, Liver cancer, Energy metabolism, Aldose reductase
INTRODUCTION
Primary liver cancer has been classified as the fifth most common cause of cancer and the fourth most common cause of cancer mortality in the world. One of the main pathological subtypes of liver cancer is hepatocellular carcinoma, which constitutes a major contributor to cancer incidence and mortality[1]. The population of Egypt has a heavy burden of liver disease, mostly due to chronic infection with hepatitis C virus. Since the liver offers a very important site for detoxification of xenobiotics, the use of synthetic chemoprotective agents offers potential risk factors[2,3]. Several reports have stressed the importance of many dietary habits in modifying the initiation, promotion and progression stages in carcinogenesis[4]. Garlic (Allium sativum), an important flavoring agent, exhibits medicinal properties that include immunomodulatory, hepatoprotective, antioxidant, antimutagenic, and anticarcinogenic effects[5,6]. The anticarcinogenic property of garlic has been documented from both epidemiological and experimental studies which suggests that the consumption of garlic can decrease the incidence of several cancers[2,7,8]. The ability of garlic to reduce the incidence of cancer has been attributed to its content of organosulfur compounds which reportedly suppress carcinogen-induced tumors in various organs of animals including the colorectum, breast and liver[9-11]. A major constituent of garlic, diallyl sulfide (DAS), has been shown to inhibit chemical toxicity and tumorigenesis in several animal models[12]. Nonetheless, the possibility that DAS may exert a protective role against N-nitrosodiethylamine (NDEA)-induced liver tumorigenesis cannot be ruled out. In this study, we investigated the cellular and molecular mechanisms of the protective effects of DAS against liver damage induced by NDEA, a potent inducer of liver cancer. We determined the histopathological effect of DAS on liver tissue, as well as on enzymatic and non-enzymatic liver functions. In addition, we investigated the possibility that DAS might act on maintaining liver tissue functions, which were assessed by investigating energy metabolism, membrane disintegration and DNA integrity indices.
MATERIALS AND METHODS
Animals
We used a total of 36 male albino rats of the Wistar strain, weighing 170-200 g, that were obtained from the central animal facility at the Faculty of Pharmacy, Cairo University, Cairo, Egypt. All rats were housed in a room with a controlled environment, at a constant temperature of 23 ± 1°C, humidity of 60% ± 10%, and a 12 h light/dark cycle. The animals were housed in groups and kept at constant nutritional conditions throughout the experimental period. The experimental protocols were approved by the Ethical Committee of Cairo University.
Drugs and chemicals
NDEA, DAS, enzymes and coenzymes were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Other chemicals were of Analar grade. NDEA was prepared as 8 mg/mL saline, whereas DAS was prepared as 80 mg/mL corn oil.
Induction of liver cancer
Each rat received an oral dose of 20 mg/kg per day NDEA for 5 d per week for 9 wk, followed by 10 mg/kg per day for 5 d per week for another 6 wk.
Protocols and experimental groups
Animals were divided into three groups: Group I was the NDEA-induced cancer group, and Group II was the DAS-treated group. Cancer was induced in this group by the same protocol. In addition, DAS was co-administered at a daily oral dose of 200 mg/kg per day for 5 d per week for the total period of the experiment (i.e. 15 wk). Group III consisted of normal rats that received an oral dose of vehicles (saline, corn oil) for the total period of the experiment.
Biochemical estimations
Blood analysis: At the end of the experimental period, all animals were killed by cervical dislocation. The separated plasma was analyzed for total protein[13] and albumin[14]. The separated serum was analyzed for aspartate aminotransferase (AST), using a kit provided by Bicon, Germany[15]; alkaline phosphatase (ALP) using a kit provided by Biolabo, France[16,17], and gamma glutamyltransferase (GGT) using a kinetic photometric method[18].
Tissue analysis: The liver was removed, rinsed with ice-cold saline and blotted dry. Accurately weighed pieces of liver tissue were treated differently for the separation and estimation of the studied parameters.
Measurement of liver malondialdehyde (MDA) content: A 10% homogenate was prepared in 1.15% KCl, centrifuged at 1000 × g at 4°C for 20 min, and the resultant supernatant was used for the assay of liver MDA content[19].
Measurement of liver cytochrome c oxidase activity and nitric oxide (NO) content: Liver tissue was homogenized in Tris-sucrose buffer, pH 7.4 (5% homogenate), using Potter-Elvejhem glass homogenizer, and centrifuged at 2000 × g at 4°C for 10 min. The resultant supernatant was used for the estimation of cytochrome c oxidase activity[20] and NO content[21].
Measurement of liver glutathione-S-transferase (GST), lactate dehydrogenase (LDH), glucose-6-phosphate dehydrogenase (G6PD), superoxide dismutase (SOD) and catalase (CAT) activities: A 10% homogenate was obtained in Tris-sucrose buffer, pH 7.4, and centrifuged at 105 000 × g at 4°C for 30 min, using a Dupont Sorvall Ultracentrifuge (USA), to isolate the cytosolic fraction which was used for the assay of GST[22], SOD[23], LDH[24], G6PD[25] and CAT[26] activities.
Estimation of liver pyruvate and lactate concen-trations: Liver tissue was homogenized in 5% meta-phosphoric acid, and centrifuged at 3000 × g at 4°C for 15 min. The resultant supernatant was used for the estimation of pyruvate and lactate concentrations according to the method of Mohun and Cook[27] and David[28], respectively.
Estimation of liver ATP content: Liver tissue was homogenized with 3 mL ice-cold 3 mol/L perchloric acid, using Potter-Elvejhem glass homogenizer. Following that, 12.5 mL of 1 mmol/L EDTA was added and the mixture was centrifuged at 1000 × g at 4°C for 1 h. The supernatant was further treated for the estimation of ATP[29].
Determination of liver glucose-6-phosphatase (G6Pase) activity: Liver tissue was homogenized in ice-cold solution containing 0.15mol/L KCl; 4 mmol/L MgSO4; 4 mmol/L EDTA and 4 mmol/L N-acetylcysteine, pH 7, and centrifuged at 12 000 × g at 4°C for 10 min. The resultant supernatant was analyzed for G6Pase activity[30].
Estimation of liver DNA content: Liver tissue was homogenized in 0.25 mol/L sucrose/in TKM buffer (0.05 mol/L Tris-HCl, 0.025 mol/L KCl, 0.005 mol/L MgCl2), pH 7.5, to prepare a 15% homogenate. Then 0.1 mL of 0.3 mol/L perchloric acid was added, left to stand at 0°C for 15 min, centrifuged at 2000 × g at 4°C for 10 min, and the precipitate was used for the estimation of DNA content[31].
