Abstract
Silica thin films and nanoparticles prepared using sol–gel chemistry are derivatized with active molecules to generate new functional materials. The mild conditions associated with sol–gel processing allow for the incorporation of a range of dopants including organic or inorganic dyes, biomolecules, surfactants, and molecular machines. Silica nanoparticles embedded with inorganic nanocrystals, and films containing living cells have also been synthesized. Silica templated with surfactants to create mesostructure contains physically and chemically different regions that can be selectively derivatized using defined techniques to create dynamic materials. Using two different techniques, donor–acceptor pairs can be doped into separated regions simultaneously and photo-induced electron transfer between the molecules can be measured. Mesoporous silica materials are also useful supports for molecular machines. Machines including snap-tops and nanoimpellers that are designed to control the release of guest molecules trapped within the pores are described. Mesoporous silica nanoparticles are promising materials for drug delivery and other biomedical applications because they are nontoxic and can be taken up by living cells. Through appropriate design and synthesis, multifunctional mesoporous silica nanoparticles for sophisticated bio-applications are created.
I. Introduction
Silica glass has been utilized for centuries because of its inertness and transparency. It is difficult to expand its range of functionality because the high temperatures involved in its production prohibit incorporation of many types of additives that would impart additional new properties. This limitation changed since the invention of sol–gel method.1,2 Owing to the mild conditions of this process it is possible to introduce various organic, inorganic, and bio-molecules into the silica material. In this article we review new properties and functionalities induced by the incorporation of molecular dopants into silica matrices. First, the use of single dopants to give silica new optical, chemical, and structural properties is discussed. Second, systems based on multiple dopants, where interaction between these molecules give rise to new set of properties that are not possessed by any of the components alone, are presented. A novel type of nanomachine is described. Finally, applications of these types of systems for biological and medical purposes, especially drug delivery, are illustrated.
II. Properties Induced by Individual Components
New optical properties can be induced in sol–gel silica glass by incorporation of appropriate molecules such as organic dyes. Dye molecules can also function as probes of the sol–gel processing. Biomolecules can be stabilized in glass and can provide multiple functions including action as biosensors. Surfactant molecules can be used to template desired mesostructures into the glass. Examples of these molecules and the induced functions are described in this section.
(1) Organic or Inorganic Molecules
One way of creating a new generation of optical materials is based on doping-specific organic and organometallic molecules in sol–gel-derived matrices.3 The sol–gel process is sufficiently benign that the molecules encapsulated in the inorganic glass matrix effectively retain their characteristic solution properties. Using the sol–gel doping approach, a wide variety of materials with designed optical properties were produced. Initial studies involved the use of spectroscopic probes to characterize the chemical and physical changes that occur during the various stages of the sol–gel process.4–8 Some of the more significant properties demonstrated by dye-doped sol–gel materials are photochromism,9 laser action,10 surface-enhanced Raman emission, 11 sensing,9,12,13 and a variety of nonlinear optical properties. 14 In addition, dye-doped silica particles are gaining a wide use as tags in biological systems.15,16 As recent review papers indicate,14,17 this field continues to generate considerable interest, with particular emphasis on the technological development of optical communication devices that use sol–gel-derived inorganic/organic hybrid materials.
(2) Biomolecules
From the research on encapsulating organic and organometallic molecules it was abundantly clear that the ability to dope sol–gel glasses was not limited to just a few systems. As long as the dopant was soluble in the aqueous or alcohol-based solvent, it could be incorporated within the sol–gel matrix and, in most cases, effectively impart its properties to the resulting solid. It was recognized that an analogous approach would be feasible for biomolecular dopants. Today, the field of sol–gel immobilization of biomolecules is a very active one.18 The field has grown dramatically as there is now the ability to routinely use sol–gel methods to encapsulate biomolecules ranging in size from small proteins of a few nanometers, to whole cells of several micrometers. The field has diversified, from soluble proteins to membrane-bound proteins, from sensor applications to new directions such as high-throughput drug screening and energy storage.
The earliest examples of biomolecule encapsulation in sol–gel matrices addressed the issue whether proteins retained their characteristic reactivity and function when encapsulated in the sol–gel-derived SiO2 matrix.19–22 These studies demonstrated that it was possible to encapsulate proteins in sol–gel matrices without altering protein structure. It should be noted, however, that the sol–gel encapsulation process does not always trap the protein in its native conformation, as there are instances where intermediate conformations are trapped.23 Additionally, as long as synthesis conditions that avoided protein denaturation were used, immobilized enzymes retained their catalytic ability, metalloproteins exhibited their metal exchange properties, heme proteins displayed redox behavior and ligand binding and antibodies retained their binding affinity.9,24
An important contribution in these early studies was the development of relatively simple synthetic procedures that successfully avoid protein denaturation. A two-step approach was devised in which tetramethoxysilicate was prehydrolyzed, generally without the addition of alcohol, followed by the addition of buffer to bring the pH to a range where the protein was stable. 20,25 A benefit of this approach was the ability to produce transparent monoliths, usually formed in spectroscopy cuvettes, to facilitate in situ monitoring of the spectroscopic properties of the protein. As the biomolecule immobilization field has continued to grow, there is interest in developing matrices for biosystems (e.g., phospholipids and whole cells) that cannot tolerate alcohols. As a result, a number of approaches have emerged in recent years that greatly lower the alcohol content and generally provide improvements in microstructure control. These include aqueous synthesis routes based on sodium silicate, 26 and the use of biocompatible silane precursors containing bound sugar moieties.27
Other important discoveries include enhanced stability of the protein that arises from the encapsulation process. Trapping the protein in the silica cage effectively suppresses protein unfolding and avoids denaturation, yet is able to respond to small analyte molecules because of the inherent porosity of the sol–gel matrix. In addition, the matrix prevents contact with proteases or microorganisms. 19,28–31 Membrane-bound proteins have been encapsulated including the transmembrane peptide ion-channel gramicidin A, a ligand-gated ion channel (nicotinic acetylcholine receptor), and a G-protein coupled receptor (dopamine D2 receptor). 32 The encapsulation of both bacteriorhodopin and F0F1-ATP synthase successfully demonstrated the use of a photo-induced proton gradient for the biosynthesis of ATP.33 This work underscores the prospect of using membrane-associated proteins to design multifunctional biocomposite materials that enable biological modes of power generation and energy storage.
Biosensors have proven to be an extremely successful direction for sol–gel-encapsulated biomolecules.34 Despite being trapped in the matrix, biological molecules retain the catalytic, recognition, and transduction functions that make the resulting sol–gel materials ideal for the design of sensors over a broad range of areas including medical and health care, environmental monitoring, industrial processing, food quality, toxic chemicals, and explosives. A great variety of enzymatic and antibody-based systems have been explored.20,24,34–37 The sol–gel approach has an advantage of being easily adapted to optical and electro-chemical detection methods. Finally, it is important to mention that sol–gel-based biosensors have moved beyond the research stage and commercialization efforts are well underway.
