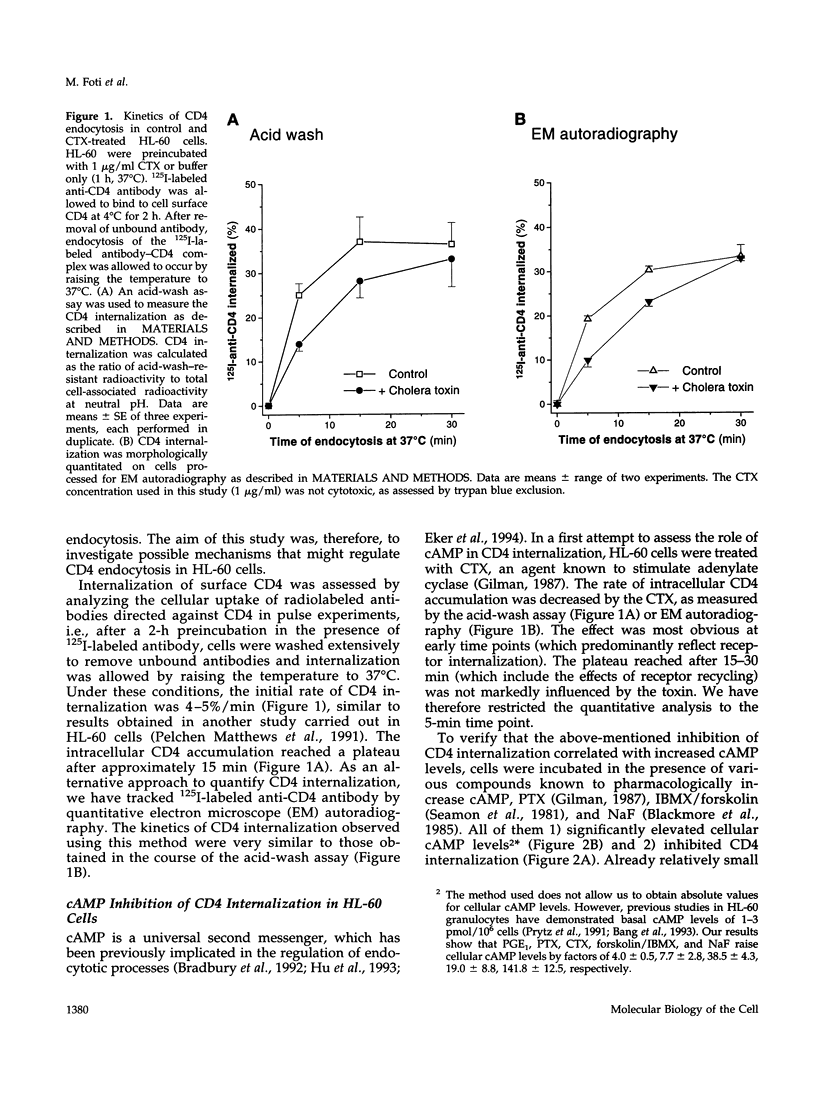
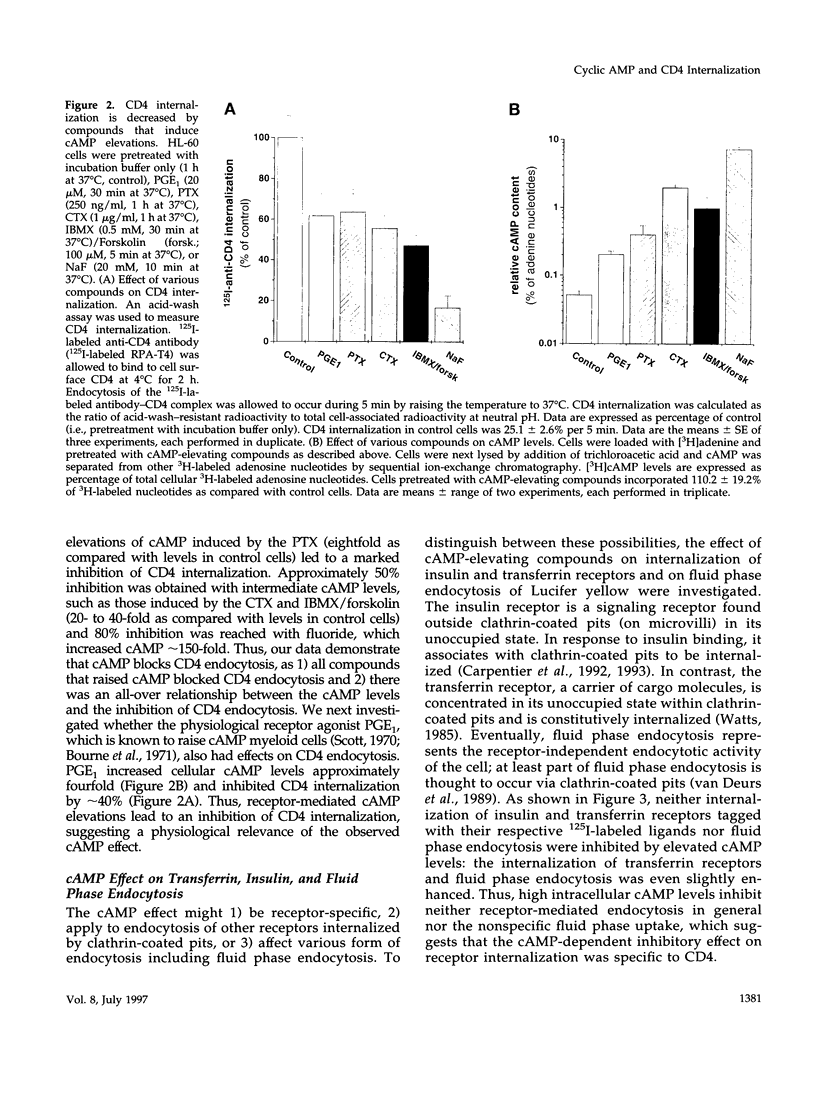
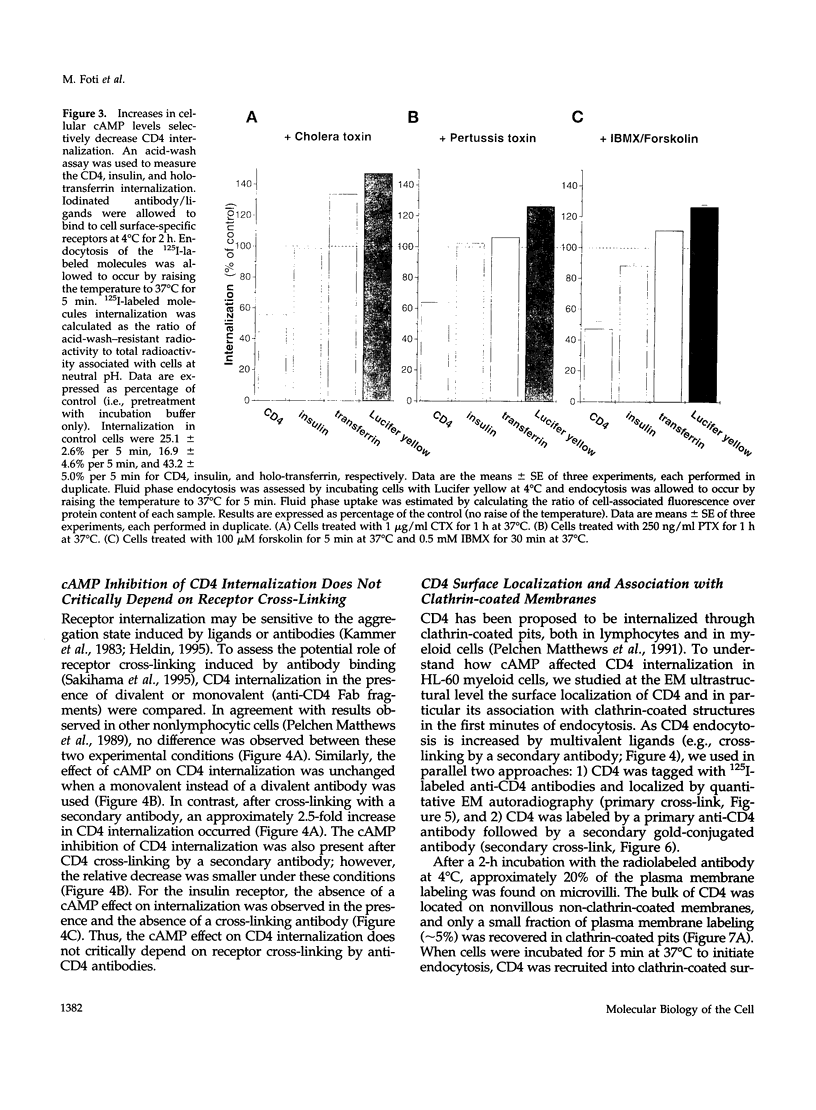
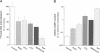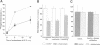Abstract
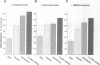
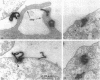
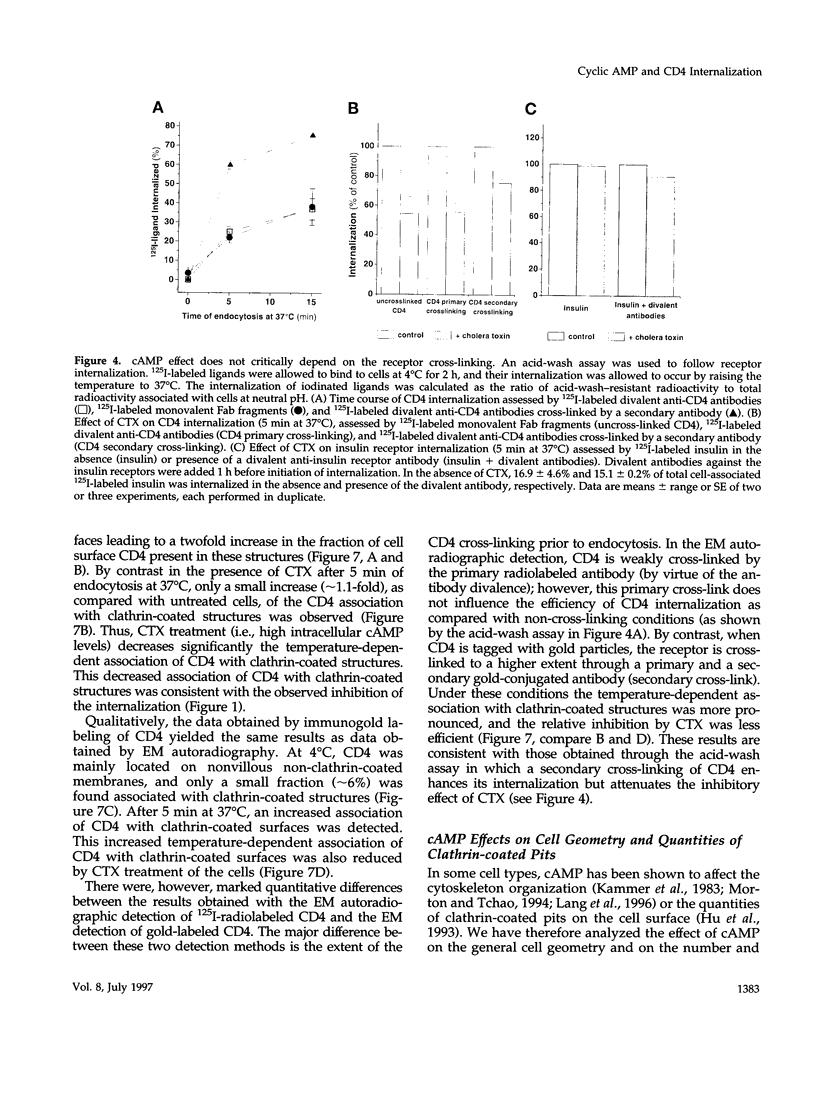
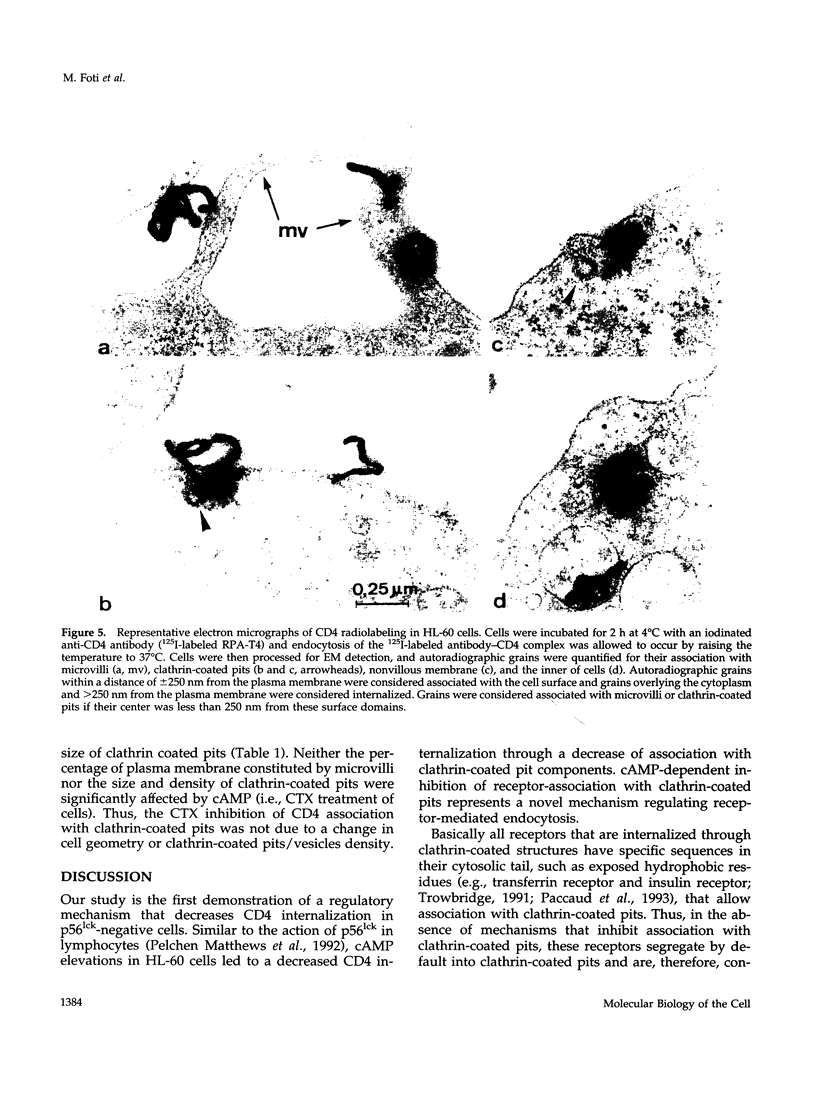
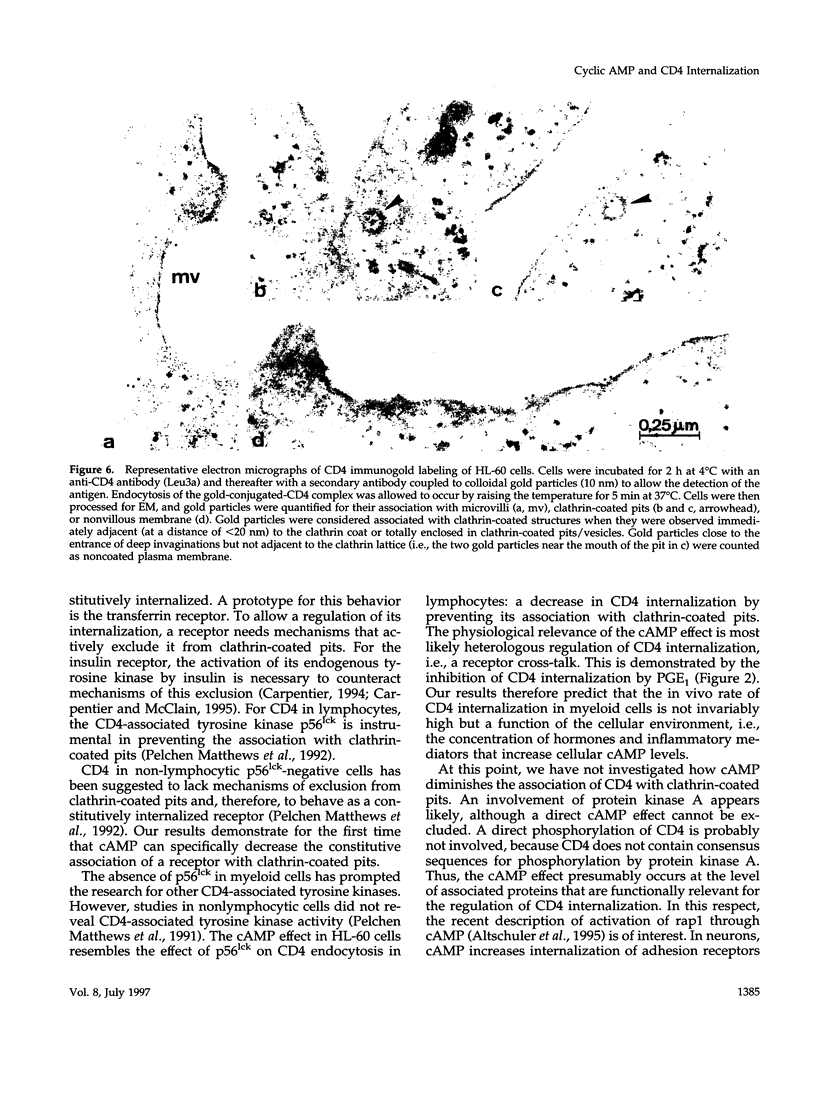
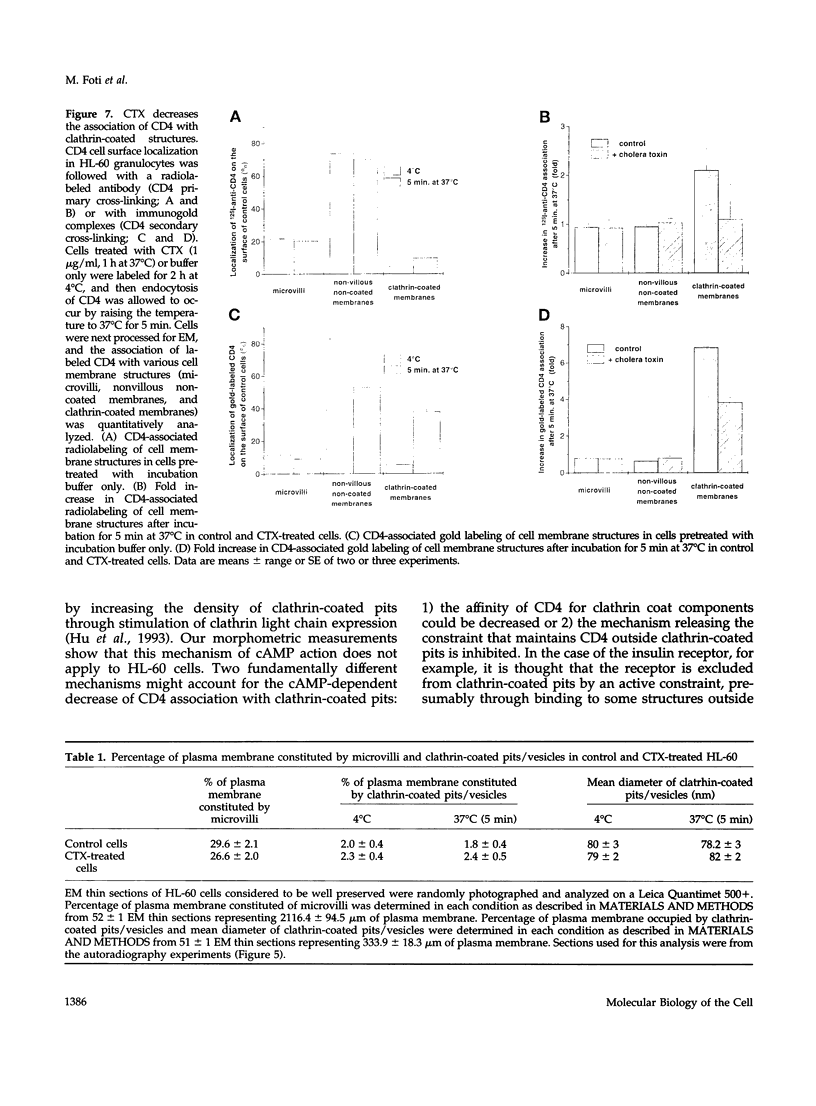
CD4, a member of the immunoglobulin superfamily, is not only expressed in T4 helper lymphocytes but also in myeloid cells. Receptor-mediated endocytosis plays a crucial role in the regulation of surface expression of adhesion molecules such as CD4. In T lymphocytes p56lck, a CD4-associated tyrosine kinase, prevents CD4 internalization, but in myeloid cells p56lck is not expressed and CD4 is constitutively internalized. In this study, we have investigated the role of cyclic AMP (cAMP) in the regulation of CD4 endocytosis in the myeloid cell line HL-60. Elevations of cellular cAMP were elicited by 1) cholera toxin, 2) pertussis toxin, 3) forskolin and IBMX, 4) NaF, or 5) the physiological receptor agonist prostaglandin E1. All five interventions led to an inhibition of CD4 internalization. Increased cAMP levels did not inhibit endocytosis per se, because internalization of insulin receptors and transferrin receptors and fluid phase endocytosis were either unchanged or slightly enhanced. The mechanism of cAMP inhibition was further analyzed at the ultrastructural level. CD4 internalization, followed either by quantitative electron microscopy autoradiography or by immunogold labeling, showed a rapid and temperature-dependent association of CD4 with clathrin-coated pits in control cells. This association was