Determination of liver aldose reductase (AR) activity: Liver tissue was homogenized in potassium phosphate buffer, pH 7 (20% homogenate), centrifuged at 105 000 × g for 45 min at 4°C, and the resultant supernatant was used for the estimation of AR activity[32].
Determination of protein concentrations: Protein concentrations of the above supernatants were estimated by the method of Lowry et al[33].
Histopathological examination
The portions of liver tissue embedded in paraffin were sectioned at 5 μm. Following sectioning, liver tissue was stained with hematoxylin and eosin. Light microscopy was used to evaluate the pathological changes in liver tissue.
Statistical analysis
The values were expressed as mean ± SE. Statistical analysis was performed by one way analysis of variance (ANOVA) followed by Kruskal-Wallis comparison test. P ≤ 0.05 was considered significant.
RESULTS
The results showed that there were no differences between the various control groups (vehicle-treated groups). Thus, the data from all of the control animals were po-oled and are shown as one normal group.
Effect of NDEA in absence or presence of DAS on non-enzymatic liver functions
NDEA significantly decreased total protein, albumin, and A/G ratio. Co-administration of DAS restored total protein, albumin and A/G ratio (Figure 1). Moreover, NDEA significantly increased total bilirubin (2.43 ± 0.151 mg/dL), compared to their control counterparts (1.19 ± 0.090 mg/dL). DAS significantly decreased total bilirubin (1.68 ± 0.078 mg/dL, P = 0.045) when compared to the NDEA-treated group value.
Figure 1.
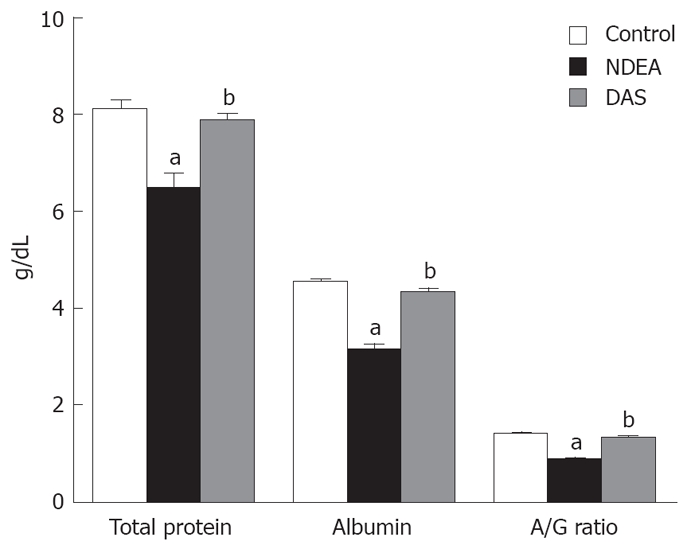
Non-enzymatic liver functions in rats treated with NDEA in absence or presence of DAS (mean ± SE) differed significantly compared to the control and NDEA-treated group (aP = 0.045, bP = 0.042, respectively).
Effect of NDEA in absence or presence of DAS on enzymatic liver functions
NDEA significantly elevated serum AST, ALP and GGT activities when compared to the normal group. DAS co-administration restored serum AST and GGT activities, together with a significant decrease in ALP activity, compared to the NDEA group value (P = 0.048) (Figure 2).
Figure 2.
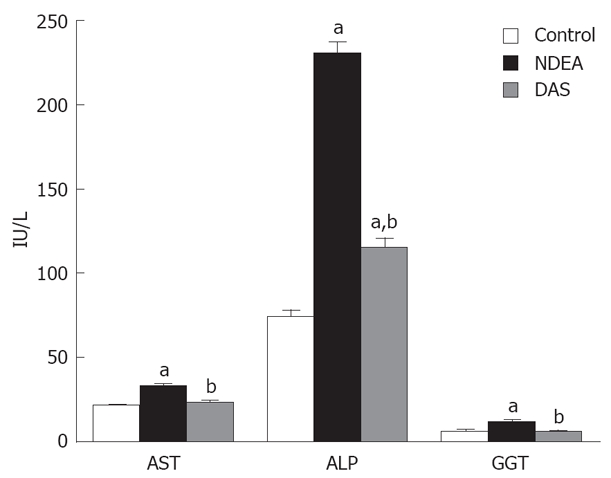
Enzymatic liver functions in rats treated with NDEA in absence or presence of DAS (mean ± SE) differed significantly compared to the control and NDEA-treated group (aP = 0.048, bP = 0.044, respectively).
Changes in liver energy metabolism indices following NDEA in absence or presence of DAS
Table 1 showed that NDEA significantly decreased ATP level and increased G6PD activity compared to normal group values (P = 0.046). Treatment with DAS resulted in a significant increase in ATP level compared to the NDEA-treated group value. In addition, DAS significantly elevated and reduced pyruvate and lactate levels respectively, with a consequent significant reduction in lactate/pyruvate ratio compared to both the NDEA and control counterparts. Also, DAS significantly increased LDH and G6PD activities compared to either NDEA or control groups.
Table 1.
Effect of NDEA in absence or presence of DAS on liver energy metabolism indices (mean ± SE)
| Parameters | Normal | NDEA | DAS |
| Lactate (μmol/g liver) | 2.27 ± 0.16 | 2.08 ± 0.094 | 1.41 ± 0.08ab |
| Pyruvate (μmol/g liver) | 0.11 ± 0.007 | 0.103 ± 0.008 | 0.15 ± 0.007ab |
| Lac/Pyr | 20.6 ± 1.65 | 17.37 ± 0.9 | 11.31 ± 0.76ab |
| LDH (μmol/mg protein per min) | 0.9 ± 0.021 | 0.9 ± 0.043 | 1.05 ± 0.028ab |
| ATP (μmol/g liver) | 5.69 ± 0.32 | 4.04 ± 0.12a | 4.91 ± 0.085ab |
| G6PD (μmol/mg protein per min) | 27.9 ± 2.29 | 47.4 ± 1.48a | 54.1 ± 4.04ab |
Significantly different from baseline values at
P = 0.046; Significantly different from NDEA treatment at
P = 0.043.