(3) Surfactants to Template Mesostructure
Since its discovery in 1992, mesostructured silicate particles have been a subject of intensive research due to the large surface areas of the materials and the simplicity in modifying both the particle and the pore size.38,39 Various organic templates (e.g., cationic surfactants and triblock copolymers) and pore swelling agents have successfully been used in the particle synthesis to give a wide range of well-defined pore sizes and mesostructures.40,41 Furthermore, the versatility of sol–gel chemistry to introduce organic functionalities on the pore walls and surfaces makes the mesoporous silica suitable as host materials to incorporate guest molecules.42 Although the original research and applications of these host–guest materials were mainly used for catalysis purposes, recent progress in synthesizing mesoporous particles of nano-sized dimensions has developed new potential in the biomedical field.43–45
In addition to powders, mesostructured silica can be prepared as films. Typically the films are prepared using a method known as Evaporation-Induced Self-Assembly.46 This process utilizes a sol containing all of the components needed for the formation of final film: silica precursor (tetraethoxysilane or tetramethoxysilane), a catalyst (hydrochloric acid), templating agent (Cetyltrimethylammonium bromide [CTAB], Pluronic, etc.), and solvents (water, ethanol). The sol is deposited as a thin liquid layer onto a suitable substrate by dip-coating or spin-coating. The evaporation of the solvent drives the formation of surfactant micelles, which further assemble into a liquid crystal. At the same time the silica condenses around the micelles. By choosing a specific composition of the sol, environmental conditions, and the method of deposition mesostructured films with hexagonal, lamellar, and cubic structures possessing high degree of long-range order can be produced. The thickness of the final film can vary between 50 and 500 nm. The surfactant molecules can be removed from the pores of the film by calcination or solvent extraction.
III. Placement of Multiple Components to Induce Functionality
The use of surfactant molecules during sol–gel synthesis has led to new techniques for the incorporation of active molecules to induce functionality in silicate materials. Surfactants can be used to template mesostructure within the materials. Different regions exist within the surfactant-templated mesostructure, enabling the simultaneous incorporation of different types of molecules. Pairs of molecules that are spatially separated from one another can be simultaneously doped into the mesostructured materials, and the dynamic interplay between the molecular pairs can be studied. Mesoporous structures can be achieved when the surfactant is removed following sol–gel synthesis, and molecular machines that take advantage of the porosity can be attached to the silica surface. One additional use of surfactants is the solubilization of inorganic nanocrystals so that they can be embedded into the core of silica nanoparticles, as will be discussed in the upcoming section.
(1) Placement of Objects
By mixing the surfactant template with inorganic nanocrystals, the versatility of mesostructured silica was extended by embedding additional functional materials at the core of the particles. Because the process of synthesizing mesoporous silica nanoparticles is done in aqueous solution, the hydrophobic nanocrystals need to be transferred into the water phase by coating them with the amphiphilic CTAB molecules.47–50 The hydrophobic interaction between CTAB surfactants and aliphatic ligands on the surface of the nanocrystals renders the materials water soluble. Mesoporous silica spheres were formed around the nanocrystals by mixing the silica source tetraethylorthosilicate (TEOS) with the aqueous solution containing CTAB-coated nanocrystals, CTAB, and base catalyst.50 The electrostatic interaction between the hydrolyzed TEOS molecules, the CTAB-coated nanocrystals, and the free surfactant micelles helped promote the base-catalyzed condensation of TEOS to form the mesostructure. By using this general procedure, iron oxide,51 gold,52 and silver53 nanocrystals were embedded at the center of the mesoporous silica.
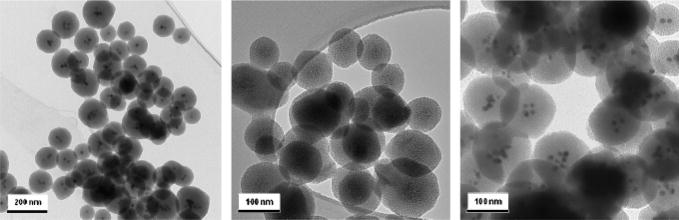
As an example of the aforementioned procedure, superparamagnetic iron oxide nanocrystals were incorporated within the mesoporous silica nanoparticles to provide magnetic resonance (MR) imaging and magnetic manipulation capabilities.51,54–58 Because the morphology of the iron oxide–mesoporous silica nanoparticles is dependent on the synthetic condition and temperature of the solution, it was necessary to form the spherical shape at higher temperature, with vigorous stirring and dilute precursor solution. The transmission electron microscope images show the dark inorganic nanocrystals at the center of the nanoparticles and also the mesostructured pores (Fig. 1). The biological applications of these nanoparticles will be described in Section IV.
Fig. 1.
Transmission electron microscope images of iron oxide (left), silver (middle), and gold (right) nanocrystals embedded within the mesoporous silica nanoparticles.50
Alternatively, metals can be deposited inside or on the surface of the silica particles. Porous silica material with palladium clusters embedded inside is an example of a potential material for hydrogen storage.59 Nanoparticles with thin metal shells (typically gold) around silica core can absorb or scatter light over a wide spectral range.60,61 Such particles have applications in imaging and anticancer therapy.
Living organisms, in addition to inanimate objects, have also been incorporated into sol–gel silica monoliths and films. The earliest studies involved encapsulation of whole yeast cells.62–66 Synthesizing mesostructured silica containing living cells is a more difficult challenge because the common surfactants that are used as templating agents kill the cells. The first example of mesostructured silica and living cells used diacylphosphatidylcholine as the structure-directing agent and either yeast or bacterial cells.67 The cell surfaces organized multilayered phospholipid vesicles that interfaced coherently with the silica host. The resulting structures maintained cell accessibility, addressability, and viability in the absence of buffer or external fluids. These new materials open the possibility of using the cell’s molecular recognition, amplification, and signal transduction properties for miniaturized, stand-alone environmental, or physiological sensors. With the ability of cells to be used in a variety of additional areas including biosynthesis and cell and tissue growth, the cell encapsulation area will be an active one in the coming years.
Optical functionality such as energy transfer68 and electron transfer69 can be achieved in mesostructured silicates by simultaneously incorporating different types of photo-active molecules into the material. The different types of molecules can be spatially separated from one another within the material as a result of the fact that mesostructured thin films and particles contain three chemically and physically distinct regions that can be selectively derivatized.70–73 These regions include the silicate framework, the organic region, and ionic interface region (Table I). The framework, organic region, and ionic interface region can each be derivatized using different one-pot approaches where a molecule of interest is included in the initial sol and is designed to assemble into a region during material synthesis. A fourth region of the material, the exterior surface, can be derivatized postsynthetically either before or after removal of templating agents.
Table I.
The Different Structural Regions of Mesostructured Silicates and Techniques for Derivatization
| Structural region | Method of derivatization | Description of method | Examples of utility |
|---|---|---|---|
| Framework | Bonding | Active molecules themselves form a part of the silica framework. They undergo hydrolysis and condensation during synthesis to become incorporated into the final framework. | Placement of photoactive and electroactive metal complexes for energy and electron transfer. |
 |
|||
| Organic | Philicity | The local solubility of the active molecules determines their placement. Hydrophobic molecules assemble into the micelle interior during synthesis | Placement of pyrene to probe micelle formation; placement of acceptor molecules for energy and electron transfer. |
 |
|||
| Ionic interface | Bifunctional | An active molecule is chemically bonded to the outside of the framework. The active molecule usually contains one end that is a trialkoxysilane capable of bonding to the framework, and the other end that is hydrophobic. | Placement of azobenzene-based nanoimpellers. |
 |
|||
| Exterior surface | Postsynthetic grafting | The exposed surfaces are derivatized after synthesis by treating the material with a molecule capable of reacting with silica. | Placement of nanovalves and snap-tops. |
 |
Mesoporous silica thin films have been used as substrates to study photo-induced electron transfer between spatially separated donor and acceptor molecules.69 In this study, a methyl viologen derivatized with a hexadecyl tail was chosen as the electron acceptor, and it was deliberately placed in the nanostructure using the philicity strategy (Fig. 2). The electroactive part of the molecule (methyl viologen) was positioned at the ionic interface region as a result of the association of the hexadecyl tail with the micellar core. Using the bonding strategy, a silylated derivative of ruthenium-based photoelectron donor was incorporated into the silica framework. To verify the successful placement of the ruthenium molecule into the silica framework, emission spectra of the complex in mesostructured and amorphous thin films were compared. The observed emission maxima were nearly identical for the structured (λmax = 615 nm) and unstructured (λmax = 614 nm) films, indicating that the molecule was incorporated into the silica region in both cases. To study electron transfer from the Ru donor to the methyl viologen acceptor, luminescence decay traces and luminescence spectra were collected for the electron donor in the presence of varying concentrations of the electron acceptor. Electron transfer was verified by observing a decrease in the luminescence lifetime of the electron donor as the concentration of the acceptor increased. Because the electron donor and acceptor are confined to separate regions, electron transfer occurs through tunneling, and the electron transfer rate decreases exponentially with the donor–acceptor distance.
