Abstract
Aberrant crypt foci (ACF), the earliest identified monoclonal lesions in the colon, provide insights into changes that promote and/or accompany the transformation of normal colonic epithelial cells to colorectal cancer. Fatty acid synthase (FAS), the primary enzyme involved in de novo lipogenesis from carbohydrates, is expressed at low levels in most normal human tissues but is elevated in several human neoplasms including colorectal adenomas and carcinomas. To mdetermine if this pathway is altered even earlier in colorectal tumorigenesis, 35 human ACF from 21 patients were evaluated for the immunohistochemical expression of FAS. Sections of colon cancer served as positive controls, and normal colonic mucosa distant from cancer or ACF served as negative controls. FAS expression was increased in 30 (86%) ACF compared with that in adjacent normal colonic mucosa. The expression of FAS in ACF was not related to the degree of dysplasia or to the number of crypts in the ACF. The over expression of FAS in a high proportion of ACF suggests that this enzyme plays an important role very early in colorectal tumorigenesis and may be a target for chemoprevention.
Keywords: human aberrant crypt foci, colon cancer precursors, biomarkers, chemoprevention, colorectal cancer
Fatty acid synthase (FAS) is the only mammalian enzyme capable of de novo long-chain fatty acid synthesis from smaller carbohydrate substrates.1 In adults, it is expressed primarily in hormone-sensitive tissues and those with high lipid metabolism.2 The expression and activity of FAS in normal tissues is highly regulated by diet, hormones and growth factors: carbohydrates, insulin, and transforming growth factor-beta all upregulate the expression and activity of FAS (reviewed in ref 3). In humans on a normal diet in industrialized countries, the expression and activity of FAS is low, even in lipogenic organs like liver and adipose tissue, due to its suppression by small amounts of dietary fatty acids.3,4 Consequently, FAS plays only a minor role in human lipogenesis.4 In contrast, FAS is highly expressed by many human cancers including prostate, breast, ovarian, endometrial, thyroid, colorectal (reviewed in ref 5); bladder,6 lung,7 oral mucosa,8 tongue,9 esophageal,10 and stomach11 carcinomas as well as melanoma,12 retinoblastoma,13 and nephroblastoma.14 Increased expression of FAS has also been reported in some benign or preinvasive lesions of the prostate, colon, breast (reviewed in refs 5,15); lung,7 cutaneous nevi,12 and stomach.11 We evaluated the expression of FAS in aberrant crypt foci (ACF), the earliest microscopically identified monoclonal lesions in the colon.16 Increased expression of FAS was seen in 30 of 35 (86%) of ACF from 21 patients with sporadic colorectal cancer or familial adenomatous polyposis (FAP).
Materials and methods
Paraffin-embedded sections of ACF with adjacent normal colonic mucosa or samples of human colorectal cancers were stored at 4°C for several months prior to immunohistochemical analysis as detailed previously.17 These studies on discarded tissues were performed after approval by our Institutional Review Board for Human Subjects and in accord with an assurance filed with and approved by the U.S. Department of Health and Human Services. Sections were heated in a pressure cooker with 0.1 M Tris buffer, pH 8.6 for 5 minutes at full pressure and incubated with affinity purified rabbit anti-human FAS antibody (Assay Designs, Inc, Ann Arbor, MI) diluted 1:100 and 1:200 in 0.1 M Tris buffered saline. This antibody, raised against a conjugate of a synthetic peptide from human FAS, was developed and characterized by Kusakabe et al.2 Colorectal tumor sections provided positive controls for FAS expression; rabbit normal IgG (Dako, Carpinteria, CA) at a similar protein concentration provided negative controls. All 35 ACF were stained twice; their expression of FAS was scored as positive if it appeared increased relative to the surrounding normal crypts by two investigators and negative if the ACF did not stain or stained similarly to the surrounding normal crypts.
Of the ACF evaluated in this study, 33 were from 20 patients with sporadic colorectal cancer; two were from one patient with FAP without colon cancer. The sporadic colorectal cancer patients were 66 ± 10 (range: 42–81) years of age; 8 female, 12 males; 2 black, 16 Caucasian, and 2 unidentified race. The FAP patient was a 31-year-old, black female. One ACF was from the proximal colon, 34 ACF from the distal colon; 11 had mild dysplasia, 24 were without dysplasia; and the ACF had a mean of 64 ± 48 (range: 13–200) crypts per focus.