Changes in oxidative stress and membrane disintegration indices following NDEA in absence or presence of DAS
As shown in Table 2, NDEA treatment significantly (P = 0.048) elevated MDA and NO contents and reduced G6Pase, CAT and SOD activities when compared to control counterparts. Administration of DAS restored MDA level and G6Pase activity, significantly decreased NO level, and non-significantly changed SOD and CAT activities when compared to NDEA-treated rats.
Table 2.
Effect of NDEA in absence or presence of DAS on oxidative stress and membrane disintegration indices (mean ± SE)
| Parameters | Normal | NDEA | DAS |
| MDA (nmol/g liver) | 55.6 ± 3.4 | 90.4 ± 8.01a | 66.8 ± 2.4b |
| NO (nmol/g liver) | 139 ± 9.7 | 344 ± 14.9a | 187 ± 9.2ab |
| G6Pase (nmol/mg protein/min) | 6.39 ± 0.46 | 3.38 ± 0.26a | 7.36 ± 0.3b |
| CAT (IU/mg protein) | 173 ± 9.1 | 141 ± 6.44a | 138 ± 6.64a |
| SOD (IU/mg protein) | 88.3 ± 6.55 | 55.7 ± 2.7a | 57.8 ± 5.38a |
Significantly different from baseline values at
P = 0.048; Significantly different from NDEA treatment at
P = 0.044.
Effect of NDEA in absence or presence of DAS on DNA fragmentation indices
NDEA significantly elevated DNA level (3.97 ± 0.189 mg/gt) compared to control level (2.5 ± 0.199 mg/gt) (P = 0.043). DAS normalized DNA levels (2.38 ± 0.08 mg/gt). NDEA significantly elevated cytochrome c oxidase and GST activities. DAS significantly lowered GST , which approached the normal values. However, DAS significantly enhanced cytochrome c oxidase activity when compared to either control or NDEA-treated group levels (Figure 3).
Figure 3.
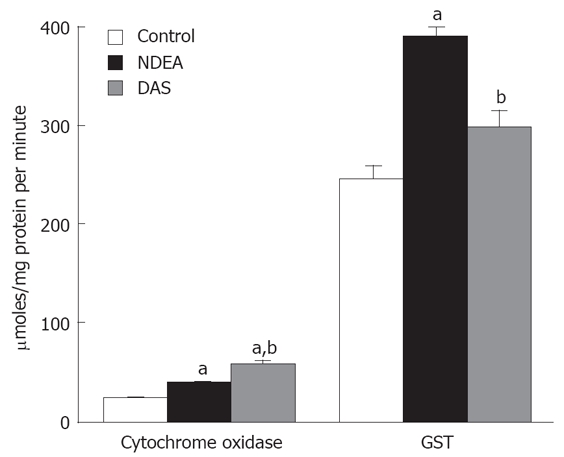
Changes in cytochrome oxidase and GST activities in rats treated with NDEA in absence or presence of DAS (mean ± SE) differed significantly compared to the control and NDEA-treated group values (aP = 0.043, bP = 0.045, respectively).
Effect of NDEA in absence or presence of DAS on AR activity
NDEA significantly elevated AR activity (P = 0.045). Co-administration of DAS restored such enzymatic activity (Figure 4).
Figure 4.
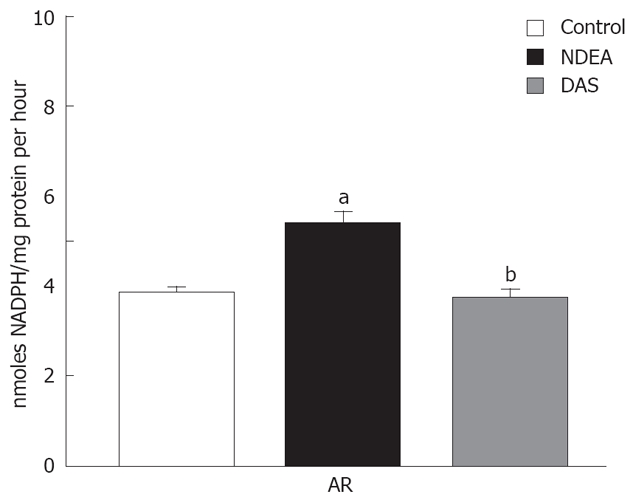
Changes in AR in rats treated with NDEA in absence or presence of DAS (mean ± SE) differed significantly compared to the control and NDEA-treated group values (aP = 0.045, bP = 0.046, respectively).
Histopathological findings
Examination of liver sections of the different groups illustrated that: Liver tissue of the normal group showed hepatic lobules with normal architecture (Figure 5A). Liver tissue of the NDEA-treated rats showed pleomorphism. Some cells exhibited multiple nucleoli, some of the cells were pyknotic, while others showed intranuclear vacuoles and cellular infiltration (Figure 5B). Other sections showed massive areas of vacuolated hepatocytes, cellular infiltration, and some cells possessed pyknotic nuclei (Figure 5C). Other section showed hyperchromatic nuclei and numerous Kupffer cells (Figure 5D). In other sections, hyperchromatic malignant nuclei were evident (Figure 5E). Liver tissue from the DAS-treated rats showed fewer degenerative changes, such as vacuolated cytoplasm, few pyknotic nuclei and dilated sinusoids (Figure 5F). Other section showed more or less normal hepatic lobular architecture (Figure 5G).
Figure 5.