Fig. 2.
Spatial separation of electron transfer pairs is achieved by doping the molecules into different regions of a mesostructured film. Photo-induced electron transfer from the Ru donor to the methyl viologen acceptor occurs through tunneling.
Luminescence lifetime data for the Ru donor were collected experimentally, and a theoretical model was used to fit the experimental data in order to calculate a value of k0, the contact quenching rate, as well as β, the decay constant for the contact quenching rate. A calculated value of β = 2.5±0.4 Å−1 demonstrated that mesostructured silica is a highly insulating material.
(2) Molecular Machines that Control Mass Transport through Nanopores
Molecules that undergo large amplitude motions in response to a designed stimulus can be used to do useful work when they are attached to the exterior surfaces or pore interiors of mesoporous silicate materials. One primary utility of machines supported on mesoporous silicates is controlled release applications: the machines are designed to block the nanopores in one configuration to trap guest molecules, but unblock the nanopores in another configuration so that the guest molecules can be released on command. Controlled release systems supported on mesoporous silicate materials include those that rely on the photo-driven dimerization of coumarin molecules,74,75 reductive cleavage of nanoparticle caps,76,77 the photoisomerization of azobenzene derivatives,78,79 and also the switching motion of supramolecular machines called nanovalves.80–87 Nanovalves are machines based on [2]rotaxanes or [2]pseudorotaxanes; the functionality of these devices results from the switching motion of a moveable ring component that slides along a tethered stalk in response to light,83 pH,84 redox,85,86 or competitive binding82 activation. A related motif is found in a different class of supramolecular controlled-release machines called snap-tops, which can be designed to respond to different stimuli including enzymes.88
Snap-tops based on [2]rotaxanes can be assembled onto the surface of mesoporous silica nanoparticles to achieve devices that are capable of encapsulating guest molecules within the nanopores and releasing them on command. Snap-top systems contain a triethylene glycol thread encircled by an α-cyclodextrin (CD) macrocycle that is held in place by a cleavable stopper; when intact, the bulky macrocycle traps guest molecules within the pores, but the contents can be released upon cleavage of the stopper and dethreading of the CD. The preparation of snaptops is a step-wise process: beginning with solvent-extracted mesoporous silica nanoparticles, amine linkers are attached and the amine functionalized material is then treated with a triethyleneglycol monotosylated monoazide thread to achieve an azide-terminated surface. The empty nanopores are then loaded with guest molecules by diffusion, and the particles are then incubated with CD, which threads onto the triethylene glycol stalks to block the nanopores. Finally, bulky stoppers are chemically attached through Cu(I)-catalyzed azide–alkyne cycloaddition. Snap-tops have a divergent synthetic design that enables facile preparation of a wide variety of systems with different modes of activation: the azide functionality serves as a handle onto which various stoppering units with different reactivities can be attached. In one study, snap-tops designed to respond to enzymes were prepared on the surface of mesoporous silica nanoparticles, and their ability to release guest molecules upon activation by porcine liver esterase (PLE) was demonstrated.88 The operation of the system was verified using luminescence spectroscopy: rhodamine B (RhB) was loaded into the pores, and the release of the dye into solution was monitored as a function of time. When an adamantyl ester stopper was attached, an increase in RhB luminescence was observed following the addition of PLE, indicating that the guest molecules were successfully released following cleaveage of the stoppers by PLE. Snap-top systems that were stoppered with adamantyl amide units showed no release of RhB upon the PLE, a result consistent with enzyme-specific hydrolysis of the adamantyl ester stoppers (Fig. 3).
Fig. 3.
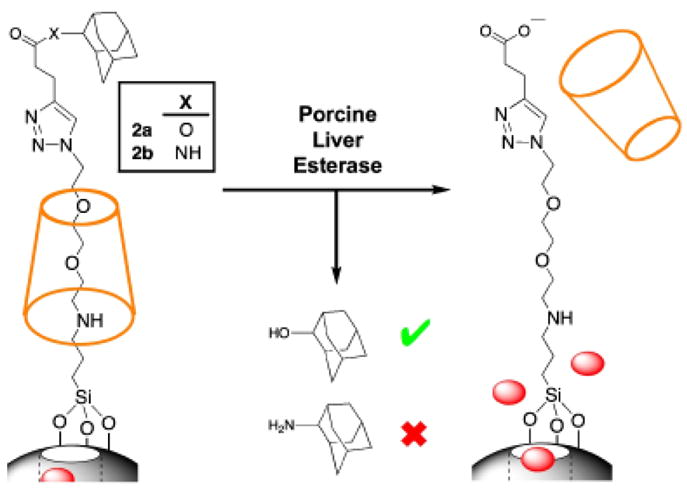
Snap-tops attached to the surface of mesoporous silica nanoparticles are able to store guest molecules within the pores while intact. Guest molecules are released upon selective cleavage of ester-linked adamantyl stoppers by porcine liver esterase (PLE).
A second type of molecular machine called a “nanoimpeller” has been developed for the photocontrolled transport of molecules through and out of the mesopores.79,89 It was made possible by immobilizing an active molecule having photoresponsive behaviors such as azobenzene derivatives to the mesostructured silica framework. Detailed photophysical studies on cis–trans isomerization of azobenzenes in nanostructured silica have been reported.90,91 The bifunctional strategy68,70,72,73 was used to attach a small azobenzene to the interiors of the pores templated by surfactant. This method involved the coupling reaction of the azobenzene with a silane linker, isocyanatopropylethoxysilane followed by cocondensation with the TEOS silica precursor. 92 In the surfactant-removed form, particles contained azobenzenes with one side bonded to the inner pore walls and the other free to undergo reversible isomerization which creates a large amplitude wagging motion capable of functioning as nanoimpellers to release pore contents from the particles (Fig. 4).
Fig. 4.

Azobenzene-derivatized mesostructured silica particles loaded with guest molecules and release of the molecules from the pores by the back-and-forth wagging motion of light-activated azobenzenes.
Photo-driven expulsion of molecules was monitored by luminescence spectroscopy of RhB.89 The fluorescence intensity of the probe released into water was recorded as a function of time. No dye was released from unexcited particles. When the particles were irradiated with 413 nm light, a wavelength where both cis and trans isomers have almost the same extinction coefficient, the dyes were released out of the pores and the increase of fluorescence was recorded. Based on the successful operation of the impeller in water, use of nanoimpeller-based silica particles as an on-demand drug delivery system has been demonstrated in living cells,89 which will be described in the next section.
IV. Bioapplications
Mesoporous silica particles that are <300 nm in diameter are ideal for biomedical applications. Owing to the versatility of incorporating multiple functionalities to the nanoparticles and the biocompatibility of silica, these materials have been successfully used as gene transfection reagents, cell markers, and carriers of molecules.44,93–95 Recent advancements in modifying the nanoparticles show that they can have dual-imaging functionalities, target-specific cells, and release anticancer drugs on command.
