Abstract
Background
Intrauterine exposure to gestational diabetes mellitus (GDM) may promote offspring obesity and higher systolic blood pressure (SBP) by adolescence. Few studies have examined adiposity or SBP in younger children exposed to GDM. This study’s objective was to examine associations of maternal glucose tolerance during pregnancy with offspring adiposity and SBP at age 3 years.
Methods
We studied 1,238 mother-child pairs in Project Viva, a prospective prebirth cohort study. Exposures were maternal blood glucose following oral glucose load, and GDM confirmed by 3-h glucose tolerance test. Main child outcomes were age 3-year body mass index (BMI) z-score, the sum (SS+TR) and ratio (SS/TR) of subscapular (SS) and tricep (TR) skinfold thicknesses, and SBP. We performed adjusted multivariable analyses.
Results
Fifty-one (4%) mothers had GDM. 9.3% of 3 year-old children were obese and mean (s.d.) SBP was 92 (11) mm Hg. Children exposed to GDM had higher SBP (3.2 mm Hg, 95% confidence interval (CI): 0.4, 5.9, P = 0.02) and greater adiposity when assessed by the sum of skinfolds (SS+TR 1.31 mm, 95% CI: 0.08, 2.55, P = 0.04) but not by BMI z-score (−0.08 units, 95% CI: −0.37, 0.22, P = 0.61). After additional adjustment for the sum of skinfold thicknesses (SS+TR), the relationship between GDM and SBP was attenuated and no longer significant (2.6 mm Hg, 95% CI: −0.2, 5.4, P = 0.07).
Conclusions
Children exposed to GDM have higher adiposity, which may mediate the higher SBP in these children. These findings extend to younger children the adverse effects of GDM previously found among adolescents and adults.
Forty years ago, Freinkel1 called gestational diabetes a “teratogen” causing higher birth weight and increased risk of congenital defects.2,3 More recently, prospective cohort studies have demonstrated metabolic changes among offspring exposed to gestational diabetes. Prenatal exposure to gestational diabetes was a strong predictor of impaired glucose tolerance (IGT) and obesity by adolescence.4–6 In these children, increased risk of obesity and diabetes was not generally seen in early childhood, but seemed to develop over time.
Fewer studies have examined associations of exposure to gestational diabetes with offspring blood pressure. These studies were generally focused on adolescents,7 limited by small sample size,8,9 and did not adequately account for the contemporaneous effects of adiposity. With prevalence of gestational diabetes mellitus (GDM) at ~4% and increasing,10 intrauterine exposure to gestational diabetes affects a large number of children. Demonstrating increased cardiovascular risk in the younger child born to mothers with GDM has important implications for limiting the cumulative impact of chronic disease.
The purpose of this study was to investigate the relationship between maternal glucose tolerance test results and offspring adiposity and systolic blood pressure (SBP) at age 3 years. We hypothesized that GDM would be associated with increases in central and overall adiposity, as well as with higher blood pressure independent of adiposity.
Methods
Population and study design
We studied participants in Project Viva, a prospective prebirth cohort study of pregnant women and their children.11 Between April 1999 and July 2002 we recruited pregnant women at their initial obstetric visit to a large multispecialty urban/suburban group practice in eastern Massachusetts. All mothers provided informed consent and were eligible to participate if they could answer questions in English, planned to stay in the area until after delivery, presented for prenatal care before 22 weeks gestation, and had a singleton pregnancy. We performed in-person study visits with the mother after the first and second trimesters of pregnancy, with both mother and child in the hospital after delivery, and at 6 months and 3 years postpartum in a research office or at home. Institutional review boards of participating institutions approved the study, and all procedures were in accordance with ethical standards for human experimentation.12
Of the 2,128 women who delivered a live infant, 1,579 were eligible for 3-year follow up by virtue of having completed prenatal nutritional assessments and consenting for their children to be followed up. We excluded those with missing or incomplete records on glucose tolerance testing (n = 16), and with a history of previous type 1 or type 2 DM or polycystic ovary syndrome with glucose intolerance (n = 11). Of the 1,552 mothers who remained, 1,254 of their children had anthropometric measurements recorded at age 3 years. After excluding 16 pregnancies with gestation <34 weeks, we included 1,238 Viva mother-child pairs in this study. Mothers included in this analysis (n = 1,238) were older (32.5 years vs. 30.9 years, P < 0.0001), more likely to be white (73% vs. 57%, P < 0.0001) and have household income >$70,000/year (65% vs. 56%, P = 0.0001), and had lower mean prepregnancy body mass index (BMI) (24.6 kg/m2 vs. 25.4 kg/m2, P = 0.0002) when compared with those not included (n = 890).
