Abstract
In this paper we report our efforts to enhance the immunogenicity of Pfs28, a transmission blocking vaccine candidate of Plasmodium falciparum, using a strategy of chemical conjugation. With an improved procedure Pfs28 was covalently coupled to the mutant and nontoxic ExoProtein A of Pseudomonas aeruginosa by the reaction between thiolated antigen and maleimide modified carrier protein. The optimized process resulted in a higher antigen-carrier conjugation ratio, and the conjugation product could be purified using single-step size-exclusion chromatography. A significant increase in immunogenicity measured by ELISA was observed in mice immunized with conjugated Pfs28 as compared to unconjugated Pfs28.
Keywords: Conjugation, Pfs28, rEPA, Malaria, Transmission blocking vaccine
1. Introduction
The malarial parasite is a mosquito-borne pathogen with a complex life cycle encompassing different developmental stages in both humans and mosquitoes. Transmission blocking vaccines, targeting molecules on malaria parasites mainly expressed at the mosquito stage, may block the transmission of malaria parasites to humans thus reducing the spread of the disease in endemic regions. Two leading transmission blocking vaccine candidates of Plasmodium falciparum, Pfs25 and Pfs28 [1,2], are however poor immunogens especially when formulated on alum [3]. We have previously shown that conjugation of Pfs25 to a carrier protein, such as outer-membrane protein complex of Neisseria meningitidis or recombinant ExoProtein A (rEPA) of Pseudomonas aeruginosa, demonstrated a significant enhancement in its immunogenicity without an apparent disruption of critical B cell epitopes [4–6].
With the success in enhancing the immunogenicity of Pfs25 by conjugation, the same attempt was made on Pfs28. The procedure previously reported for conjugating Pfs25 to rEPA [6] has been improved, and this improved procedure was applied to the conjugation of Pfs28 to rEPA. Optimization of this process focused on two aspects: (1) using equal moles of free thiols and maleimide groups in the reaction to stringently control the conjugation reaction and limit the unreacted linker groups on the conjugation products; (2) increasing the number of maleimide groups on rEPA to achieve two goals: (A) to generate conjugates with a higher antigen to carrier ratio, and (B) to simplify the purification of conjugation products by the activation and conjugation of all rEPA molecules in the reaction. Immune responses to the conjugated Pfs28 were assessed in mice. A significant increase in antibody titers of the Pfs28 conjugates was observed as compared to the unconjugated Pfs28.
2. Materials and Methods
2.1. Antigen and carrier protein
The recombinant Pfs28 is a Pichia pastoris expressed protein with molecular mass about 21 kDa, the purification of which will be introduced elsewhere in detail (D.L. Narum, manuscript in preparation). The recombinant rEPA used in this study is a non-toxic 67 kDa protein produced in Escherichia coli as previously described [6].
2.2. Thiolation of antigen
The recombinant Pfs28 was buffer-exchanged into thiolation buffer (0.1 M NaHCO3, 5 mM EDTA, pH 8.3) using 5 kDa molecular weight cut off (MWCO) spin filters (Millipore, Billerica, MA), and the concentration was adjusted to 2 mg/ml. DL-N-acetylhomocysteine thiolactone (NAHT) (Sigma Aldrich Inc., St Louis, MO) dissolved in thiolation buffer was added to a final concentration of 10 mM. After 24 h incubation at 4 °C, the thiolated antigen was buffer-exchanged into conjugation buffer (1 × PBS, 5 mM EDTA, pH 7.2). The content of free thiols on the activated antigen molecules (moles of free thiols per mole of antigen) was determined with Ellman’s reagent [5,5′-diothio-bis-(2-nitrobenzoic acid), Pierce Inc., Rockford, IL] by comparison to the L-cysteine·HCl (Pierce Inc., Rockford, IL) used as standard.