markedly inhibited in cells with elevated cAMP levels. Thus these findings suggest a second-messenger regulation of CD4 internalization through an inhibition of CD4 association with clathrin-coated pits in p56lck-negative cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken C., Konner J., Landau N. R., Lenburg M. E., Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994 Mar 11;76(5):853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Altschuler D. L., Peterson S. N., Ostrowski M. C., Lapetina E. G. Cyclic AMP-dependent activation of Rap1b. J Biol Chem. 1995 May 5;270(18):10373–10376. doi: 10.1074/jbc.270.18.10373. [DOI] [PubMed] [Google Scholar]
- Bang B. E., Aarbakke J., Sager G. Epinephrine induces beta-adrenergic desensitization and differentiation of HL-60 cells. Scand J Clin Lab Invest. 1993 Jul;53(4):311–315. doi: 10.3109/00365519309086621. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Bocckino S. B., Waynick L. E., Exton J. H. Role of a guanine nucleotide-binding regulatory protein in the hydrolysis of hepatocyte phosphatidylinositol 4,5-bisphosphate by calcium-mobilizing hormones and the control of cell calcium. Studies utilizing aluminum fluoride. J Biol Chem. 1985 Nov 25;260(27):14477–14483. [PubMed] [Google Scholar]
- Bourne H. R., Lehrer R. I., Cline M. J., Melmon K. L. Cyclic 3',5'-adenosine monophosphate in the human lukocyte: synthesis, degradation, andeffects n neutrophil candidacidal activity. J Clin Invest. 1971 Apr;50(4):920–929. doi: 10.1172/JCI106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury N. A., Jilling T., Kirk K. L., Bridges R. J. Regulated endocytosis in a chloride secretory epithelial cell line. Am J Physiol. 1992 Mar;262(3 Pt 1):C752–C759. doi: 10.1152/ajpcell.1992.262.3.C752. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Amherdt M., Van Obberghen E., Kahn C. R., Orci L. 125I-insulin binding to cultured human lymphocytes. Initial localization and fate of hormone determined by quantitative electron microscopic autoradiography. J Clin Invest. 1978 Apr;61(4):1057–1070. doi: 10.1172/JCI109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Anderson R. G., Goldstein J. L., Brown M. S., Cohen S., Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982 Oct;95(1):73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L. Insulin receptor internalization: molecular mechanisms and physiopathological implications. Diabetologia. 1994 Sep;37 (Suppl 2):S117–S124. doi: 10.1007/BF00400835. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Lew D. P., Paccaud J. P., Gil R., Iacopetta B., Kazatchkine M., Stendahl O., Pozzan T. Internalization pathway of C3b receptors in human neutrophils and its transmodulation by chemoattractant receptors stimulation. Cell Regul. 1991 Jan;2(1):41–55. doi: 10.1091/mbc.2.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., McClain D. Insulin receptor kinase activation releases a constraint maintaining the receptor on microvilli. J Biol Chem. 1995 Mar 10;270(10):5001–5006. doi: 10.1074/jbc.270.10.5001. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Paccaud J. P., Backer J., Gilbert A., Orci L., Kahn C. R., Baecker J [corrected to Backer J. ]. Two steps of insulin receptor internalization depend on different domains of the beta-subunit. J Cell Biol. 1993 Sep;122(6):1243–1252. doi: 10.1083/jcb.122.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Paccaud J. P., Gorden P., Rutter W. J., Orci L. Insulin-induced surface redistribution regulates internalization of the insulin receptor and requires its autophosphorylation. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):162–166. doi: 10.1073/pnas.89.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L. Robert Feulgen Prize Lecture 1993. The journey of the insulin receptor into the cell: from cellular biology to pathophysiology. Histochemistry. 1993 Sep;100(3):169–184. doi: 10.1007/BF00269090. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L. The cell biology of the insulin receptor. Diabetologia. 1989 Sep;32(9):627–635. doi: 10.1007/BF00274248. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Van Obberghen E., Gorden P., Orci L. Surface redistribution of 125I-insulin in cultured human lymphocytes. J Cell Biol. 1981 Oct;91(1):17–25. doi: 10.1083/jcb.91.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992 Feb 5;267(4):2318–2324. [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Eker P., Holm P. K., van Deurs B., Sandvig K. Selective regulation of apical endocytosis in polarized Madin-Darby canine kidney cells by mastoparan and cAMP. J Biol Chem. 1994 Jul 15;269(28):18607–18615. [PubMed] [Google Scholar]
- Fan J. Y., Carpentier J. L., Gorden P., Van Obberghen E., Blackett N. M., Grunfeld C., Orci L. Receptor-mediated endocytosis of insulin: role of microvilli, coated pits, and coated vesicles. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7788–7791. doi: 10.1073/pnas.79.24.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion L. G., Izaguirre C. A., Garber G. E., Huebsh L., Aye M. T. Detection of surface and cytoplasmic CD4 on blood monocytes from normal and HIV-1 infected individuals. J Immunol Methods. 1990 Dec 31;135(1-2):59–69. doi: 10.1016/0022-1759(90)90256-u. [DOI] [PubMed] [Google Scholar]
- Foti M., Lew D. P., Carpentier J. L., Krause K. H. CD4 in nonlymphocytic cells: more than an HIV receptor? J Lab Clin Med. 1995 Sep;126(3):233–239. [PubMed] [Google Scholar]