Results
FAS showed immunohistochemically demonstrable increased expression in 30 of 35 (86%) of ACF compared with the very low or absent expression of FAS in the adjacent normal mucosa (Figure 1). As seen in this figure, some ACF (Figure 1C,D) from sporadic colorectal cancer patients had uniformly high expression of FAS throughout the crypts of the ACF while other ACF (Figure 1E,F) showed a gradient of expression from the bottom of the crypt, where it is highest, to the top of the crypt. This type of gradient of expression was seen in 20 of 30 ACF with increased expression of FAS. The two ACF from an FAP patient also showed a gradient of expression, but here the highest expression was at the top of the crypts. Since only a single FAP patient was evaluated, we do not know if this is characteristic of the FAP phenotype.
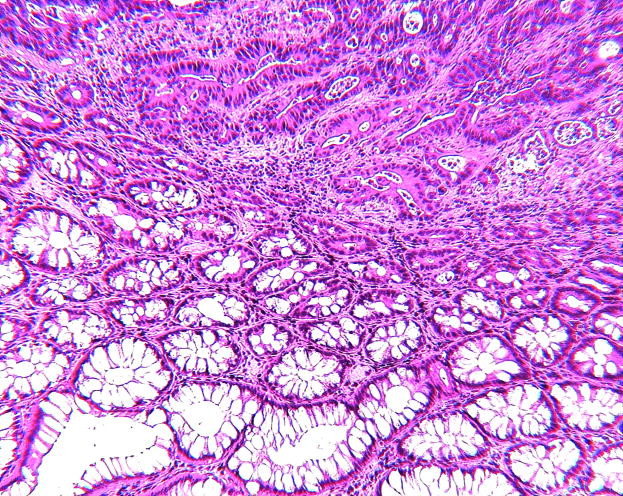
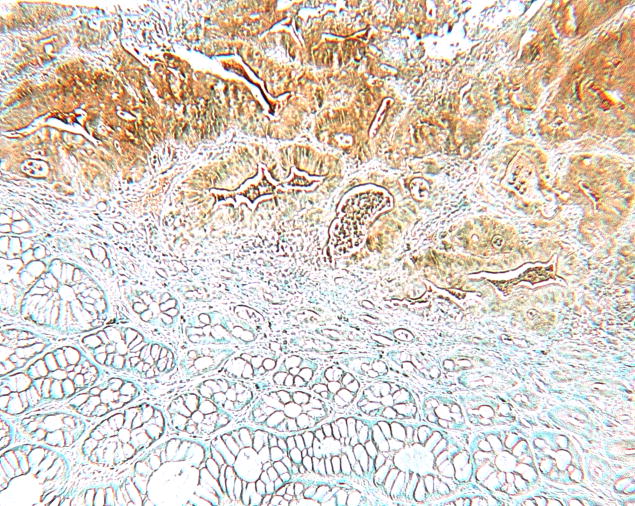
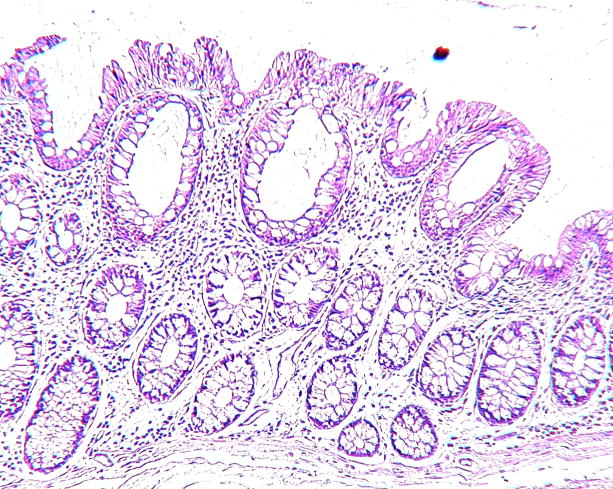
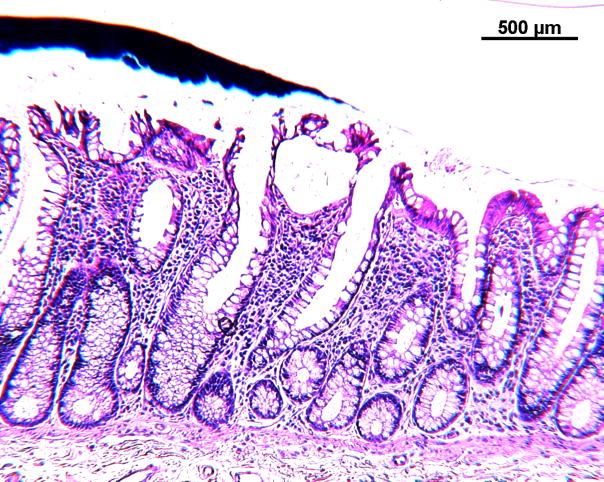
Figure 1.
