Abstract
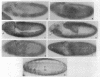
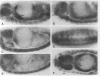
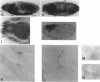
A Drosophila cDNA encoding a structural homologue of the mammalian coated vesicle component alpha-adaptin (AP2 adaptor complex) has been cloned and sequenced. The mammalian and invertebrate sequences are highly conserved, especially within the amino terminal region, a domain that mediates interactions with other components within the AP2 complex and with specific receptors tails. Mammalian alpha-adaptins are encoded by two genes; however, Drosophila alpha-adaptin has a single gene locus, within polytene bands 21C2-C3 on the left arm of the chromosome 2, closely adjacent to the paired homeobox gene aristaless. There seem to be at least two Drosophila alpha-adaptin transcripts expressed, plausibly by alternative splicing. One of the transcripts is more abundant during early embryogenesis and may be of maternal origin. We have studied the distribution of the alpha-adaptin protein throughout embryogenesis and at the neuromuscular junction of the third instar larva. During cellularization of the blastoderm embryo, the protein is seen between and ahead of the elongating nuclei, and then redistributes to the cell surface during gastrulation. These observations suggest a role for endocytosis in cellularization and are consistent with the finding that dynamin (the shibire gene product), another component of the endocytic mechanism, is required for cellularization. At later stages of embryogenesis, alpha-adaptin is expressed in complex and dynamic patterns. It is strongly induced in elements of the central and peripheral nervous system (e.g., in neuroblasts, the presumptive stomatogastric nervous system, and the lateral chordotonal sense organs), in the Garland cells, the adult midgut precursors, the antenno-maxillary complex, the endoderm, the fat bodies, and the visceral mesoderm. In the larva, alpha-adaptin is localized at the plasma membrane in the synaptic boutons of the neuromuscular junctions. The cells expressing high levels of alpha-adaptin are known or expected to support high levels of endocytosis; thus, this coated vesicle protein seems to be an excellent marker for endocytic activity. The expression patterns of dynamin, detected in the embryo by in situ hybridization methods, are very similar to those reported here for alpha-adaptin reflecting the likely coordinated expression of endocytic components. Taken together with previous evidence, our results suggest that endosomal vesicle trafficking, membrane recycling, and the regulation of endocytosis play critical roles in the wide range of developmental processes.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J., Smith M., Merakovsky J., Coulson M., Hannan F., Kelly L. E. The stoned locus of Drosophila melanogaster produces a dicistronic transcript and encodes two distinct polypeptides. Genetics. 1996 Aug;143(4):1699–1711. doi: 10.1093/genetics/143.4.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball C. L., Hunt S. P., Robinson M. S. Expression and localization of alpha-adaptin isoforms. J Cell Sci. 1995 Aug;108(Pt 8):2865–2875. doi: 10.1242/jcs.108.8.2865. [DOI] [PubMed] [Google Scholar]
- Bazinet C., Katzen A. L., Morgan M., Mahowald A. P., Lemmon S. K. The Drosophila clathrin heavy chain gene: clathrin function is essential in a multicellular organism. Genetics. 1993 Aug;134(4):1119–1134. doi: 10.1093/genetics/134.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedigheimer M., Laughon A. Expanded: a gene involved in the control of cell proliferation in imaginal discs. Development. 1993 Aug;118(4):1291–1301. doi: 10.1242/dev.118.4.1291. [DOI] [PubMed] [Google Scholar]
- Camidge D. R., Pearse B. M. Cloning of Drosophila beta-adaptin and its localization on expression in mammalian cells. J Cell Sci. 1994 Mar;107(Pt 3):709–718. [PubMed] [Google Scholar]
- Chen M. S., Burgess C. C., Vallee R. B., Wadsworth S. C. Developmental stage- and tissue-specific expression of shibire, a Drosophila gene involved in endocytosis. J Cell Sci. 1992 Nov;103(Pt 3):619–628. doi: 10.1242/jcs.103.3.619. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Obar R. A., Schroeder C. C., Austin T. W., Poodry C. A., Wadsworth S. C., Vallee R. B. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991 Jun 13;351(6327):583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMario P. J., Mahowald A. P. Female sterile (1) yolkless: a recessive female sterile mutation in Drosophila melanogaster with depressed numbers of coated pits and coated vesicles within the developing oocytes. J Cell Biol. 1987 Jul;105(1):199–206. doi: 10.1083/jcb.105.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L. H., Rushlow C. A group of genes required for maintenance of the amnioserosa tissue in Drosophila. Development. 1996 May;122(5):1343–1352. doi: 10.1242/dev.122.5.1343. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Keith F. J. Regulation of translation and proteolysis during the development of embryonic dorso-ventral polarity in Drosophila. Homology of easter proteinase with Limulus proclotting enzyme and translational activation of Toll receptor synthesis. Biochim Biophys Acta. 1992 Oct 20;1132(3):290–296. doi: 10.1016/0167-4781(92)90163-t. [DOI] [PubMed] [Google Scholar]