- Frederickson G. G., Basch R. S. L3T4 antigen expression by hemopoietic precursor cells. J Exp Med. 1989 Apr 1;169(4):1473–1478. doi: 10.1084/jem.169.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féger J., Gil-Falgon S., Lamaze C. Cell receptors: definition, mechanisms and regulation of receptor-mediated endocytosis. Cell Mol Biol (Noisy-le-grand) 1994 Dec;40(8):1039–1061. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hanna Z., Simard C., Laperrière A., Jolicoeur P. Specific expression of the human CD4 gene in mature CD4+ CD8- and immature CD4+ CD8+ T cells and in macrophages of transgenic mice. Mol Cell Biol. 1994 Feb;14(2):1084–1094. doi: 10.1128/mcb.14.2.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H. Dimerization of cell surface receptors in signal transduction. Cell. 1995 Jan 27;80(2):213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Hu Y., Barzilai A., Chen M., Bailey C. H., Kandel E. R. 5-HT and cAMP induce the formation of coated pits and vesicles and increase the expression of clathrin light chain in sensory neurons of aplysia. Neuron. 1993 May;10(5):921–929. doi: 10.1016/0896-6273(93)90207-8. [DOI] [PubMed] [Google Scholar]
- Iacopetta B., Carpentier J. L., Pozzan T., Lew D. P., Gorden P., Orci L. Role of intracellular calcium and protein kinase C in the endocytosis of transferrin and insulin by HL60 cells. J Cell Biol. 1986 Sep;103(3):851–856. doi: 10.1083/jcb.103.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Alvarez R., Salomon Y. Determination of adenylyl cyclase catalytic activity using single and double column procedures. Methods Enzymol. 1994;238:31–56. doi: 10.1016/0076-6879(94)38005-8. [DOI] [PubMed] [Google Scholar]