2.3. Maleimide activation of rEPA
The carrier protein rEPA was buffer-exchanged into conjugation buffer using 5 kDa MWCO spin filters and its concentration was adjusted to 2 mg/ml. N-[ε-maleimidocaproyloxy] sulfosuccinimide ester (Sulfo-EMCS) (Pierce Inc., Rockford, IL) dissolved in conjugation buffer was added to a final concentration of 1 mM. The mixture was incubated at 22 °C for 1 h (instead of a 20-min incubation used in the previous reaction [6]). After reaction, the mixture was immediately buffer-exchanged into conjugation buffer. The content of maleimide groups on the molecules of maleimide derivatized rEPA (moles of maleimide groups per mole of rEPA) was determined indirectly with Ellman’s reagent by measuring the consumption of free thiol of L-cysteine·HCl used to react with the activated protein.
2.4. Conjugation reaction between thiolated antigen and maleimide-rEPA
A series of tests with different ratios of thiolated antigen to maleimide-rEPA were performed to determine a ratio for preparative-scale conjugation, where the moles of maleimide groups were equal to the moles of free thiols. The approximate molar ratio was determined to be 5:1 (thiolated Pfs28:maleimide-rEPA) after SDS-PAGE analysis of the test samples. The preparative-scale conjugation reaction between thiolated antigen and maleimide-rEPA with determined molar ratio (instead of excess thiolated antigen used in the previous reaction [6]) was conducted at room temperature for 1 hour. The purification was run on a 16/60 Superdex 200 size-exclusion chromatography (SEC) column (GE Healthcare, Piscataway, NJ) at a flow rate of 1 ml/min and with PBS (pH 7.2) as mobile phase. The Pfs28-rEPA conjugates were obtained by pooling the different collected fractions according to the SDS-PAGE analysis, and arbitrarily divided into two parts based on their size, Pfs28-rEPA fraction 1 (Pfs28-rEPA F1) and Pfs28-rEPA fraction 2 (Pfs28-rEPA F2).
2.5. Characterization of conjugate
The migration pattern of the conjugates was analyzed by SDS-PAGE. The molecular mass of the conjugates in solution was determined using SEC-HPLC with inline multiangle light scattering (MALS) as described [6,7]. The antigen-carrier conjugation ratio (molar ratio of antigen to carrier within a conjugate) was calculated following the method as described [8] and based on the results of amino acid analysis performed by the W. M. Keck Facility (Yale University, New Haven, CT). The concentration of Pfs28 within the conjugate was then calculated based on the conjugation ratio and the absorbance of conjugates at 280 nm. Two anti-Pfs28 monoclonal antibodies, 2D8 and 2A6 (recognizing conformational and linear epitopes respectively), were used in Western blot to analyze the conjugates. Endotoxin levels of both fractions of the conjugates were measured using Limulus amoebocyte lysate in a 96-well plate with chromogenic reagents and PyroSoft software (Associates of Cape Cod Inc., East Falmouth, MA). The endotoxin values were all less than 0.052 EU/μg of Pfs28.
2.6. Animal study
The conjugated or unconjugated Pfs28 was formulated on 1600 μg/ml Alhydrogel (Brenntag Biosector, Denmark), and the adsorption of the antigens to Alhydrogel was examined by SDS-PAGE [9]. A mouse study was carried out in compliance with the NIH guidelines and an Animal Care and Use Committee-approved protocol. BALB/c mice (Charles River Laboratories, Frederick, MD) were used in 9 groups of 10. The vaccine formulations containing the doses of Pfs28 at 0.1, 0.5 and 2.5 μg per 50 μl were administered through the anterior tibialis muscle on days 0 and 28, and the sera were collected on days 42, 56 and 70.
The antibody titers of the sera were examined using ELISA performed following a standardized protocol previously reported [10,11]. Kruskal-Wallis One-Way ANOVA followed by Student-Newman-Keuls was performed to test for a significant enhancement of antibody titers among the groups. If the P value was less than 0.05, the difference was considered significant.