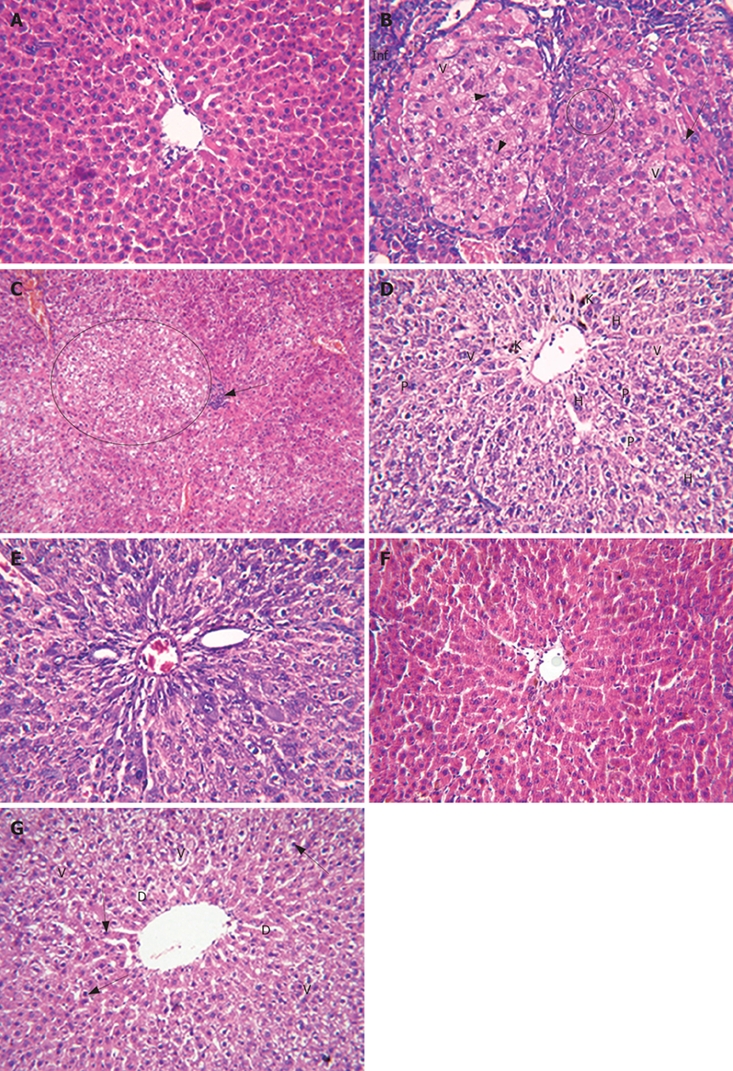
Liver sections of the different groups. A: Liver tissue of the normal group (control) showed hepatic lobule having normal architecture; B: Liver tissue of the NDEA-treated rats showed nuclear pleomorphism; some cells exhibited multiple nucleoli (encircled), pyknotic cells (short arrows), intranuclear vacuoles (arrow), some showed cytoplasmic vacuoles (V) and cellular infiltration (Inf); C: Other sections showed massive areas of vacuolated hepatocytes (encircled); cellular infiltration (arrow) and some cells possessed pyknotic nuclei; D: Other slides showed vacuolated cytoplasm, hyperchromatic nuclei, pyknotic nuclei and numerous Kupffer cells; E: Other section showed hyperchromatic malignant nuclei (H); F: Liver tissue of the DAS-treated rats showed some degenerative changes, vacuolated cytoplasm (V), few pyknotic nuclei (arrows) and dilated sinusoids (D); G: Other slides showed more or less normal hepatic lobular architecture.
DISCUSSION
The liver is a multifunctional organ that plays essential roles in metabolism, biosynthesis, excretion, secretion and detoxification. These processes require energy, making the liver a highly aerobic, oxygen-dependent tissue. These processes also cause vulnerability of the liver to anoxia, increased susceptibility to noxious insults, and create a demand for cell replacement after tissue loss. Enhanced liver cell death and impaired regeneration are indeed features of most liver disorders. Proteins play a big role in fighting off infections and building or repairing muscle tissue. Low albumin is a sign of poor health and a predictor of a bad outcome. Thus, a decrease in the A/G ratio often indicates the presence of impaired liver function. As shown in our results, NDEA decreased significantly total protein, albumin and A/G levels, which was indicative of poor liver function and inability to fight infections. On the contrary, DAS administration normalized total protein, albumin and A/G levels, indicating the ability of DAS to improve liver function in face of NDEA-induced liver damage. Serum AST, ALP and GGT are sensitive indicators of hepatic injury. Several reports have shown an increase in the activities of AST and ALT during NDEA-induced hepatocarcinogenesis[34]. Elevated activities of serum AST, ALP and GGT observed in NDEA-treated rats may be due to the NDEA-induced hepatic damage and the subsequent leakage of these enzymes into the circulation. Administration of DAS restored the activities of these enzymes to near normal values, which may be an indication of the hepatoprotective role of DAS.
As shown in our results, NDEA produced a significant decrease in hepatic ATP level. Oncotic necrosis is most often the consequence of metabolic injury, leading to ATP depletion. ATP depletion in hepatocytes is associated with ATP-depletion-dependent cytoskeletal alterations, after which a metastable state develops, characterized by mitochondrial depolarization and lysosomal breakdown. This metastable state culminates in outright rupture of plasma membrane, irreversible breakdown of the plasma membrane permeability barrier, and leakage of cytosolic enzymes and metabolic intermediates[35]. Treatment with DAS resulted in a significant decrease in lactate and lactate/pyruvate ratio, along with significant elevation of pyruvate, ATP levels and liver LDH activity, compared to NDEA-treated rats. The increased activity of LDH could favor pyruvate (aerobic carbohydrate metabolism) against lactate (anaerobic), thus enhancing energy metabolism in the cell and reflecting restoration of normal cellular/metabolic function. The histopathological findings observed in this study support the biochemical ones that liver tissue of the NDEA-treated rats showed drastic changes in the morphology of the liver cells, whereas the DAS-treated rats showed more or less normal hepatic lobular architecture. Accordingly, we presented evidence that DAS substantially improved the liver cell metabolic indices, as well as its synthetic capacity, and further protected against its malignant transformation.
The present data revealed that NDEA produced significant increases in the activity of liver G6PD, which agrees with findings from other studies[36,37]. G6PD is a housekeeping enzyme that produces riboses, which are incorporated into nucleotides and nucleic acids, and NADPH, the major cytoplasmic reducing compound[38]. NADPH is necessary for reduction of oxidized glutathione by glutathione reductase[39], and is a substrate for phase I and II biotransformation and detoxification enzymes[36]. G6PD is elevated in response to external stimuli, toxic and oxidative stress[40,41]. G6PD activity is strongly upregulated in proliferating cells such as malignant cells[42]. There is increasing evidence that G6PD activity is of major importance for NADPH production for defense against oxidative stress, rather than for ribose production during proliferation[43]. Interestingly, treatment with DAS resulted in further elevation in the activity of G6PD. This result provides new evidence that DAS might exhibit a compensatory mechanism in enhancing the production of NADPH as a further defense mechanism against proliferating cancer cells, as well as for enhancing cellular antioxidant capacity.