- Kammer G. M., Boehm C. A., Rudolph S. A., Schultz L. A. Mobility of the human T lymphocyte surface molecules CD3, CD4, and CD8: regulation by a cAMP-dependent pathway. Proc Natl Acad Sci U S A. 1988 Feb;85(3):792–796. doi: 10.1073/pnas.85.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer G. M., Smith J. A., Mitchell R. Capping of human T cell specific determinants: kinetics of capping and receptor re-expression and regulation by the cytoskeleton. J Immunol. 1983 Jan;130(1):38–44. [PubMed] [Google Scholar]
- Krischer J., Gilbert A., Gorden P., Carpentier J. L. Endocytosis is inhibited in hepatocytes from diabetic rats. Diabetes. 1993 Sep;42(9):1303–1309. doi: 10.2337/diab.42.9.1303. [DOI] [PubMed] [Google Scholar]
- Lang P., Gesbert F., Delespine-Carmagnat M., Stancou R., Pouchelet M., Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996 Feb 1;15(3):510–519. [PMC free article] [PubMed] [Google Scholar]
- Louache F., Debili N., Marandin A., Coulombel L., Vainchenker W. Expression of CD4 by human hematopoietic progenitors. Blood. 1994 Nov 15;84(10):3344–3355. [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Molineaux S. M., Maddon D. E., Zimmerman K. A., Godfrey M., Alt F. W., Chess L., Axel R. Structure and expression of the human and mouse T4 genes. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9155–9159. doi: 10.1073/pnas.84.24.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Morton D. M., Tchao R. Regulation of motility and cytoskeletal organization of rat bladder carcinoma cells by cyclic AMP. Cell Motil Cytoskeleton. 1994;29(4):375–382. doi: 10.1002/cm.970290410. [DOI] [PubMed] [Google Scholar]
- Paccaud J. P., Reith W., Johansson B., Magnusson K. E., Mach B., Carpentier J. L. Clathrin-coated pit-mediated receptor internalization. Role of internalization signals and receptor mobility. J Biol Chem. 1993 Nov 5;268(31):23191–23196. [PubMed] [Google Scholar]