3. Results and discussion
3.1. Preparation of Pfs28-rEPA
Using the following three reaction steps, a protein antigen can be covalently conjugated to rEPA. (1) Thiolation of the antigen using NAHT. The nucleophilic reaction between NAHT and primary amines on the antigen opens the ring of NAHT, forms an amide bond between the linker and antigen, and creates a free thiol. (2) Maleimide activation of rEPA using Sulfo-EMCS. The NHS ester of Sulfo-EMCS reacts with primary amines on rEPA via a nucleophilic reaction. With the release of the NHS group and the formation of an amide bond between the linker and the rEPA, the maleimide group is added. (3) The maleimide group on rEPA reacts with the sulfhydryl on the antigen, resulting in a stable thioether linkage between two proteins. Thus antigen-rEPA conjugates are formed.
Reaction parameters such as buffer content, pH, reaction time and linker concentration greatly affect the modification of both antigen and carrier protein. Higher levels of thiolation can be achieved at the strong alkaline pH (pH 11) in the reaction mixture, but protein aggregation was observed at the high pH. Based on the results of preliminary experiments, the reaction parameters were determined for Pfs28 thiolation and rEPA maleimide modification as described in the section of materials and methods. Each mole of Pfs28 contained ~ 0.8 moles of free thiols, and each mole of rEPA contained ~ 3.8 moles of maleimide groups. Equal moles of free thiols on Pfs28 and maleimide groups on rEPA (thus 5: molar ratio for thiolated Pfs28:maleimide-rEPA) were mixed. As expected, at the end of the reaction, the mixture contained high molecular mass conjugation products and un-conjugated Pfs28, but no visible un-conjugated rEPA presented on the SDS-PAGE gel (Fig. 1A). The difference in molecular mass between conjugated and un-conjugated Pfs28 allowed for a complete separation by SEC. In previous studies on Pfs25, an additional step of purification with immobilized metal affinity chromatography was used to capture both Pfs25 and Pfs25-rEPA conjugates and thereby remove the unmodified rEPA [6]. With the optimization of the process reported here, this step was no longer necessary.
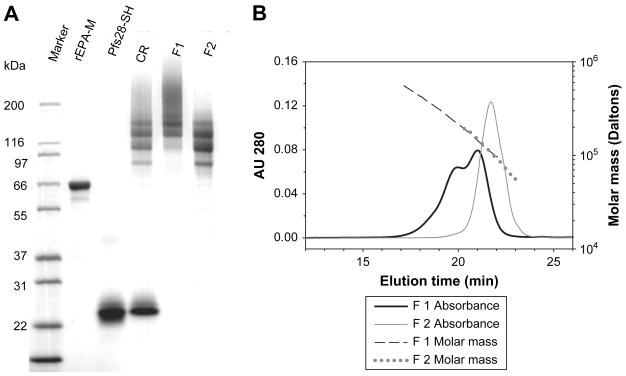
Fig. 1.
SDS-PAGE and SEC-HPLC-MALS analysis of the conjugates. (A) SDS-PAGE of Pfs28-rEPA. Marker: molecular weight markers; rEPA-M: maleimide-rEPA; Pfs28-SH: thiolated Pfs28; CR: unpurified conjugation reaction mixture; F1: Pfs28-rEPA fraction 1; F2: Pfs28-rEPA fraction 2. (B) SEC-HPLC-MALS to determine molecular mass of Pfs28-rEPA fraction 1 and 2 in solution.