In hepatocellular carcinoma, there is disequilibrium between oxidant and antioxidant balance, which is tilted towards the oxidant side[44]. Reactive oxygen species (ROS) are believed to cause genetic oxidation and damage to DNA and other macromolecules. Unchecked, this oxidative damage may lead to a host of conditions including cancer. Normally, this process is held in check by elaborate endogenous or exogenous antioxidant processes. Various enzymatic and non-enzymatic systems have been developed by the cell to cope with ROS and other free radicals[45]. Since many of the anomalies that are induced by NDEA can arise from oxidative stress, which is also known to accompany cancer development, it was of a prime interest to evaluate oxidative stress levels under those circumstances. As shown in the present study, NDEA produced a significant increase in hepatic MDA and NO levels, along with significant decreases in SOD and CAT activities. MDA was one of the main lipid peroxidation products; its elevated levels can reflect the degree of lipid-peroxidation-induced injury in hepatocytes[46]. On the other hand, it has been reported that SOD and CAT constitute a mutually supportive defense against ROS[47]. The decreased activity of SOD in liver of NDEA-treated rats may have been due to the enhanced lipid peroxidation or inactivation of the antioxidative enzymes. This may have caused an increased accumulation of superoxide radicals, which could have further stimulated lipid peroxidation[48]. Decreased activities of SOD and CAT in NDEA-treated rats, which is in agreement with other reported studies[49], could have been due to over-utilization of these enzymatic antioxidants to scavenge the products of lipid peroxidation. Tumor cells have been reported to sequester essential antioxidants from the circulation in order to meet the demands of the growing tumor cells. On the other hand, the current data demonstrated the ability of DAS to reduce formation of ROS and reactive nitrogen species, measured as MDA and NO, which agrees with findings from other laboratories[7]. These findings conform to previous results on the established, specific antioxidant profile for DAS as an inhibitor of the hepatic ROS generating enzyme CYP2E1. The latter enzyme is known as a prominent trigger of hepatic oxidative stress[50]. Treatment with DAS showed no significant enhancement of the activity of the endogenous antioxidant enzyme SOD or CAT.
G6Pase plays a critical role in blood glucose homoeostasis and its activity can also be considered as an index of the stability of the microsomal membrane[51]. Decreased activity of liver G6Pase was shown in the NDEA-treated rats, which might be attributed to the increased lipid peroxidation caused by NDEA. Consistent with previous studies[52], DAS administration enhanced G6Pase activity significantly, compared to the NDEA-treated group value, suggesting the ability of DAS to preserve membrane integrity.
In this study, an increased activity of liver GST was observed in NDEA-treated rats, with respect to their control counterparts. In addition, we also showed an increased activity of serum GGT in the NDEA-treated rats, which might have been responsible for the increased level of GST in this group of animals[44]. Initial reports from nitrogen-mustard-resistant cell lines have shown these cells to over-express GST, which also holds true for a number of tumors. The increased level of GST is likely to be the key mediator of drug resistance in cancer chemotherapy[44]. Restoration of GST activity was observed with DAS treatment, suggesting a preservation of the redox system, which reflects a decrease in free-radical production, as well as improving cancer cell sensitivity to chemotherapy.
Cancer is well known to induce uncontrolled cellular proliferation. In this context, our results demonstrated that NDEA increased total DNA level, suggesting enhanced cellular proliferation. Notably, treatment with DAS significantly reduced DNA levels to near normal values, suggesting interference with mitotic pathways and enhancing apoptosis of cancer cells[53]. In addition, the current results showed an enhanced activity of cytochrome c oxidase enzyme in the NDEA-treated rats, which was further enhanced by the co-administration of DAS. However, for this investigation, we measured total cytochrome c oxidase, so it was difficult to delineate whether the increase we observed in the levels of cytochrome c oxidase was attributed to the mitochondrial or cytosolic fraction. According to the observed elevation in hepatic MDA and NO contents in the NDEA-treated group, a state of oxidative stress can exist in such animals, which contributes to mitochondrial membrane leakage and in turn, allows the translocation of cytochrome c oxidase to the cytosolic fractions. Thus, we suggest that the increase in cytochrome c oxidase with NDEA treatment might be of cytosolic origin. Interestingly, we demonstrated, and to the best of our knowledge, for the first time, that DAS markedly enhanced the activity of cytochrome c oxidase. We suggest that such increased cytochrome c oxidase activity might be attributed to a mitochondrial rather than cytosolic origin, which is supported by the observed increase in ATP and decrease in oxidative-stress biomarkers shown in DAS-treated animals. DAS might induce direct perturbation of mitochondria, resulting in apoptotic damage of the cancer cells. This effect has been reported recently with some anticancer agents[54,55].
Our results showed an almost 1.5-fold increase in AR activity in NDEA-treated animals. AR belongs to the aldo-keto reductase (AKR) superfamily. Most of the AKR superfamily proteins are involved in the detoxification of a wide variety of substrates. Several reports have shown that over-expression of AR, in many tumor cells, renders these cells resistant to chemotherapy, and also demonstrate that inhibition of AR enhances cancer cell sensitivity to chemotherapeutic drugs[56,57]. In addition, over-expression of AR enhances production of ROS, which cause membrane damage and cellular leakage[58]. To the best of our knowledge, the present study is the first to show enhanced production of AR in an in vivo model of liver tumorigenesis. To our knowledge, this is the first report that identifies the ability of DAS to reduce the expression of AR in NDEA-treated rats. This provides new evidence for its very important potential role in cancer protection. The ability of DAS to reduce the expression of AR suggests that DAS is effective against in vivo tumorigenesis by suppressing AR production and subsequently lowering the production of ROS, as well as enhancing cancer cell sensitivity to chemotherapeutic drugs.
Our findings were further supported by the histopathological examination of liver sections, which illustrated that liver tissue of NDEA-treated rats showed damage, manifest as nuclear pleomorphism, intranuclear vacuoles, cellular infiltration, hyperchromatic nuclei, pyknotic nuclei, numerous Kupffer cells and hyperchromatic malignant nuclei. On the contrary, liver tissue of the DAS-treated rats showed more or less normal hepatic lobular architecture.