- Parnes J. R. Molecular biology and function of CD4 and CD8. Adv Immunol. 1989;44:265–311. doi: 10.1016/s0065-2776(08)60644-6. [DOI] [PubMed] [Google Scholar]
- Pelchen-Matthews A., Armes J. E., Griffiths G., Marsh M. Differential endocytosis of CD4 in lymphocytic and nonlymphocytic cells. J Exp Med. 1991 Mar 1;173(3):575–587. doi: 10.1084/jem.173.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews A., Armes J. E., Marsh M. Internalization and recycling of CD4 transfected into HeLa and NIH3T3 cells. EMBO J. 1989 Dec 1;8(12):3641–3649. doi: 10.1002/j.1460-2075.1989.tb08538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews A., Boulet I., Littman D. R., Fagard R., Marsh M. The protein tyrosine kinase p56lck inhibits CD4 endocytosis by preventing entry of CD4 into coated pits. J Cell Biol. 1992 Apr;117(2):279–290. doi: 10.1083/jcb.117.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prytz P. S., Bang B. E., Endresen P. C., Møller C., Aarbakke J. Elevation of cyclic AMP levels in HL-60 cells accumulated in G1 or G2 by transmethylation inhibitors. Biochem Pharmacol. 1991 Oct 9;42(9):1761–1766. doi: 10.1016/0006-2952(91)90513-5. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E. CD4, CD8 and the TCR-CD3 complex: a novel class of protein-tyrosine kinase receptor. Immunol Today. 1990 Nov;11(11):400–406. doi: 10.1016/0167-5699(90)90159-7. [DOI] [PubMed] [Google Scholar]