3.2. Characterization of Pfs28-rEPA
The Pfs28-rEPA F1 containing high molecular mass conjugates appeared as a smear on the gel, whereas the Pfs28-rEPA F2 mainly contained low molecular mass conjugates with less cross-linkage (Fig. 1A). To measure the molecular mass of the conjugates in solution, SEC-HPLC-MALS was performed on the two fractions of the Pfs28–rEPA (Fig. 1B). The molecular mass range of the Pfs28-rEPA F1 was from ~ 91 kDa to 524 kDa, with weighted average masses of 144 kDa and 279 kDa for the two peaks; the molecular mass of Pfs28-rEPA F2 ranged from ~ 56 kDa to 191 kDa with a single peak of weighted average mass of 111 kDa. Western blot analysis showed that all bands as well as the smear of the Pfs28-rEPA conjugates were recognized by two anti-Pfs28 monoclonal antibodies, one of which (mAb 2D8) was conformation-dependent and had transmission-blocking activity (data not shown).
Conjugation ratios were calculated as 3.5 and 2.3 for the Pfs28-rEPA F1 and Pfs28-rEPA F2, respectively. The values of conjugation ratio presented here were higher than those of the Pfs25-rEPA conjugates obtained in the previous study [6], despite excess thiolated antigens used in the previous study. The major difference between the number of linker groups added on Pfs28 and rEPA led to the increase of the conjugation ratio. The remaining unreacted free thiols or maleimide groups on the purified conjugates were lower than the levels of detection by direct or indirect Ellman’s method.
3.3. Significant higher antibody titers induced by Pfs28-rEPA in mice
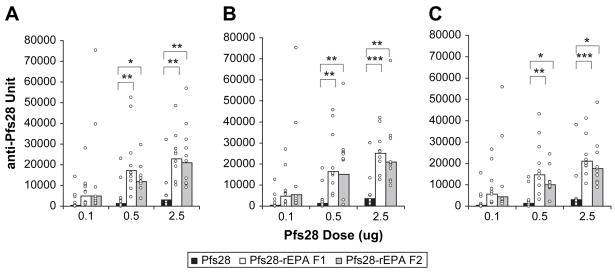
A mouse study was performed to compare the immunogenicity of rEPA-conjugated and unconjugated Pfs28. At the doses of 0.5 and 2.5 μg, both F1 and F2 fractions of the conjugated Pfs28 induced significantly higher antibody titers than the unconjugated Pfs28 on any of three sera collection days (Fig. 2). Increases in antibody titer (total IgG, predominantly IgG1) were 8- to 14-fold and 6- to 7-fold, respectively, at the two doses. The differences between antibody titers induced by F1 and F2 fractions of Pfs28-rEPA were slight and not as apparent as those observed in the previous study of Pfs25-rEPA [6]. The duration of antibody response showed that the antibody titers had already reached the plateau on day 42, two weeks post second immunization, and maintained that level without significant decrease during the next 4 weeks (Fig. 2).
Fig. 2.
Pfs28-specific antibody responses in mice. ELISA was performed to examine the Pfs28 specific antibody titers of the immune mouse sera collected on days 42 (A), 56 (B) and 70 (C), two, four and six weeks after second immunization, respectively. Results are expressed as geometric mean of ELISA units of the group with each animal represented by an open circle. The asterisks indicate the significant difference in antibody titer between two immunization groups tested by Student Newman-Keuls. *: P< 0.05; **: P< 0.01; ***: P< 0.001.
In a previous transmission-blocking assay using the mouse antisera made by high doses of unconjugated Pfs28 formulated with Montanide ISA720, only about 60% oocyst reduction was achieved when the antibody titer of the sera fed to mosquitoes was 5600 units (unpublished data). Based on the experience obtained from Pfs25 studies, at least 4-fold higher anti-Pfs28 antibody titer (23000 units) would be required to achieve about 90% oocyst reduction. However, only 11 out of 20 mice at the dose of 2.5 μg on day 42 and 8 out of 20 mice at the dose of 0.5 μg on day 56 in two Pfs28-rEPA immunization groups had antibody titers above 23000 units. Hence the level of antibody titer induced by conjugated Pfs28, although greatly increased, is still not high enough with regard to the number of responders achieving high titers at the doses set in this study. Further enhancing the immunogenicity of Pfs28 is necessary, which is possible by using combined adjuvants such as Alhydrogel plus CpG ODN [12].