(1) Uptake of Particles
Several studies have shown that mesostructured silica nanoparticles are able to undergo cellular uptake without inducing cytotoxicity. 44,45,96 Various factors contribute to the cellular uptake efficacy of the materials, namely the particle size, the dispersibility in aqueous solution, and the surface functional groups. When an aqueous suspension of the nanoparticles was added to the mammalian cells, the uptake of the nanoparticles occurred in a relatively short time. By using fluorescence microscopy, the mesoporous silica nanoparticles could be observed inside the cells within 30 min of incubation. The nanoparticles were mainly located in the acidic organelles such as the lysosomes, which has been observed for other types of nanoparticles as well.50
(2) Imaging, Targeting, and Drug Delivery
Fluorescent silica nanoparticles have a great potential application for imaging, sensing, targeting, and detection of molecules and single sells. The silica enhances the fluorescence and lifetime of the dye molecules encapsulated inside and at the same time provides a surface that can be easily functionalized with a variety of biomolecules.97–99
Magnetic core nanoparticles can be used for separation and purification of biomolecules, such as DNA, RNA, and proteins. 98 This can be done by bioconjugation of recognition elements onto the silica surface of the nanoparticles. The target molecules will interact with the nanoparticles, which can be preferentially localized by exposure to a magnetic field.
The multifunctional iron oxide–mesoporous silica nanoparticles are designed for cancer cell-specific delivery of hydrophobic anticancer drugs and have dual fluorescence and MR imaging capability.50 For the imaging functionalities, organic dye molecules were used for fluorescence imaging purposes and the iron oxide nanocrystals were used for MR imaging applications. Fluorescent dye molecules were functionalized onto the iron oxide–mesoporous silica nanoparticles using a co-condensation method.44,93,100 The MR contrast effect of the iron oxide–mesoporous silica nanoparticles in solution and inside cells was tested using a clinical MR imaging instrument. Superparamagnetic iron oxides are used as contrast agents in MR imaging because of their negative enhancement effect on T2-weighted sequences.54,101–104 As a result, the suspension or the cells containing the iron oxide–mesoporous silica nanoparticles appeared dark in the T2-weighted MR image. The nanoparticles were used to store and deliver water-insoluble anticancer drugs into cells.93 The porous materials were filled with either camptothecin (CPT) or paclitaxel by mixing the nanoparticles in a DMSO solution containing the drugs. The drug-loaded nanoparticles were then collected by centrifugation to remove the supernatant and dried under vacuum before resuspending them in water. It was observed that only small amounts of the stored drug molecules were released in the buffered aqueous solution. However, once the drug-loaded nanoparticles were dispersed in the organic solvents, all of the drugs were released from the mesopores. The nanoparticles alone were not toxic to the cells at the concentrations used in the experiment, but the drug-loaded nanoparticles caused apoptotic cancer cell death, as shown by Caspse3 cleavage.93 Based on these results, the nanoparticles can potentially be used as a vehicle to store and deliver anticancer drugs that are both highly toxic and water-insoluble into different types of cancer cells.
Folic acid was conjugated to the surface of the iron oxide–mesoporous silica nanoparticles in order to introduce the targeting functionality.105 The effect of folic acid modification on the cellular uptake of nanoparticles was demonstrated using the PANC-1 cancer cells and the HFF fibroblasts. Although the use of folic acid as a targeting ligand may have limitations for in vivo experiments, its use in vitro can be demonstrated as a proof of concept to increase the uptake and delivery of anticancer drugs into cells. The attachment of folic acid to the drug-loaded nanoparticles is expected to increase the cellular uptake of nanoparticles and the delivery of drugs to the PANC-1 cells that overexpress α-folate receptor (Fig. 5). Because the nanoparticles can enter both PANC-1 and HFF, the cytotoxicity of the drug-loaded nanoparticles was observed for both cell lines. However, there was a noticeable difference in the cytotoxicity of folate-modified drug-loaded nanoparticles to PANC-1 cells, which correlated with the increased particle uptake. On the other hand, the cytotoxicity between the folate-modified and the unmodified drug-loaded nanoparticles was similar for the HFF because these cells do not overexpress the receptors.
Fig. 5.
Fluorescence microscope images of particle uptake into cells. HFF treated with (a) nanoparticles or (b) folate-modified nanoparticles. PANC-1 treated with (c) nanoparticles or (d) folate-modified nanoparticles. Increased uptake of the folate-modified nanoparticles was observed for the PANC-1 cells (overexpressed folate receptors), but not on the HFF. Red fluorescence: membrane stained with WGA-Alexa Fluor 594; blue fluorescence: nuclei stained with DAPI; green fluorescence: nanoparticles. The fluorescence of DAPI is increased by ~20-fold when it binds to the nucleic acids in the nucleus. WGA-Alexa Fluor 594 binds to the glycoproteins in the cell membrane.50
(3) Nanoimpeller-Functionalized Silica Particles for Photocontrolled Cell Apoptosis
Nanoimpeller-functionalized silica described has been demonstrated to deliver and release anticancer drugs into living cells under photocontrol.89 The PANC-1 cancer cells were treated with a suspension of the particles loaded with the anticancer drug CPT. After 3 h of incubation, the cells were washed with buffer to remove the particles that were not taken up by the cells. The cells containing the drug-loaded particles were either left in the dark or illuminated for 5 min with a laser light at different wavelengths, and then examined by confocal microscopy. For the fluorescence imaging, the irradiated cells were double stained with a 1:1 mixture of a PI and Hoechst 33342 dye to confirm that the cell death resulted from the CPT release. When the treated cells were irradiated with 413 nm light, a wavelength that produces a continuous cis/trans isomerization of azobenzenes, the CPT molecules were released out of the particles, which induced nuclear fragmentation and cell death. In the dark, however, the CPT remained inside the particles and the cells were not damaged (Fig. 6). No cell apoptosis was observed also when they were irradiated with 676 nm light, a wavelength that the azobenzenes do not absorb. A cytotoxicity assay of the particles showed that cell survival decreased about to half after 10 min of the impeller activation with ~0.1 W/cm2 at 413 nm.
Fig. 6.
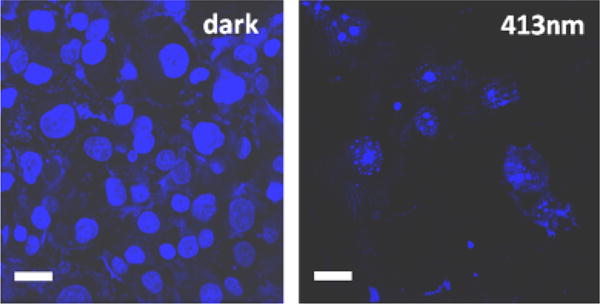
Confocal fluorescence microscope images of the PANC-1 cancer cells that were treated with a suspension of the nanoimpeller-functionalized particles loaded with the camptothecin (CPT), kept in the dark (left) and 5 min illuminated at 413 nm of a light (right). Photoactivated impellers released the CPT from the particles inducing cell apoptosis (right) while unexcited machines did not cause the CPT release and cells were intact (left). Scale bar: 30 μm.
V. Summary
Silicate materials prepared by sol–gel chemistry are versatile frameworks that can be derivatized with various active molecules ranging from biological entities to molecular machines. The materials can be synthesized in different forms including bulk monoliths, thin films, or nanoparticles. Because of the mild conditions associated with the sol–gel process, living cells and other biomolecules can be encapsulated within the silica matrix and the biological activities of the dopants are preserved despite immobilization inside the glass. The sol–gel process can also be tailored to allow for the incorporation of inorganic nanocrystals at the core of silica nanoparticles. Silica nanoparticles embedded with superparamagnetic iron oxide nanocrystals have been prepared, and these materials are useful because they can be externally manipulated using magnetic fields and can also act as MR imaging contrast agents.