Photomicrographs of a sigmoid colon cancer (Figures 1A and B) from an 89-year-old female and two aberrant crypt foci (ACF) without dysplasia from two different patients with sporadic colorectal cancer in the distal colon all taken at the same magnification. The top row (Figures 1A, C, E) after staining with hematoxylin and eosin; the bottom row (Figures 1B, D, F), nearby sections from the same tissues as above, after antibody to human FAS with diaminobenzidine as substrate. Note the strong, though somewhat heterogeneous, expression of FAS throughout the cancer (top portion of Figure 1B) compared to the normal glands (bottom portion of Figure 1B). Figures 1C and D show a portion of an ACF with 180 crypts, Figures 1E and F, an ACF with 63 crypts. The immunohistochemical expression of FAS in Figures 1D and F clearly delineates the ACF (left portion of the Figures) from the normal adjacent glands (right portion of the Figures). Some ink, used to mark ACF in the intact tissue, is visible above the ACF in Figures 1E and F.
In our study, increased expression of FAS was seen in ACF with 13–200 crypts per focus and in 21 of 30 (70%) ACF without dysplasia, i.e., the expression of FAS was independent of the number of crypts in the ACF and whether or not the ACF was dysplastic. With a very large proportion of ACF showing increased expression of FAS, it would require a very large sample size to detect any variation with these parameters. Overexpression of FAS in 86% of ACF is second only to the overexpression of carcinoembryonic antigen (CEA) in 93% of human ACF (see ref 18 for review of altered phenotypes in human ACF).
Discussion
Various mechanisms have been proposed to explain the increase of FAS in a wide range of malignancies and their putative precursors. The requirements of increased proliferation seen in cancer cells and their precursors may lead to up regulation of FAS to satisfy the demands for fatty acids as a source of energy and of phospholipids for membranes.2,3 In addition, the high levels of circulating insulin, associated with increased colon cancer risk,19 may contribute to the stimulation of the expression and activity of FAS in the colon. Since increased levels of insulin are not associated with many other tumors and precancerous lesions, this is unlikely to be the major factor affecting FAS expression in tumors and their precursors. The microenvironments of most tumors include areas of hypoxia and low pH. Another interesting hypothesis (discussed in ref 3) is that the induction of FAS may be a means of producing oxidized intermediates, under limited oxygen conditions, through its utilization of NADPH. For every palmitate generated, the predominant product of FAS, 14 equivalents of NADPH are consumed (see ref 5). The ability of cancer cells to supply oxidizing equivalents under hypoxic conditions may provide a major growth advantage to these cells.
Increased FAS expression in cancer is often associated with poor prognosis (reviewed in refs 5,13,14,20,21). While increased FAS expression was reported in 81%20 to 100%22 of colorectal cancers, neither study found FAS expression to be an independent prognostic indicator of survival. Both studies20,22 found increased expression of FAS in colonic polyps; Visca et al20 found increased FAS expression in 18% of 100 adenomas while Rashid et al22 found increased FAS expression in all 36 adenomas evaluated as well as microadenomas in FAP patients and 3 of 3 hyperplastic polyps. Our studies extend these observations to microscopically identified ACF, the earliest monoclonal lesions identified in the colorectum.
FAS has been proposed as a drug target for cancer due to its limited distribution in humans. Cerulenin, a natural antibiotic and an inhibitor of FAS, inhibited the growth of a wide variety of cancer cell lines, but did not inhibit the growth of normal human fibroblasts in vitro (reviewed in ref 5). In a series of human breast cancer cell lines, demonstration that the cytotoxic effect of cerulenin was proportional to the level of FAS activity provided further evidence that neoplastic growth was dependent on FAS (reviewed in ref 5). Cerulenin and C75, a small molecule inhibitor of FAS, are also selectively cytotoxic for human cancer xenografts in vivo in rodents, but both cause significant weight loss as well (reviewed in refs 1,5). A more recent synthetic FAS inhibitor, C93, is cytotoxic to cancer cell xenografts without causing significant weight loss or cytotoxicity to proliferating cells.23 While there appears to be little toxicity with the inhibition of FAS in normal cells, its inhibition in human cancer cells both in vitro and in vivo induces apoptosis (reviewed in ref 1).