Measures
Exposures—maternal glucose tolerance test
Clinicians at Harvard Vanguard Medical Associates routinely screened all women for gestational diabetes at 26–28 weeks of gestation with a nonfasting oral glucose challenge test, in which venous blood was sampled 1 h after a 50 g oral glucose load. If this screening test result was abnormal (blood glucose value of >140 mg/dl) the woman was referred for a fasting 3-h 100 g oral glucose tolerance test. Abnormal results were a blood glucose >95 mg/dl at baseline, >180 mg/dl at 1 h, >155 mg/dl at 2 h, or >140 mg/dl at 3 h.13 We categorized women with two or more abnormal fasting glucose tolerance test results as having GDM, those who failed the nonfasting screening test but had 0 or 1 abnormal results on the fasting glucose tolerance test as having IGT, and those with normal screening glucose challenge as having normal glucose tolerance. Those who were diagnosed with GDM were followed by a nutritionist, instructed to check their fasting blood sugar daily, and treated with diet, exercise, and in some cases insulin. Those with IGT did not have any further screening and were managed in the same way as women with normal glucose challenge test results.
Outcomes—child age 3-year anthropometry and blood pressure
During in-person study visits, trained research assistants measured children’s heights and weights using a calibrated stadiometer (Shorr Productions, Olney, MD) and scale (Seca model 881; Seca, Hanover, MD). We calculated age- and sex-specific BMI percentiles and z-scores using US national reference data.14 We measured subscapular (SS) and triceps (TR) skinfold thicknesses using Holtain calipers (Holtain, Cross-well, UK), and calculated the sum (SS+TR) and the ratio (SS/TR) of skinfolds. BMI z-score and SS+TR represent overall adiposity, whereas SS/TR is a measure of central or truncal adiposity.15 Research assistants followed standardized techniques and participated in biannual in-service training to ensure measurement validity (IJ Shorr; Shorr Productions).16 Inter- and intrarater error for skinfold measurements were within published reference ranges for all measurements.17
At a single visit, we recorded child blood pressure up to five times at 1-min intervals using biannually calibrated Dinamap Pro-100 oscillometric automated monitors (GE Medical Services, Tampa, FL), and also conditions of measurement including order of readings, cuff size, limb, and child position and activity. In total, 1,020 infants had 5 measurements, 62 had 4, 28 had 3, 30 had 2, and 33 had 1, for a total of 5,525 measurements. We used systolic rather than diastolic blood pressure for all analyses because of the validity of its measurement and its superior prediction of later blood pressure.18,19
Covariates
Using questionnaires and interviews, we collected information about the mother’s race/ethnicity, age, education, marital status, parity, smoking status, and household income; paternal hypertension; and history of diabetes or GDM in the mother’s mother. Mothers also reported their own prepregnancy weight and height and paternal weight and height. We obtained information from the prenatal medical record on serial pregnancy weights and blood pressure, glucose tolerance test results, infant birth weight and delivery date. We calculated gestational weight gain as the difference between self-reported pre-pregnancy weight and the last clinically recorded weight before delivery. We calculated gestational age from the last menstrual period or from the second trimester ultrasound if the two estimates differed by >10 days. We determined infant sex-specific birth weight for gestational age (fetal growth) z-value based on US national natality data.20 Research assistants measured maternal blood pressure at 3-year postpartum.