The use of surfactants to template mesostructure within silica thin films and nanoparticles has opened up a host of applications for sol–gel materials. The mesostructure that is generated when surfactants are used contains spatially separated regions that are physically and chemically different from one another. These differences allow different dopant molecules to be selectively localized in specific locations and multiple types of molecules can be doped into the material simultaneously. Various strategies exist for deliberately placing dopant molecules in specific regions of the mesostructure. These strategies have been used to derivatize mesostructured thin films with different donor and acceptor molecules that exhibit photo-induced energy or electron transfer capabilities.
Mesoporous silica materials are also useful supports for molecular machines that undergo large amplitude motions in response to a designed stimulus. The existence of nanopores makes these materials useful for controlled release applications, where molecular machines can be used to control the entrances to pore orifices and operate by releasing encapsulated guest molecules on command. Nano-impellers based on azobenzene derivatives and supramolecular snap-tops are two examples of controlled release mechanisms that respond to light or enzyme activation, respectively.
The biological compatibility of silicate materials extends beyond their ability to encapsulate biomolecules: it has been demonstrated mesoporous silica nanoparticles are nontoxic to cells and undergo cellular uptake by endocytosis. This type of biological compatibility is one property that makes mesoporous silica materials popular candidates for drug delivery and other biomedical applications. While the nanoparticles themselves are nontoxic, they can be loaded with water-insoluble anticancer drugs and used to deliver the drugs into cells to induce apoptosis. By making modifications to the nanoparticles, steps can be made to enhance the sophistication of nanoparticles as drug delivery agents. One such modification involves the attachment of targeting moieties to the surface of the nanoparticles to enhance their uptake by cancer cells in relation to that of noncancerous cells. It has also been demonstrated that particles modified with photoresponsive nanoimpellers can be used to deliver drug molecules to cells under photocontrol. In general, unmodified silica is a stable, inert, and generally inactive material. However, it can be derivatized by a range of active molecules to produce an immense range of interesting and useful functions.
Acknowledgments
We would like to acknowledge Michael Kovochich, Jie Lu, Kaushik Patel, Tian Xia, and Ying-Wei Yang for their contributions.
This work was supported by the National Science Foundation (CHE 0809384) and the University of California (UC) Lead Campus for Nanotoxicology Training and Research, funded by the UC TSR&TP.
Footnotes
G. Messing—contributing editor
Presented at the 10th International Conference on Ceramic Processing Science, May 25–28, 2008, Inuyama, Japan.
References
- 1.Iler RK. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry. John Wiley and Sons; Chichester, Engl: 1979. p. 892. [Google Scholar]
- 2.Zsigmondy R. The Chemistry of Colloids. John Wiley and Sons Inc; New York, NY: 1917. [Google Scholar]
- 3.Dunn B, Zink JI. Optical Properties of Sol–Gel Glasses Doped with Organic Molecules. J Mater Chem. 1991;1(6):903–13. [Google Scholar]
- 4.Dunn B, Zink JI. Probes of Pore Environment and Molecule–Matrix Interactions in Sol–Gel Materials. Chem Mater. 1997;9(11):2280–91. [Google Scholar]
- 5.Franville AC, Dunn B, Zink JI. Molecular Motion and Environmental Rigidity in the Framework and Ionic Interface Regions of Mesostructured Silica Thin Films. J Phys Chem B. 2001;105(42):10335–9. [Google Scholar]
- 6.Huang MH, Soyez HM, Dunn BS, Zink JI. In Situ Fluorescence Probing of Molecular Mobility and Chemical Changes During Formation of Dip-Coated Sol–Gel Silica Thin Films. Chem Mater. 2000;12(1):231–5. [Google Scholar]
- 7.Huang MH, Dunn BS, Zink JI. In Situ Luminescence Probing of the Chemical and Structural Changes During Formation of Dip-Coated Lamellar Phase Sodium Dodecyl Sulfate Sol–Gel Thin Films. J Am Chem Soc. 2000;122(15):3739–45. [Google Scholar]
- 8.Huang MH, Dunn BS, Soyez H, Zink JI. In Situ Probing by Fluorescence Spectroscopy of the Formation of Continuous Highly-Ordered Lamellar-Phase Mesostructured Thin Films. Langmuir. 1998;14(26):7331–3. [Google Scholar]
- 9.Avnir D. Organic Chemistry within Ceramic Matrixes: Doped Sol–Gel Materials. Acc Chem Res. 1995;28(8):328–34. [Google Scholar]
- 10.McKiernan JM, Yamanaka SA, Dunn B, Zink JI. Spectroscopy and Laser Action of Rhodamine 6G Doped Aluminosilicate Xerogels. J Phys Chem. 1990;94(15):5652–4. [Google Scholar]
- 11.Akbarian F, Dunn BS, Zink JI. Surface-Enhanced Raman Spectroscopy Using Photodeposited Gold Particles in Porous Sol–Gel Silicates. J Phys Chem. 1995;99(12):3892–4. [Google Scholar]
- 12.MacCraith BD, McDonagh C. Enhanced Fluorescence Sensing Using Sol–Gel Materials. J Fluoresc. 2002;12(34):333–42. [Google Scholar]
- 13.Pouxviel JC, Dunn B, Zink JI. Fluorescence Study of Aluminosilicate Sols and Gels Doped with Hydroxy Trisulfonated Pyrene. J Phys Chem. 1989;93(5):2134–9. [Google Scholar]
- 14.Innocenzi P, Lebeau B. Organic-Inorganic Hybrid Materials for Non-Linear Optics. J Mater Chem. 2005;15(35–36):3821–31. [Google Scholar]
- 15.Ostafin AE, Siegel M, Wang Q, Mizukami H. Fluorescence of Cascade Blue Inside Nano-Sized Porous Shells of Silicate. Microporous Mesoporous Mater. 2003;57(1):47–55. [Google Scholar]
- 16.Ow H, Larson DR, Srivastava M, Baird BA, Webb WW, Wiesner U. Bright and Stable Core–Shell Fluorescent Silica Nanoparticles. Nano Lett. 2005;5(1):113–7. doi: 10.1021/nl0482478. [DOI] [PubMed] [Google Scholar]
- 17.Reisfeld R, Weiss A, Saraidarov T, Yariv E, Ishchenko AA. Solid-State Lasers Based on Inorganic–Organic Hybrid Materials Obtained by Combined Sol–Gel Polymer Technology. Polym Adv Technol. 2004;15(6):291–301. [Google Scholar]
- 18.Avnir D, Coradin T, Lev O, Livage J. Recent Bio-Applications of Sol– Gel Materials. J Mater Chem. 2006;16(11):1013–30. [Google Scholar]
- 19.Braun S, Rappoport S, Zusman R, Avnir D, Ottolenghi M. Biochemically Active Sol–Gel Glasses: The Trapping of Enzymes. Mater Lett. 1990;10(1–2):1–5. [Google Scholar]