To conclude, we provide evidence that DAS exerts a protective role on liver tissue in face of enhanced tumorigenesis caused by NDEA, as demonstrated by the following points: (1) DAS could normalize almost all the non-enzymatic and enzymatic liver function tests, indicating its ability to improve liver functions; (2) DAS significantly decreased lactate and lactate/pyruvate ratio, along with elevating pyruvate, ATP levels and liver LDH activity, thus enhancing energy metabolism in the liver tissue and reflecting restoration of normal cellular/metabolic functions; (3) DAS elevated the activity of G6PD, so it might exhibit a compensatory mechanism in enhancing the production of NADPH as a further defense mechanism against proliferating cancer cells, as well as enhancing cellular antioxidant capacity; (4) DAS reduced the formation of free radicals, measured as MDA and NO, providing specific antioxidant profiles for DAS as an inhibitor of the hepatic ROS-generating enzyme; (5) DAS enhanced G6Pase activity significantly, suggesting its ability to preserve liver cell membrane integrity; (6) DAS restored GST activity, suggesting a preservation of the redox system, as well as improving cancer cell sensitivity to chemotherapy; (7) DAS significantly reduced DNA level comparable to that in the NDEA-treated group, and close to the normal value, suggesting interference with mitotic pathways and enhancing apoptosis of cancer cells; (8) DAS markedly enhanced cytochrome c oxidase activity, thus, DAS might induce direct perturbation of mitochondria, resulting in apoptotic damage of the cancer cells; (9) to the best of our knowledge, this is the first report that identifies the ability of DAS to reduce the expression of liver AR in NDEA-treated rats, which suggests a very important potential role in cancer protection and subsequently lowering the production of ROS, as well as enhancing cancer cell sensitivity to chemotherapeutic drugs; (10) our biochemical findings were further supported by the histopathological examination of liver sections, which illustrated that liver tissue of the NDEA-treated rats showed damage, resulting in malignant cell formation. On the contrary, liver tissue of the DAS-treated rats showed more or less normal hepatic lobular architecture.
COMMENTS
Background
Diallyl sulfide (DAS), a biologically active garlic constituent, has been demonstrated as a potential cytoprotective agent in many animal models. Garlic (Allium sativum), an important flavoring agent, exhibits medicinal properties that include immunomodulatory, hepatoprotective, antioxidant, antimutagenic and anticarcinogenic effects.
Research frontiers
Oncotic necrosis is most often the consequence of metabolic injury, leading to ATP depletion, culminating in leakage of cytosolic enzymes and metabolic intermediates. In hepatocellular carcinoma, disequilibrium exists between oxidant and antioxidant balance, which is tilted towards oxidants. Tumor cells sequester essential antioxidants from the circulation to meet the demands of the growing tumor cells. We showed that DAS reduced formation of ROS, which agrees with reported findings. Aldose reductase (AR) belongs to the aldo-keto reductase (AKR) superfamily involved in the detoxification processes of a wide variety of substrates. Over-expression of AR, in many tumor cells, renders these cells resistant to chemotherapy.
Innovations and breakthroughs
DAS protects against N-nitrosodiethylamine (NDEA)-induced liver cancer. DAS markedly enhanced, cytochrome c oxidase activity, thus inducing direct perturbation of mitochondria, culminating in apoptotic damage of the cancer cells. The expression of liver AR in NDEA-treated rats was reduced by DAS, subsequently lowering the production of ROS and enhancing cancer cell sensitivity to chemotherapeutic drugs. Histopathological examination illustrated that liver tissue of the NDEA-treated rats showed malignant cell formation, which was prevented by DAS.
Applications
The population of Egypt has a heavy burden of liver disease and the use of synthetic chemoprotective agents has potential risks in this population. Dietary habits may modify carcinogenesis initiation, promotion and progression. Hence, this study indicates the potential protective effect of DAS against NDEA-induced liver cancer. This could be used as a protective method to prevent exacerbation of cancer in developed countries that cannot afford the burden of expensive chemotherapy.
Terminology
DAS: Diallyl sulfide (a major constituent of garlic); NDEA: N-nitrosodiethylamine (inducer of liver cancer); LDH: Lactate dehydrogenase; G6PD: Glucose-6-phosphate dehydrogenase; MDA: Malondialdehyde; NO: Nitric oxide; G6Pase: Glucose-6-phosphatase; CAT: Catalase; SOD: Superoxide dismutase; GST: Glutathione-S-transferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; GGT: Gamma-glutamyl transferase; AR: Aldose reductase.
Peer review
The authors demonstrated in this study that DAS had anti-oxidant properties in NDEA-treated rats. This work is very interesting. The authors bring some novelty and innovation to their research. The references are appropriate and updated.
Acknowledgments
The authors thank Dr. Sohair Assad, Professor of Histology, Faculty of Medicine, Cairo University, Cairo, Egypt, for her valuable assistance with the staining and the interpretation of the histological slides.
Footnotes
Peer reviewer: Dr. Cintia Siqueira, Center of Gastroenterology, Institute of Molecular Medicine, Av. Prof. Egas Moniz, Lisboa 1649-028, Portugal
S- Editor Tian L L- Editor Roberts SE E- Editor Lin YP
References
- 1.McGlynn KA, Tsao L, Hsing AW, Devesa SS, Fraumeni JF Jr. International trends and patterns of primary liver cancer. Int J Cancer. 2001;94:290–296. doi: 10.1002/ijc.1456. [DOI] [PubMed] [Google Scholar]