3.4. Conclusion
In summary, Pfs28 was successfully conjugated to rEPA following the improved procedure. The conjugation products had a desired higher conjugation ratio and could be purified by one-step SEC. The immunogenicity of Pfs28 in mice was significantly enhanced by conjugation. The improved procedure reported here can be employed not only for conjugation of Pfs28 but also for conjugation of other malarial antigens as well as protein antigens from other pathogens to the carrier protein rEPA.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 2.Duffy PE, Kaslow DC. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun. 1997;65:1109–1113. doi: 10.1128/iai.65.3.1109-1113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saul A. Mosquito stage, transmission blocking vaccines for malaria. Curr Opin Infect Dis. 2007;20:476–481. doi: 10.1097/QCO.0b013e3282a95e12. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Przysiecki C, Flanagan E, Bello-Irizarry SN, Ionescu R, Muratova O, Dobrescu G, Lambert L, Keister D, Rippeon Y, Long CA, Shi L, Caulfield M, Shaw A, Saul A, Shiver J, Miller LH. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc Natl Acad Sci USA. 2006;103:18243–18248. doi: 10.1073/pnas.0608545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubler-Kielb J, Majadly F, Wu Y, Narum DL, Guo C, Miller LH, Shiloach J, Robbins JB, Schneerson R. Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc Natl Acad Sci USA. 2007;104:293–298. doi: 10.1073/pnas.0609885104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian F, Wu Y, Muratova O, Zhou H, Dobrescu G, Duggan P, Lynn L, Song G, Zhang Y, Reiter K, MacDonald N, Narum DL, Long CA, Miller LH, Saul A, Mullen GE. Conjugating recombinant proteins to Pseudomonas aeruginosa ExoProtein A: a strategy for enhancing immunogenicity of malaria vaccine candidates. Vaccine. 2007;25:3923–3933. doi: 10.1016/j.vaccine.2007.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimp RL, Jr, Martin LB, Zhang Y, Henderson BS, Duggan P, MacDonald NJ, Lebowitz J, Saul A, Narum DL. Production and characterization of clinical grade Escherichia coli derived Plasmodium falciparum 42 kDa merozoite surface protein 1 (MSP1(42)) in the absence of an affinity tag. Protein Expr Purif. 2006;50:58–67. doi: 10.1016/j.pep.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Shuler KR, Dunham RG, Kanda P. A simplified method for determination of peptide-protein molar ratios using amino acid analysis. J Immunol Methods. 1992;156:137–149. doi: 10.1016/0022-1759(92)90020-t. [DOI] [PubMed] [Google Scholar]
- 9.Aebig JA, Mullen GE, Dobrescu G, Rausch K, Lambert L, Ajose-Popoola O, Long CA, Saul A, Miles AP. Formulation of vaccines containing CpG oligonucleotides and alum. J Immunol Methods. 2007;323:139–146. doi: 10.1016/j.jim.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullen GE, Giersing BK, Ajose-Popoola O, Davis HL, Kothe C, Zhou H, Aebig J, Dobrescu G, Saul A, Long CA. Enhancement of functional antibody responses to AMA1-C1/Alhydrogel, a Plasmodium falciparum malaria vaccine, with CpG oligodeoxynucleotide. Vaccine. 2006;24:2497–2505. doi: 10.1016/j.vaccine.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26:193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian F, Rausch KM, Muratova O, Zhou H, Song G, Diouf A, Lambert L, Narum DL, Wu Y, Saul A, Miller LH, Long CA, Mullen GE. Addition of CpG ODN to recombinant Pseudomonas aeruginosa ExoProtein A conjugates of AMA1 and Pfs25 greatly increases the number of responders. Vaccine. 2008;26:2521–2527. doi: 10.1016/j.vaccine.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]