- 20.Ellerby LM, Nishida CR, Nishida F, Yamanaka SA, Dunn B, Valentine JS, Zink JI. Encapsulation of Proteins in Transparent Porous Silicate Glasses Prepared by the Sol–Gel Method. Science. 1992;255(5048):1113–5. doi: 10.1126/science.1312257. [DOI] [PubMed] [Google Scholar]
- 21.Dave BC, Miller JM, Dunn B, Valentine JS, Zink JI. Encapsulation of Proteins in Bulk and Thin Film Sol–Gel Matrixes. J Sol–Gel Sci Technol. 1997;8(123):629–34. [Google Scholar]
- 22.Miller JM, Dunn B, Valentine JS, Zink JI. Synthesis Conditions for Encapsulating Cytochrome c and Catalase in SiO2 Sol–Gel Materials. J Non-Cryst Solids. 1996;202(3):279–89. [Google Scholar]
- 23.Khan I, Shannon CF, Dantsker D, Friedman AJ, Perez-Gonzalez-de-Apodaca J, Friedman JM. Sol–Gel Trapping of Functional Intermediates of Hemoglobin: Geminate and Bimolecular Recombination Studies. Biochemistry. 2000;39(51):16099–109. doi: 10.1021/bi000536x. [DOI] [PubMed] [Google Scholar]
- 24.Dave BC, Dunn B, Valentine JS, Zink JI. Sol–Gel Encapsulation Methods for Biosensors. Anal Chem. 1994;66(22):1120A–1127A. [Google Scholar]
- 25.Dunn B, Miller JM, Dave BC, Valentine JS, Zink JI. Strategies for Encapsulating Biomolecules in Sol–Gel Matrixes. Acta Mater. 1998;46(3):737–41. [Google Scholar]
- 26.Bhatia RB, Brinker CJ, Gupta AK, Singh AK. Aqueous Sol–Gel Process for Protein Encapsulation. Chem Mater. 2000;12(8):2434–41. [Google Scholar]
- 27.Sui X, Cruz-Aguado JA, Chen Y, Zhang Z, Brook MA, Brennan JD. Properties of Human Serum Albumin Entrapped in Sol–Gel-Derived Silica Bearing Covalently Tethered Sugars. Chem Mater. 2005;17(5):1174–82. [Google Scholar]
- 28.Chen Q, Kenausis GL, Heller A. Stability of Oxidases Immobilized in Silica Gels. J Am Chem Soc. 1998;120(19):4582–5. [Google Scholar]
- 29.Eggers DK, Valentine JS. Molecular Confinement Influences Protein Structure and Enhances Thermal Protein Stability. Protein Sci. 2001;10(2):250–61. doi: 10.1110/ps.36201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan EH, Dave BC, Fukuto JM, Dunn B, Zink JI, Valentine JS. Synthesis of Sol–Gel Encapsulated Heme Proteins with Chemical Sensing Properties. J Mater Chem. 1999;9(1):45–53. [Google Scholar]
- 31.Nguyen DT, Smit M, Dunn B, Zink JI. Stabilization of Creatine Kinase Encapsulated in Silicate Sol–Gel Materials and Unusual Temperature Effects on its Activity. Chem Mater. 2002;14(10):4300–6. [Google Scholar]
- 32.Besanger TR, Easwaramoorthy B, Brennan JD. Entrapment of Highly Active Membrane-Bound Receptors in Macroporous Sol–Gel Derived Silica. Anal Chem. 2004;76(21):6470–5. doi: 10.1021/ac0488210. [DOI] [PubMed] [Google Scholar]
- 33.Luo TJM, Soong R, Lan E, Dunn B, Montemagno C. Photo-Induced Proton Gradients and ATP Biosynthesis Produced by Vesicles Encapsulated in a Silica Matrix. Nat Mater. 2005;4(3):220–4. doi: 10.1038/nmat1322. [DOI] [PubMed] [Google Scholar]
- 34.Gill I. Bio-Doped Nanocomposite Polymers: Sol–Gel Bioencapsulates. Chem Mater. 2001;13(10):3404–21. [Google Scholar]
- 35.Lan EH, Dunn B, Zink JI. Sol–Gel Encapsulated Anti-Trinitrotoluene Antibodies in Immunoassays for TNT. Chem Mater. 2000;12(7):1874–8. [Google Scholar]
- 36.Rickus JL, Dunn B, Zink JI. Optically Based Sol–Gel Biosensor Materials. Opt Biosens. 2002:427–56. [Google Scholar]
- 37.Wang R, Narang U, Prasad PN, Bright FV. Affinity of Antifluorescein Antibodies Encapsulated within a Transparent Sol–Gel Glass. Anal Chem. 1993;65(19):2671–5. [Google Scholar]
- 38.Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J Am Chem Soc. 1992;114(27):10834–43. [Google Scholar]
- 39.Stein A, Melde BJ, Schroden RC. Hybrid Inorganic–Organic Mesoporous Silicates—Nanoscopic Reactors Coming of Age. Adv Mater. 2000;12(19):1403–19. [Google Scholar]
- 40.Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science. 1998;279(5350):548–52. doi: 10.1126/science.279.5350.548. [DOI] [PubMed] [Google Scholar]
- 41.Zhao D, Huo Q, Feng J, Chmelka BF, Stucky GD. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J Am Chem Soc. 1998;120(24):6024–36. [Google Scholar]
- 42.Shephard DS, Zhou W, Maschmeyer T, Matters JM, Roper CL, Parsons S, Johnson BFG, Duer MJ. Site-Directed Surface Derivatization of MCM-41: Use of High-Resolution Transmission Electron Microscopy and Molecular Recognition for Determining the Position of Functionality within Mesoporous Materials. Angew Chem, Int Ed. 1998;37(19):2719–23. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2719::AID-ANIE2719>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Cai Q, Luo ZS, Pang WQ, Fan YW, Chen XH, Cui FZ. Dilute Solution Routes to Various Controllable Morphologies of MCM-41 Silica with a Basic Medium. Chem Mater. 2001;13(2):258–63. [Google Scholar]
- 44.Lin YS, Tsai CP, Huang HY, Kuo CT, Hung Y, Huang DM, Chen YC, Mou CY. Well-Ordered Mesoporous Silica Nanoparticles as Cell Markers. Chem Mater. 2005;17(18):4570–3. [Google Scholar]
- 45.Radu DR, Lai CY, Jeftinija K, Rowe EW, Jeftinija S, Lin VSY. A Polyamidoamine Dendrimer-Capped Mesoporous Silica Nanosphere-Based Gene Transfection Reagent. J Am Chem Soc. 2004;126(41):13216–7. doi: 10.1021/ja046275m. [DOI] [PubMed] [Google Scholar]
- 46.Lu Y, Ganguli R, Drewien CA, Anderson MT, Brinker CJ, Gong W, Guo Y, Soyez H, Dunn B, Huang MH, Zink JI. Continuous Formation of Supported Cubic and Hexagonal Mesoporous Films by Sol–Gel Dip-Coating. Nature. 1997;389(6649):364–8. [Google Scholar]
- 47.Kim J, Lee JE, Lee J, Yu JH, Kim BC, An K, Hwang Y, Shin CH, Park JG, Hyeon T. Magnetic Fluorescent Delivery Vehicle using Uniform Mesoporous Silica Spheres Embedded with Monodisperse Magnetic and Semi-conductor Nanocrystals. J Am Chem Soc. 2006;128(3):688–9. doi: 10.1021/ja0565875. [DOI] [PubMed] [Google Scholar]
- 48.Fan H, Gabaldon J, Brinker CJ, Jiang YB. Ordered Nanocrystal/Silica Particles Self-Assembled from Nanocrystal Micelles and Silicate. Chem Commun. 2006;22:2323–5. doi: 10.1039/b600923a. [DOI] [PubMed] [Google Scholar]
- 49.Fan H, Yang K, Boye DM, Sigmon T, Malloy KJ, Xu H, Lopez GP, Brinker CJ. Self-Assembly of Ordered, Robust, Three-Dimensional Gold Nanocrystal/Silica Arrays. Science. 2004;304(5670):567–71. doi: 10.1126/science.1095140. [DOI] [PubMed] [Google Scholar]