With the identification of increased FAS expression in early neoplastic lesions, including ACF, FAS inhibitors are also being considered for chemoprevention (reviewed in ref 1). The FAS inhibitor, trisclosan, reduced the incidence of mammary tumors in rats initiated with a single dose of methylnitrosourea.24 More recently, soy protein reduced the colonic expression of FAS and the circulating level of insulin one day after rats were initiated with azoxymethane. This biochemical pathway likely explains the previously demonstrated inhibition of colon cancer by soy protein in this animal model.25
In summary, the increased expression of FAS in 86% human ACF suggests its importance from very early in the development of colonic neoplasms. The selective toxicity of inhibitors of FAS makes this pathway a good therapeutic target for both chemopreventive and chemotherapeutic drugs in the colorectum.
Acknowledgments
We thank Mr Adam Kresak for the photomicrographs, Ms Nancy Edgehouse and the personnel of the Histology Core Facility for assistance with histology, the faculty and staff of the Tissue Procurement Core Facility for all the tissues used in this study. Both Core Facilities are part of the Comprehensive Cancer Center of Case Western Reserve University and University Hospitals of Cleveland. This work was supported in part by National Cancer Institute grants CA66725 (to TPP) and P30 CA43703 to the Comprehensive Cancer Center of Case Western Reserve University.
References
- 1.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–80. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 2.Kusakabe T, Maeda M, Hoshi N, Sugino T, Watanabe K, Fukuda T, Suzuki T. Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J Histochem Cytochem. 2000;48:613–22. doi: 10.1177/002215540004800505. [DOI] [PubMed] [Google Scholar]
- 3.Menendez JA, Lupu R. Oncogenic properties of the endogenous fatty acid metabolism: molecular pathology of fatty acid synthase in cancer cells. Curr Opin Clin Nutr Metab Care. 2006;9:346–57. doi: 10.1097/01.mco.0000232893.21050.15. [DOI] [PubMed] [Google Scholar]
- 4.Weiss L, Hoffmann GE, Schreiber R, Andres H, Fuchs E, Korber E, Kolb HJ. Fatty-acid biosynthesis in man, a pathway of minor importance. Purification, optimal assay conditions, and organ distribution of fatty-acid synthase. Biol Chem Hoppe Seyler. 1986;367:905–12. doi: 10.1515/bchm3.1986.367.2.905. [DOI] [PubMed] [Google Scholar]
- 5.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–8. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 6.Visca P, Sebastiani V, Pizer ES, Botti C, De Carli P, Filippi S, Monaco S, Alo PL. Immunohistochemical expression and prognostic significance of FAS and GLUT1 in bladder carcinoma. Anticancer Res. 2003;23:335–9. [PubMed] [Google Scholar]
- 7.Piyathilake CJ, Frost AR, Manne U, Bell WC, Weiss H, Heimburger DC, Grizzle WE. The expression of fatty acid synthase (FASE) is an early event in the development and progression of squamous cell carcinoma of the lung. Hum Pathol. 2000;31:1068–73. doi: 10.1053/hupa.2000.9842. [DOI] [PubMed] [Google Scholar]
- 8.Guo CB, Cui NH, Yu GY, Liu DX, Meng SC, Song Q. Effects of cerulenin on the endogenous fatty acid synthetic activity in squamous cell carcinoma of the oral cavity. J Oral Maxillofac Surg. 2003;61:909–12. doi: 10.1016/s0278-2391(03)00237-4. [DOI] [PubMed] [Google Scholar]
- 9.Krontiras H, Roye GD, Beenken SE, Myers RB, Mayo MS, Peters GE, Grizzle WE. Fatty acid synthase expression is increased in neoplastic lesions of the oral tongue. Head Neck. 1999;21:325–9. doi: 10.1002/(sici)1097-0347(199907)21:4<325::aid-hed6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Nemoto T, Terashima S, Kogure M, Hoshino Y, Kusakabe T, Suzuki T, Gotoh M. Overexpression of fatty acid synthase in oesophageal squamous cell dysplasia and carcinoma. Pathobiology. 2001;69:297–303. doi: 10.1159/000064636. [DOI] [PubMed] [Google Scholar]