Statistical analysis
Using multivariable linear regression, we analyzed relationships of three categories of maternal glucose tolerance in pregnancy (normoglycemic, IGT, and GDM) with child BMI z-score, SS+TR, and SS/TR. In secondary analyses, we used the measured glucose level following the nonfasting glucose challenge as a continuous exposure.
To assess associations between exposures and SBP, we used mixed models that incorporated each of the blood pressure measurements from each child as repeated outcome measures.21 We adjusted blood pressure models for measurement conditions, child age and sex.
In all multivariable models, we included additional covariates based on our expectation of which ones would independently predict the outcomes as demonstrated by prior studies.22,23 Included covariates were child sex and age, maternal race/ethnicity, education, parity, age, prepregnancy BMI, pregnancy weight gain, smoking status, and paternal BMI. We additionally adjusted models predicting SS/TR for child BMI z-score because we were interested in fat distribution after controlling for overall body size. For SBP models only, we included measurement conditions, maternal SBP at 3-year postpartum and paternal hypertension status. In subsequent steps, we further adjusted for factors that might be in the pathway linking gestational diabetes with attained blood pressure, namely fetal growth, attained child height and overall adiposity.
We performed all analyses using SAS version 9.1 (Cary, NC).
Results
Mean (s.d.) maternal age was 32.5 (5.0) years, and prepregnancy BMI 24.6 (5.1) kg/m2. Mothers’ race/ethnicity was 73% white, 12% black, 6% Hispanic, and 9% other; 71% were college graduates, and 65% had total household income of $70,000/year or more.
We identified 51 (4%) mothers with GDM, and an additional 152 (12%) with IGT. When compared to normoglycemic mothers, those with IGT or GDM were older and had higher prepregnancy BMI and pregnancy weight gain (Table 1). Mothers with GDM were slightly less likely to be white and were more likely to have maternal family history of DM or GDM. Among the 25 Project Viva mothers with GDM who had a recorded hemoglobin A1C value during pregnancy, mean (s.d.) was 5.3 (0.6)%, reflecting excellent control.
Table 1.
Characteristics of 1,238 mother-child pairs in Project Viva, according to maternal glycemia during pregnancy
| Maternal glycemia in pregnancy | ||||
|---|---|---|---|---|
| Normoglycemic | IGT | GDM | ||
|
N = 1,035 (85%) |
N = 152 (12%) |
N = 51 (4%) |
||
| Characteristics | Mean (s.d.) | P value* | ||
| Age (years) | 32.3 (5.1) | 33.7 (4.4) | 33.0 (4.6) | 0.003 |
| Prepregnancy BMI (kg/m2) |
24.3 (4.9) | 25.4 (5.0) | 27.7 (6.4) | <0.0001 |
| Total gestational weight gain (kg) |
15.9 (5.1) | 15.0 (5.9) | 12.2 (6.3) | <0.0001 |
| 3-year postpartum SBP (mm Hg) |
107.7 (11.1) | 109.5 (12.3) | 112.9 (15.0) | 0.002 |
| Paternal BMI (kg/m2) | 26.3 (3.9) | 26.9 (3.6) | 26.9 (3.8) | 0.18 |
|
Maternal characteristics |
N (%) | P value | ||
| Race/ethnicity | ||||
| White | 756 (73) | 113 (74) | 31 (61) | 0.32 |
| Black | 126 (12) | 16 (11) | 11 (22) | |
| Hispanic | 58 (6) | 12 (8) | 4 (8) | |
| Other | 92 (9) | 11 (7) | 5 (10) | |
| College graduate | 734 (71) | 110 (72) | 34 (67) | 0.73 |
| Annual household income ≥$70,000 |
628 (65) | 90 (62) | 30 (63) | 0.74 |
| Married/cohabiting | 961 (93) | 141 (93) | 49 (96) | 0.70 |
| Maternal smoking | ||||
| During pregnancy | 103 (10) | 13 (9) | 9 (20) | 0.29 |
| Never | 696 (69) | 103 (69) | 27 (59) | |
| Former | 211 (21) | 33 (22) | 10 (22) | |
| Maternal family history DM or GDM |
63 (7) | 17 (12) | 8 (17) | 0.003 |
| Paternal hypertension |
64 (6) | 10 (7) | 3 (6) | 0.99 |
BMI, body mass index; DM, diabetes mellitus; GDM, gestational DM; IGT, impaired glucose tolerance; SBP: systolic blood pressure.