- 2.D'Alessandro N, Poma P, Montalto G. Multifactorial nature of hepatocellular carcinoma drug resistance: could plant polyphenols be helpful? World J Gastroenterol. 2007;13:2037–2043. doi: 10.3748/wjg.v13.i14.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steiner M, Seule M, Steiner B, Bauer I, Freund M, Kohne CH, Schuff-Werner P. 5-Fluorouracil/irinotecan induced lethal toxicity as a result of a combined pharmacogenetic syndrome: report of a case. J Clin Pathol. 2005;58:553–555. doi: 10.1136/jcp.2004.022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schorah CJ. Micronutrients, vitamins, and cancer risk. Vitam Horm. 1999;57:1–23. doi: 10.1016/s0083-6729(08)60638-x. [DOI] [PubMed] [Google Scholar]
- 5.Singh SV, Mohan RR, Agarwal R, Benson PJ, Hu X, Rudy MA, Xia H, Katoh A, Srivastava SK, Mukhtar H, et al. Novel anti-carcinogenic activity of an organosulfide from garlic: inhibition of H-RAS oncogene transformed tumor growth in vivo by diallyl disulfide is associated with inhibition of p21H-ras processing. Biochem Biophys Res Commun. 1996;225:660–665. doi: 10.1006/bbrc.1996.1226. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal KC. Therapeutic actions of garlic constituents. Med Res Rev. 1996;16:111–124. doi: 10.1002/(SICI)1098-1128(199601)16:1<111::AID-MED4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Aquilano K, Filomeni G, Baldelli S, Piccirillo S, De Martino A, Rotilio G, Ciriolo MR. Neuronal nitric oxide synthase protects neuroblastoma cells from oxidative stress mediated by garlic derivatives. J Neurochem. 2007;101:1327–1337. doi: 10.1111/j.1471-4159.2006.04431.x. [DOI] [PubMed] [Google Scholar]
- 8.Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007;247:167–181. doi: 10.1016/j.canlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Jakubikova J, Sedlak J. Garlic-derived organosulfides induce cytotoxicity, apoptosis, cell cycle arrest and oxidative stress in human colon carcinoma cell lines. Neoplasma. 2006;53:191–199. [PubMed] [Google Scholar]
- 10.Arunkumar A, Vijayababu MR, Venkataraman P, Senthilkumar K, Arunakaran J. Chemoprevention of rat prostate carcinogenesis by diallyl disulfide, an organosulfur compound of garlic. Biol Pharm Bull. 2006;29:375–379. doi: 10.1248/bpb.29.375. [DOI] [PubMed] [Google Scholar]
- 11.El-Bayoumy K, Sinha R, Pinto JT, Rivlin RS. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J Nutr. 2006;136:864S–869S. doi: 10.1093/jn/136.3.864S. [DOI] [PubMed] [Google Scholar]
- 12.Herman-Antosiewicz A, Singh SV. Signal transduction pathways leading to cell cycle arrest and apoptosis induction in cancer cells by Allium vegetable-derived organosulfur compounds: a review. Mutat Res. 2004;555:121–131. doi: 10.1016/j.mrfmmm.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Weichselbaum TE, Varney PL. A new method of flame photometry. Proc Soc Exp Biol Med. 1949;71:570–572. doi: 10.3181/00379727-71-17259. [DOI] [PubMed] [Google Scholar]
- 14.Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 15.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 16.Kind PR, King EJ. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954;7:322–326. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belfield A, Goldberg DM. Colorimetric determination of alkaline phosphatase activity. Enzyme. 1971;12:561–568. doi: 10.1159/000459586. [DOI] [PubMed] [Google Scholar]
- 18.Szasz G. A kinetic photometric method for serum gamma-glutamyl transpeptidase. Clin Chem. 1969;15:124–136. [PubMed] [Google Scholar]
- 19.Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol. 1979;135:372–376. doi: 10.1016/0002-9378(79)90708-7. [DOI] [PubMed] [Google Scholar]
- 20.Smith L. Spectrophotometric assay of cytochrome c oxidase. In Methods of Biochemical Analysis. In: Glick D ed., editor. New York: John Wiley & Sons; 1955. pp. 427–434. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt HH, Warner TD, Nakane M, Forstermann U, Murad F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol. 1992;41:615–624. [PubMed] [Google Scholar]
- 22.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 23.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 24.Buhl SN, Jackson KY. Optimal conditions and comparison of lactate dehydrogenase catalysis of the lactate-to-pyruvate and pyruvate-to-lactate reactions in human serum at 25, 30, and 37 degrees C. Clin Chem. 1978;24:828–831. [PubMed] [Google Scholar]
- 25.Sie HG, Hablanian A, Fishman WH. Divergent effects of actinomycin d on cortisol and on glucose stimulation of glycogenesis in mouse liver. Biochem J. 1967;102:103–109. doi: 10.1042/bj1020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maral J, Puget K, Michelson AM. Comparative study of superoxide dismutase, catalase and glutathione peroxidase levels in erythrocytes of different animals. Biochem Biophys Res Commun. 1977;77:1525–1535. doi: 10.1016/s0006-291x(77)80151-4. [DOI] [PubMed] [Google Scholar]
- 27.Mohun AF, Cook IJ. Simple methods for measuring serum levels of the glutamic-oxalacetic and glutamic-pyruvic transaminases in routine laboratories. J Clin Pathol. 1957;10:394–399. doi: 10.1136/jcp.10.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.David BS. Carbohydrate. In: Burtis CA, Ashwood ER, eds , editors. Tietz textbook of clinical chemistry, 3rd ed. Philadelphia, Pennsylvania: W.B. Saunders Company; 1999. p. 788. [Google Scholar]
- 29.Lowry OH, Passommeau JV, Hasselberger FX, Schulz DW. Effect of Ischemia on Known Substrates and Cofactors of the Glycolytic Pathway in Brain. J Biol Chem. 1964;239:18–30. [PubMed] [Google Scholar]
- 30.Harper AE. Hormonal factors affecting glucose 6-phosphatase activity. 2. Some effects of diet and of alloxan diabetes in the rat. Biochem J. 1959;71:702–705. doi: 10.1042/bj0710702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giles KW, Myers A. An imporoved diphenylamine method for the estimation of deoxyribonucleic acid. Nature (Lond) 1965;206:93. [Google Scholar]
- 32.Chauncey B, Leite MV, Goldstein L. Renal sorbitol accumulation and associated enzyme activities in diabetes. Enzyme. 1988;39:231–234. doi: 10.1159/000469124. [DOI] [PubMed] [Google Scholar]