- Sakihama T., Smolyar A., Reinherz E. L. Oligomerization of CD4 is required for stable binding to class II major histocompatibility complex proteins but not for interaction with human immunodeficiency virus gp120. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6444–6448. doi: 10.1073/pnas.92.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E. Effects of prostaglandins, epinephrine and NaF on human leukocyte, platelet and liver adenyl cyclase. Blood. 1970 Apr;35(4):514–516. [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotzik F., Rosenberg Y., Boyd A. W., Honeyman M., Metcalf D., Scollay R., Wu L., Shortman K. Assessment of CD4 expression by early T precursor cells and by dendritic cells in the human thymus. J Immunol. 1994 Apr 1;152(7):3370–3377. [PubMed] [Google Scholar]
- Stewart S. J., Fujimoto J., Levy R. Human T lymphocytes and monocytes bear the same Leu-3(T4) antigen. J Immunol. 1986 May 15;136(10):3773–3778. [PubMed] [Google Scholar]
- Trowbridge I. S. Endocytosis and signals for internalization. Curr Opin Cell Biol. 1991 Aug;3(4):634–641. doi: 10.1016/0955-0674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Valitutti S., Müller S., Cella M., Padovan E., Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995 May 11;375(6527):148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Watts C. Rapid endocytosis of the transferrin receptor in the absence of bound transferrin. J Cell Biol. 1985 Feb;100(2):633–637. doi: 10.1083/jcb.100.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]
- Zauli G., Furlini G., Vitale M., Re M. C., Gibellini D., Zamai L., Visani G., Borgatti P., Capitani S., La Placa M. A subset of human CD34+ hematopoietic progenitors express low levels of CD4, the high-affinity receptor for human immunodeficiency virus-type 1. Blood. 1994 Sep 15;84(6):1896–1905. [PubMed] [Google Scholar]
- van Deurs B., Petersen O. W., Olsnes S., Sandvig K. The ways of endocytosis. Int Rev Cytol. 1989;117:131–177. doi: 10.1016/s0074-7696(08)61336-4. [DOI] [PubMed] [Google Scholar]