- 50.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano. 2008;2(5):889–96. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park J, An K, Hwang Y, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat Mater. 2004;3(12):891–5. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- 52.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of Thiol-Derivatised Gold Nanoparticles in a Two-Phase Liquid–Liquid System. Chem Commun. 1994:801–2. [Google Scholar]
- 53.Hiramatsu H, Osterloh FE. A Simple Large-Scale Synthesis of Nearly Monodisperse Gold and Silver Nanoparticles with Adjustable Sizes and with Exchangeable Surfactants. Chem Mater. 2004;16(13):2509–11. [Google Scholar]
- 54.Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J. Artificially Engineered Magnetic Nanoparticles for Ultra-Sensitive Molecular Imaging. Nat Med. 2007;13(1):95–9. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 55.Lu AH, Salabas EL, Schüth F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew Chem, Int Ed. 2007;46(8):1222–44. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 56.Na HB, Lee JH, An K, Park YI, Park M, Lee IS, Nam DH, Kim ST, Kim SH, Kim SW, Lim KH, Kim KS, Kim SO, Hyeon T. Development of a T1 Contrast Agent for Magnetic Resonance Imaging Using MnO Nanoparticles. Angew Chem, Int Ed. 2007;46(28):5397–401. doi: 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- 57.Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J Am Chem Soc. 2004;126(1):273–9. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 58.Yu WW, Falkner JC, Yavuz CT, Colvin VL. Synthesis of Monodisperse Iron Oxide Nanocrystals by Thermal Decomposition of Iron Carboxylate Salts. Chem Commun. 2004;20:2306–7. doi: 10.1039/b409601k. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Nelson JA, White WB, Eklund PC, Adair JH. Palladium Infiltration in High Surface Area Microporous Silica Nanoparticles. Mater Lett. 2006;60(29–30):3573–6. [Google Scholar]
- 60.Lin AWH, Lewinski NA, West JL, Halas NJ, Drezek RA. Optically Tunable Nanoparticle Contrast Agents for Early Cancer Detection: Model-Based Analysis of Gold Nanoshells. J Biomed Opt. 2005;10(6):064035. doi: 10.1117/1.2141825. [DOI] [PubMed] [Google Scholar]
- 61.Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted Nanoshells for Integrated Cancer Imaging and Therapy. Nano Lett. 2005;5(4):709–11. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 62.Baca HK, Carnes E, Singh S, Ashley C, Lopez D, Brinker CJ. Cell-Directed Assembly of Bio/Nano Interfaces—A New Scheme for Cell Immobilization. Acc Chem Res. 2007;40(9):836–45. doi: 10.1021/ar600027u. [DOI] [PubMed] [Google Scholar]
- 63.Carturan G, Campostrini R, Dire S, Scardi V, De Alteriis E. Inorganic Gels for Immobilization of Biocatalysts: Inclusion of Invertase-Active Whole Cells of Yeast (Saccharomyces cerevisiae) into Thin Layers of Silica Gel Deposited on Glass Sheets. J Mol Catal. 1989;57(1):L13–6. [Google Scholar]
- 64.Carturan G, Dal Toso R, Boninsegna S, Dal Monte R. Encapsulation of Functional Cells by Sol–Gel Silica: Actual Progress and Perspectives for Cell Therapy. J Mater Chem. 2004;14(14):2087–98. [Google Scholar]
- 65.Chia S, Urano J, Tamanoi F, Dunn B, Zink JI. Patterned Hexagonal Arrays of Living Cells in Sol–Gel Silica Films. J Am Chem Soc. 2000;122(27):6488–9. [Google Scholar]
- 66.Coradin T, Livage J. Aqueous Silicates in Biological Sol–Gel Applications: New Perspectives for Old Precursors. Acc Chem Res. 2007;40(9):819–26. doi: 10.1021/ar068129m. [DOI] [PubMed] [Google Scholar]
- 67.Baca HK, Ashley C, Carnes E, Lopez D, Flemming J, Dunphy D, Singh S, Chen Z, Liu N, Fan H, Lopez GP, Brozik SM, Werner-Washburne M, Brinker CJ. Cell-Directed Assembly of Lipid-Silica Nanostructures Providing Extended Cell Viability. Science (Washington, DC) 2006;313(5785):337–41. doi: 10.1126/science.1126590. [DOI] [PubMed] [Google Scholar]
- 68.Minoofar PN, Dunn BS, Zink JI. Multiply Doped Nanostructured Silicate Sol–Gel Thin Films: Spatial Segregation of Dopants, Energy Transfer, and Distance Measurements. J Am Chem Soc. 2005;127(8):2656–65. doi: 10.1021/ja045185e. [DOI] [PubMed] [Google Scholar]
- 69.Johansson E, Zink JI. Nanostructured Silica Thin Films Self-Assembled with Electron Donors and Acceptors to Measure Electron Tunneling. J Am Chem Soc. 2007;129(46):14437–43. doi: 10.1021/ja075323a. [DOI] [PubMed] [Google Scholar]
- 70.Hernandez R, Franville AC, Minoofar P, Dunn B, Zink JI. Controlled Placement of Luminescent Molecules and Polymers in Mesostructured Sol– Gel Thin Films. J Am Chem Soc. 2001;123(6):1248–9. doi: 10.1021/ja003634e. [DOI] [PubMed] [Google Scholar]
- 71.Hernandez R, Minoofar P, Huang M, Franville AC, Chia S, Dunn B, Zink JI. Strategies for Spatially Separating Photoactive Molecules in Mesostructured Sol–Gel Silicate Films. Stud Surf Sci Catal. 2003;146:413–8. [Nanotechnology in Mesostructured Materials] [Google Scholar]
- 72.Minoofar P, Hernandez R, Franville AC, Chia S, Dunn B, Zink JI. Strategies for Spatially Separating Molecules in Mesostructured Sol–Gel Silicate Films. J Sol–Gel Sci Technol. 2003;26(123):571–5. [Google Scholar]
- 73.Minoofar PN, Hernandez R, Chia S, Dunn B, Zink JI, Franville AC. Placement and Characterization of Pairs of Luminescent Molecules in Spatially Separated Regions of Nanostructured Thin Films. J Am Chem Soc. 2002;124(48):14388–96. doi: 10.1021/ja020817n. [DOI] [PubMed] [Google Scholar]
- 74.Mal NK, Fujiwara M, Tanaka Y, Taguchi T, Matsukata M. Photo-Switched Storage and Release of Guest Molecules in the Pore Void of Coumarin-Modified MCM-41. Chem Mater. 2003;15(17):3385–94. [Google Scholar]
- 75.Mal NK, Fujiwara M, Tanaka Y. Photocontrolled Reversible Release of Guest Molecules from Coumarin-Modified Mesoporous Silica. Nature. 2003;421(6921):350–3. doi: 10.1038/nature01362. [DOI] [PubMed] [Google Scholar]
- 76.Giri S, Trewyn BG, Stellmaker MP, Lin VSY. Stimuli-Responsive Controlled-Release Delivery System Based on Mesoporous Silica Nanorods Capped with Magnetic Nanoparticles. Angew Chem, Int Ed. 2005;44(32):5038–44. doi: 10.1002/anie.200501819. [DOI] [PubMed] [Google Scholar]
- 77.Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, Lin VSY. A Mesoporous Silica Nanosphere-Based Carrier System with Chemically Removable CdS Nanoparticle Caps for Stimuli-Responsive Controlled Release of Neurotransmitters and Drug Molecules. J Am Chem Soc. 2003;125(15):4451–9. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 78.Liu NG, Dunphy DR, Atanassov P, Bunge SD, Chen Z, Lopez GP, Boyle TJ, Brinker CJ. Photoregulation of Mass Transport Through a Photoresponsive Azobenzene-Modified Nanoporous Membrane. Nano Lett. 2004;4(4):551–4. [Google Scholar]