- González-Gaitán M., Jäckle H. Role of Drosophila alpha-adaptin in presynaptic vesicle recycling. Cell. 1997 Mar 21;88(6):767–776. doi: 10.1016/s0092-8674(00)81923-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Gerttula S., Anderson K. V. Plasma membrane localization of the Toll protein in the syncytial Drosophila embryo: importance of transmembrane signaling for dorsal-ventral pattern formation. Development. 1991 Apr;111(4):1021–1028. doi: 10.1242/dev.111.4.1021. [DOI] [PubMed] [Google Scholar]
- Kim Y. T., Wu C. F. Reversible blockage of neurite development and growth cone formation in neuronal cultures of a temperature-sensitive mutant of Drosophila. J Neurosci. 1987 Oct;7(10):3245–3255. doi: 10.1523/JNEUROSCI.07-10-03245.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T., Ikeda K. Reversible blockage of membrane retrieval and endocytosis in the garland cell of the temperature-sensitive mutant of Drosophila melanogaster, shibirets1. J Cell Biol. 1983 Aug;97(2):499–507. doi: 10.1083/jcb.97.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K., Gay N. J. The dorsal protein enhances the biosynthesis and stability of the Drosophila I kappa B homologue cactus. Nucleic Acids Res. 1995 Aug 25;23(16):3111–3118. doi: 10.1093/nar/23.16.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Jongeward G. D., Sternberg P. W. unc-101, a gene required for many aspects of Caenorhabditis elegans development and behavior, encodes a clathrin-associated protein. Genes Dev. 1994 Jan;8(1):60–73. doi: 10.1101/gad.8.1.60. [DOI] [PubMed] [Google Scholar]
- Lewis E. B. The Relation of Repeats to Position Effect in Drosophila Melanogaster. Genetics. 1945 Mar;30(2):137–166. doi: 10.1093/genetics/30.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. M., Goodman C. S. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994 Sep;13(3):507–523. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- O'Connell P. O., Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984 Jul 11;12(13):5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Stewart J., Fournier M. C., Bosshart H., Rhee I., Miyatake S., Saito T., Gallusser A., Kirchhausen T., Bonifacino J. S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995 Sep 29;269(5232):1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Page L. J., Robinson M. S. Targeting signals and subunit interactions in coated vesicle adaptor complexes. J Cell Biol. 1995 Nov;131(3):619–630. doi: 10.1083/jcb.131.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnambalam S., Robinson M. S., Jackson A. P., Peiperl L., Parham P. Conservation and diversity in families of coated vesicle adaptins. J Biol Chem. 1990 Mar 25;265(9):4814–4820. [PubMed] [Google Scholar]
- Poodry C. A., Hall L., Suzuki D. T. Developmental properties of Shibire: a pleiotropic mutation affecting larval and adult locomotion and development. Dev Biol. 1973 Jun;32(2):373–386. doi: 10.1016/0012-1606(73)90248-0. [DOI] [PubMed] [Google Scholar]
- Poole S. J., Kauvar L. M., Drees B., Kornberg T. The engrailed locus of Drosophila: structural analysis of an embryonic transcript. Cell. 1985 Jan;40(1):37–43. doi: 10.1016/0092-8674(85)90306-x. [DOI] [PubMed] [Google Scholar]
- Rickoll W. L. Cytoplasmic continuity between embryonic cells and the primitive yolk sac during early gastrulation in Drosophila melanogaster. Dev Biol. 1976 Mar;49(1):304–310. doi: 10.1016/0012-1606(76)90278-5. [DOI] [PubMed] [Google Scholar]
- Robinson M. S. Cloning of cDNAs encoding two related 100-kD coated vesicle proteins (alpha-adaptins). J Cell Biol. 1989 Mar;108(3):833–842. doi: 10.1083/jcb.108.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. S. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994 Aug;6(4):538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Schneitz K., Spielmann P., Noll M. Molecular genetics of aristaless, a prd-type homeo box gene involved in the morphogenesis of proximal and distal pattern elements in a subset of appendages in Drosophila. Genes Dev. 1993 Jan;7(1):114–129. doi: 10.1101/gad.7.1.114. [DOI] [PubMed] [Google Scholar]
- Sorkin A., Carpenter G. Interaction of activated EGF receptors with coated pit adaptins. Science. 1993 Jul 30;261(5121):612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tucker K. L., Nathanson K., Kirchhausen T. Sequence of the rat alpha c large chain of the clathrin associated protein complex AP-2. Nucleic Acids Res. 1990 Sep 11;18(17):5306–5306. doi: 10.1093/nar/18.17.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A., Brodsky F. M. In vivo phosphorylation of adaptors regulates their interaction with clathrin. J Cell Biol. 1996 Nov;135(3):635–645. doi: 10.1083/jcb.135.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek A. M., Meyerowitz E. M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991 May 30;351(6325):411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]