- 33.Lowry OH, Rosebrough NJ, Farr AL, Ranall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 34.Dakshayani KB, Subramanian P, Manivasagam T, Essa MM, Manoharan S. Melatonin modulates the oxidant-antioxidant imbalance during N-nitrosodiethylamine induced hepatocarcinogenesis in rats. J Pharm Pharm Sci. 2005;8:316–321. [PubMed] [Google Scholar]
- 35.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 36.Winzer K, Van Noorden CJ, Kohler A. Glucose-6-phosphate dehydrogenase: the key to sex-related xenobiotic toxicity in hepatocytes of European flounder (Platichthys flesus L.)? Aquat Toxicol. 2002;56:275–288. doi: 10.1016/s0166-445x(01)00215-6. [DOI] [PubMed] [Google Scholar]
- 37.Frederiks WM, Bosch KS, De Jong JS, Van Noorden CJ. Post-translational regulation of glucose-6-phosphate dehydrogenase activity in (pre)neoplastic lesions in rat liver. J Histochem Cytochem. 2003;51:105–112. doi: 10.1177/002215540305100112. [DOI] [PubMed] [Google Scholar]
- 38.Kletzien RF, Harris PK, Foellmi LA. Glucose-6-phosphate dehydrogenase: a "housekeeping" enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J. 1994;8:174–181. doi: 10.1096/fasebj.8.2.8119488. [DOI] [PubMed] [Google Scholar]
- 39.Ninfali P, Biagiotti E, Guidi L, Malatesta M, Gazzanelli G, Del Grande P. Cytochemical and immunocytochemical methods for electron microscopic detection of glucose-6-phosphate dehydrogenase in brain areas. Brain Res Brain Res Protoc. 2000;5:115–120. doi: 10.1016/s1385-299x(99)00063-x. [DOI] [PubMed] [Google Scholar]
- 40.Spolarics Z. Endotoxemia, pentose cycle, and the oxidant/antioxidant balance in the hepatic sinusoid. J Leukoc Biol. 1998;63:534–541. doi: 10.1002/jlb.63.5.534. [DOI] [PubMed] [Google Scholar]
- 41.Salati LM, Amir-Ahmady B. Dietary regulation of expression of glucose-6-phosphate dehydrogenase. Annu Rev Nutr. 2001;21:121–140. doi: 10.1146/annurev.nutr.21.1.121. [DOI] [PubMed] [Google Scholar]
- 42.Kuo W, Lin J, Tang TK. Human glucose-6-phosphate dehydrogenase (G6PD) gene transforms NIH 3T3 cells and induces tumors in nude mice. Int J Cancer. 2000;85:857–864. doi: 10.1002/(sici)1097-0215(20000315)85:6<857::aid-ijc20>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 43.Biagiotti E, Bosch KS, Ninfali P, Frederiks WM, Van Noorden CJ. Posttranslational regulation of glucose-6-phosphate dehydrogenase activity in tongue epithelium. J Histochem Cytochem. 2000;48:971–977. doi: 10.1177/002215540004800710. [DOI] [PubMed] [Google Scholar]
- 44.Rajkapoor B, Murugesh N, Chodon D, Sakthisekaran D. Chemoprevention of N-nitrosodiethylamine induced phenobarbitol promoted liver tumors in rat by extract of Indigofera aspalathoides. Biol Pharm Bull. 2005;28:364–366. doi: 10.1248/bpb.28.364. [DOI] [PubMed] [Google Scholar]
- 45.Milner JA. Preclinical perspectives on garlic and cancer. J Nutr. 2006;136:827S–831S. doi: 10.1093/jn/136.3.727S. [DOI] [PubMed] [Google Scholar]
- 46.Yang SS, Huang CC, Chen JR, Chiu CL, Shieh MJ, Lin SJ, Yang SC. Effects of ethanol on antioxidant capacity in isolated rat hepatocytes. World J Gastroenterol. 2005;11:7272–7276. doi: 10.3748/wjg.v11.i46.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manna PR, Chandrala SP, King SR, Jo Y, Counis R, Huhtaniemi IT, Stocco DM. Molecular mechanisms of insulin-like growth factor-I mediated regulation of the steroidogenic acute regulatory protein in mouse leydig cells. Mol Endocrinol. 2006;20:362–378. doi: 10.1210/me.2004-0526. [DOI] [PubMed] [Google Scholar]
- 48.Park YJ, Choi EY, Choi JY, Park JG, You HJ, Chung MH. Genetic changes of hOGG1 and the activity of oh8Gua glycosylase in colon cancer. Eur J Cancer. 2001;37:340–346. doi: 10.1016/s0959-8049(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 49.Sundaresan S, Subramanian P. S-allylcysteine inhibits circulatory lipid peroxidation and promotes antioxidants in N-nitrosodiethylamine-induced carcinogenesis. Pol J Pharmacol. 2003;55:37–42. [PubMed] [Google Scholar]
- 50.Park KA, Kweon S, Choi H. Anticarcinogenic effect and modification of cytochrome P450 2E1 by dietary garlic powder in diethylnitrosamine-initiated rat hepatocarcinogenesis. J Biochem Mol Biol. 2002;35:615–622. doi: 10.5483/bmbrep.2002.35.6.615. [DOI] [PubMed] [Google Scholar]
- 51.van Schaftingen E, Gerin I. The glucose-6-phosphatase system. Biochem J. 2002;362:513–532. doi: 10.1042/0264-6021:3620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh A, Arora A, Shukla Y. Modulation of altered hepatic foci induction by diallyl sulphide in Wistar rats. Eur J Cancer Prev. 2004;13:263–269. doi: 10.1097/01.cej.0000127633.89678.fb. [DOI] [PubMed] [Google Scholar]
- 53.Xiao D, Pinto JT, Gundersen GG, Weinstein IB. Effects of a series of organosulfur compounds on mitotic arrest and induction of apoptosis in colon cancer cells. Mol Cancer Ther. 2005;4:1388–1398. doi: 10.1158/1535-7163.MCT-05-0152. [DOI] [PubMed] [Google Scholar]
- 54.Rotem R, Heyfets A, Fingrut O, Blickstein D, Shaklai M, Flescher E. Jasmonates: novel anticancer agents acting directly and selectively on human cancer cell mitochondria. Cancer Res. 2005;65:1984–1993. doi: 10.1158/0008-5472.CAN-04-3091. [DOI] [PubMed] [Google Scholar]
- 55.You KR, Wen J, Lee ST, Kim DG. Cytochrome c oxidase subunit III: a molecular marker for N-(4-hydroxy-phenyl)retinamise-induced oxidative stress in hepatoma cells. J Biol Chem. 2002;277:3870–3877. doi: 10.1074/jbc.M109284200. [DOI] [PubMed] [Google Scholar]
- 56.Saraswat M, Mrudula T, Kumar PU, Suneetha A, Rao Rao TS, Srinivasulu M, Reddy B. Overexpression of aldose reductase in human cancer tissues. Med Sci Monit. 2006;12:CR525–CR529. [PubMed] [Google Scholar]
- 57.Zeindl-Eberhart E, Haraida S, Liebmann S, Jungblut PR, Lamer S, Mayer D, Jager G, Chung S, Rabes HM. Detection and identification of tumor-associated protein variants in human hepatocellular carcinomas. Hepatology. 2004;39:540–549. doi: 10.1002/hep.20060. [DOI] [PubMed] [Google Scholar]
- 58.Thomas T, Rauscher F, Sanders R, Veltman J, Watkins JB 3rd. Effects of aldose reductase inhibitors on antioxidant defense in rat and rabbit liver. Toxicol Sci. 2000;53:145–149. doi: 10.1093/toxsci/53.1.145. [DOI] [PubMed] [Google Scholar]