- 11.Kusakabe T, Nashimoto A, Honma K, Suzuki T. Fatty acid synthase is highly expressed in carcinoma, adenoma and in regenerative epithelium and intestinal metaplasia of the stomach. Histopathology. 2002;40:71–9. doi: 10.1046/j.1365-2559.2002.01289.x. [DOI] [PubMed] [Google Scholar]
- 12.Innocenzi D, Alo PL, Balzani A, Sebastiani V, Silipo V, La Torre G, Ricciardi G, Bosman C, Calvieri S. Fatty acid synthase expression in melanoma. J Cutan Pathol. 2003;30:23–8. doi: 10.1034/j.1600-0560.2003.300104.x. [DOI] [PubMed] [Google Scholar]
- 13.Camassei FD, Cozza R, Acquaviva A, Jenkner A, Rava L, Gareri R, Donfrancesco A, Bosman C, Vadala P, Hadjistilianou T, Boldrini R. Expression of the lipogenic enzyme fatty acid synthase (FAS) in retinoblastoma and its correlation with tumor aggressiveness. Invest Ophthalmol Vis Sci. 2003;44:2399–403. doi: 10.1167/iovs.02-0934. [DOI] [PubMed] [Google Scholar]
- 14.Camassei FD, Jenkner A, Rava L, Bosman C, Francalanci P, Donfrancesco A, Alo PL, Boldrini R. Expression of the lipogenic enzyme fatty acid synthase (FAS) as a predictor of poor outcome in nephroblastoma: an interinstitutional study. Med Pediatr Oncol. 2003;40:302–8. doi: 10.1002/mpo.10274. [DOI] [PubMed] [Google Scholar]
- 15.Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res. 1997;3:2115–20. [PubMed] [Google Scholar]
- 16.Siu I-M, Robinson DR, Schwartz S, Kung H-J, Pretlow TG, Petersen RB, Pretlow TP. The identification of monoclonality in human aberrant crypt foci. Cancer Res. 1999;59:63–6. [PubMed] [Google Scholar]
- 17.Hao XP, Pretlow TG, Rao JS, Pretlow TP. b-catenin expression is altered in human colonic aberrant crypt foci. Cancer Res. 2001;61:8085–8. [PubMed] [Google Scholar]
- 18.Pretlow TP, Pretlow TG. Mutant KRAS in aberrant crypt foci (ACF): initiation of colorectal cancer? Biochim Biophys Acta. 2005;1756:83–96. doi: 10.1016/j.bbcan.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: A review of the evidence. J Nutr. 2001;131:3109S–20S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 20.Visca P, Alo PL, Del Nonno F, Botti C, Trombetta G, Marandino F, Filippi S, Di Tondo U, Donnorso RP. Immunohistochemical expression of fatty acid synthase, apoptotic-regulating genes, proliferating factors, and ras protein product in colorectal adenomas, carcinomas, and adjacent nonneoplastic mucosa. Clin Cancer Res. 1999;5:4111–8. [PubMed] [Google Scholar]
- 21.Visca P, Sebastiani V, Botti C, Diodoro MG, Lasagni RP, Romagnoli F, Brenna A, De Joannon BC, Donnorso RP, Lombardi G, Alo PL. Fatty acid synthase (FAS) is a marker of increased risk of recurrence in lung carcinoma. Anticancer Res. 2004;24:4169–73. [PubMed] [Google Scholar]
- 22.Rashid A, Pizer ES, Moga M, Milgraum LZ, Zahurak M, Pasternack GR, Kuhajda FP, Hamilton SR. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. American Journal of Pathology. 1997;150:201–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, Kim EK, Vadlamudi A, Medghalchi SM, El Meskini R, Ronnett GV, Townsend CA, et al. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. 2007;67:2964–71. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- 24.Lu S, Archer MC. Fatty acid synthase is a potential molecular target for the chemoprevention of breast cancer. Carcinogenesis. 2005;26:153–7. doi: 10.1093/carcin/bgh278. [DOI] [PubMed] [Google Scholar]
- 25.Xiao R, Su Y, Simmen RC, Simmen FA. Dietary soy protein inhibits DNA damage and cell survival of colon epithelial cells through attenuated expression of fatty acid synthase. Am J Physiol Gastrointest Liver Physiol. 2008;294:G868–76. doi: 10.1152/ajpgi.00515.2007. [DOI] [PubMed] [Google Scholar]