- 79.Angelos S, Choi E, Voegtle F, De Cola L, Zink JI. Photo-Driven Expulsion of Molecules from Mesostructured Silica Nanoparticles. J Phys Chem C. 2007;111(18):6589–92. [Google Scholar]
- 80.Chia SY, Cao JG, Stoddart JF, Zink JI. Working Supramolecular Machines Trapped in Glass and Mounted on a Film Surface. Angew Chem, Int Ed. 2001;40(13):2447. doi: 10.1002/1521-3773(20010702)40:13<2447::AID-ANIE2447>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 81.Hernandez R, Tseng HR, Wong JW, Stoddart JF, Zink JI. An Operational Supramolecular Nanovalve. J Am Chem Soc. 2004;126(11):3370–1. doi: 10.1021/ja039424u. [DOI] [PubMed] [Google Scholar]
- 82.Leung KCF, Nguyen TD, Stoddart JF, Zink JI. Supramolecular Nanovalves Controlled by Proton Abstraction and Competitive Binding. Chem Mater. 2006;18(25):5919–28. [Google Scholar]
- 83.Nguyen TD, Leung KCF, Liong M, Liu Y, Stoddart F, Zink JI. Versatile Supramolecular Nanovalves Reconfigured for Light Activation. Adv Funct Mater. 2007;17(13):2101–10. [Google Scholar]
- 84.Nguyen TD, Leung KCF, Liong M, Pentecost CD, Stoddart JF, Zink JI. Construction of a pH-Driven Supramolecular Nanovalve. Organ Lett. 2006;8(15):3363–6. doi: 10.1021/ol0612509. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen TD, Liu Y, Saha S, Leung KCF, Stoddart JF, Zink JI. Design and Optimization of Molecular Nanovalves Based on Redox-Switchable Bistable Rotaxanes. J Am Chem Soc. 2007;129(3):626–34. doi: 10.1021/ja065485r. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen TD, Tseng HR, Celestre PC, Flood AH, Liu Y, Stoddart JF, Zink JI. A Reversible Molecular Valve. Proc Natl Acad Sci USA. 2005;102(29):10029–34. doi: 10.1073/pnas.0504109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saha S, Leung KCF, Nguyen TD, Stoddart JF, Zink JI. Nanovalves. Adv Funct Mater. 2007;17(5):685–93. [Google Scholar]
- 88.Patel K, Angelos S, Dichtel WR, Coskun A, Yang YW, Zink JI, Stoddart JF. Enzyme-Responsive Snap-Top Covered Silica Nanocontainers. J Am Chem Soc. 2008;130(8):2382–3. doi: 10.1021/ja0772086. [DOI] [PubMed] [Google Scholar]
- 89.Lu J, Choi E, Tamanoi F, Zink JI. Light-Activated Nanoimpeller-Controlled Drug Release in Cancer Cells. Small. 2008;4(4):421–6. doi: 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sierocki P, Maas H, Dragut P, Richardt G, Voegtle F, De Cola L, Brouwer F, Zink JI. Photoisomerization of Azobenzene Derivatives in Nanostructured Silica. J Phys Chem B. 2006;110(48):24390–8. doi: 10.1021/jp0641334. [DOI] [PubMed] [Google Scholar]
- 91.Liu N, Chen Z, Dunphy DR, Jiang YB, Assink RA, Brinker CJ. Photoresponsive Nanocomposite Formed by Self-Assembly of an Azobenzene-Modified Silane. Angew Chem, Int Ed. 2003;42(15):1731–4. doi: 10.1002/anie.200250189. [DOI] [PubMed] [Google Scholar]
- 92.Liu N, Assink RA, Smarsly B, Brinker CJ. Synthesis and Characterization of Highly Ordered Functional Mesoporous Silica Thin Films with Positively Chargeable –NH2 Groups. Chem Commun. 2003;(10):1146–7. doi: 10.1039/b301910a. [DOI] [PubMed] [Google Scholar]
- 93.Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous Silica Nanoparticles as a Delivery System for Hydrophobic Anticancer Drugs. Small. 2007;3(8):1341–6. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- 94.Torney F, Trewyn BG, Lin VSY, Wang K. Mesoporous Silica Nanoparticles Deliver DNA and Chemicals into Plants. Nat Nanotechnol. 2007;2(5):295–300. doi: 10.1038/nnano.2007.108. [DOI] [PubMed] [Google Scholar]
- 95.Tsai CP, Hung Y, Chou YH, Huang DM, Hsiao JK, Chang C, Chen YC, Mou CY. High-Contrast Paramagnetic Fluorescent Mesoporous Silica Nanorods as a Multifunctional Cell-Imaging Probe. Small. 2008;4(2):186–91. doi: 10.1002/smll.200700457. [DOI] [PubMed] [Google Scholar]
- 96.Lu J, Liong M, Sherman S, Xia T, Kovochich M, Nel AE, Zink JI, Tamanoi F. Mesoporous Silica Nanoparticles for Cancer Therapy: Energy-Dependent Cellular Uptake and Delivery of Paclitaxel to Cancer Cells. Nano Biotechnol. 2007;3(2):89–95. doi: 10.1007/s12030-008-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burns A, Ow H, Wiesner U. Fluorescent Core–hell Silica Nanoparticles: Towards ‘Lab on a Particle’ Architectures for Nanobiotechnology. Chem Soc Rev. 2006;35(11):1028–42. doi: 10.1039/b600562b. [DOI] [PubMed] [Google Scholar]
- 98.Tan W, Wang K, He X, Zhao XJ, Drake T, Wang L, Bagwe RP. Bionanotechnology Based on Silica Nanoparticles. Med Res Rev. 2004;24(5):621–38. doi: 10.1002/med.20003. [DOI] [PubMed] [Google Scholar]
- 99.Fuller JE, Zugates GT, Ferreira LS, Ow HS, Nguyen NN, Wiesner UB, Langer RS. Intracellular Delivery of Core–Shell Fluorescent Silica Nanoparticles. Biomaterials. 2008;29(10):1526–32. doi: 10.1016/j.biomaterials.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 100.Slowing I, Trewyn BG, Lin VSY. Effect of Surface Functionalization of MCM-41-TypeMesoporous Silica Nanoparticles on the Endocytosis by Human Cancer Cells. J Am Chem Soc. 2006;128(46):14792–3. doi: 10.1021/ja0645943. [DOI] [PubMed] [Google Scholar]
- 101.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In Vivo Imaging of siRNA Delivery and Silencing in Tumors. Nat Med. 2007;13(3):372–7. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 102.Corot C, Robert P, Idee JM, Port M. Recent Advances in Iron Oxide Nanocrystal Technology for Medical Imaging. Adv Drug Delivery Rev. 2006;58(14):1471–504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 103.Wu SH, Lin YS, Hung Y, Chou YH, Hsu YH, Chang C, Mou CY. Multifunctional Mesoporous Silica Nanoparticles for Intracellular Labeling and Animal Magnetic Resonance Imaging Studies. Chem Biochem. 2008;9(1):53–7. doi: 10.1002/cbic.200700509. [DOI] [PubMed] [Google Scholar]
- 104.Lee J-H, Jun Y-w, Yeon S-I, Shin J-S, Cheon J. Dual-Mode Nanoparticle Probes for High-Performance Magnetic Resonance and Fluorescence Imaging of Neuroblastoma. Angew Chem, Int Ed. 2006;118(48):8340–2. doi: 10.1002/anie.200603052. [DOI] [PubMed] [Google Scholar]
- 105.Sudimack J, Lee RJ. Targeted Drug Delivery Via the Folate Receptor. Adv Drug Delivery Rev. 2000;41(2):147–62. doi: 10.1016/s0169-409x(99)00062-9. [DOI] [PubMed] [Google Scholar]