P values for comparison of rates between groups based on χ2-test; P values for comparison of means based on analysis of variance.
Three-year-old children had a mean (s.d.) BMI z-score of 0.45 (1.0) units, 17.1% were overweight (BMI >85–95th percentile), and 9.3% were obese (BMI >95th percentile), slightly lower than the contemporaneous national average of 13.9% for children aged 2–5 years.24,25 Mean child SBP was 92 (11) mm Hg, similar to the median for US children in the 50th percentile for height at age 3 years (male 91 mm Hg, female 89 mm Hg).26
In unadjusted analyses, compared with offspring of normoglycemic mothers, infants born to mothers with IGT had greater fetal growth, but offspring of mothers with GDM were not larger at birth (Table 2). At age 3 years, offspring of diabetic mothers had higher mean SBP than offspring of normoglycemic mothers.
Table 2.
Child characteristics among 1,238 mother-child pairs in Project Viva, according to maternal glycemia during pregnancy
| Maternal glycemia in pregnancy | ||||
|---|---|---|---|---|
| Normoglycemic | IGT | GDM | ||
|
N = 1,035 (85%) |
N = 152 (12%) |
N = 51 (4%) |
||
| Child | Mean (s.d.) | P value* | ||
| Characteristics | ||||
| Birth weight z-score |
0.18 (0.96) | 0.39 (0.94) | 0.26 (0.92) | 0.04 |
| Birth weight (kg) | 3.51 (0.5) | 3.6 (0.52) | 3.51 (0.52) | 0.13 |
| Age 3 child BMI z-score |
0.44 (1.02) | 0.52 (1.01) | 0.47 (1.20) | 0.68 |
| Age 3 child BMI | 16.5 (1.47) | 16.6 (1.5) | 16.6 (1.77) | 0.52 |
| Age 3 SS+TR (mm) | 16.6 (4.2) | 17.1 (4.6) | 17.5 (4.8) | 0.24 |
| Age3 SS/TR (mm) | 0.64 (0.16) | 0.67 (0.16) | 0.65 (0.13) | 0.15 |
| Age 3 SBP (mm Hg) | 92 (10) | 93 (13) | 96 (11) | 0.03 |
| Age 3 height (cm) | 97.6 (4.6) | 97.5 (5.1) | 97.6 (4.9) | 0.99 |
| N (%) | P value | |||
| Female | 503 (49) | 80 (52) | 23 (45) | 0.55 |
| Age 3 BMI percentile status | ||||
| <5th | 23 (2) | 3 (2) | 2 (4) | 0.58 |
| 5 to <85th | 738 (72) | 101 (67) | 31 (63) | |
| 85 to <95th | 169 (17) | 31 (21) | 9 (18) | |
| ≥95th | 91 (9) | 16 (11) | 7 (14) | |
BMI, body mass index; GDM, gestational diabetes mellitus; IGT, impaired glucose tolerance; SBP, systolic blood pressure; SS+TR, sum of subscapular and tricep skinfolds; SS/TR, ratio of subscapular and tricep skinfolds.
P values for comparison of rates between groups based on χ2-test; P values for comparison of means based on analysis of variance.
In analyses adjusted only for child sex and age, GDM was not associated with offspring BMI z-score at age 3 years (0.02 units, 95% confidence interval (CI) −0.27, 0.32, P = 0.87). However, when overall adiposity was assessed by the sum of the skinfolds, maternal GDM was associated with somewhat greater SS+TR (0.92 mm, 95% CI −0.27, 2.11, P = 0.13). After adjustment for maternal age, education, race/ethnicity, smoking history, BMI, pregnancy weight gain, parity, paternal BMI, and fetal growth, maternal GDM was more strongly associated with child SS+TR (1.31 mm, 95% CI 0.08, 2.55, P = 0.04) but still not associated with BMI z-score (−0.08 units, 95% CI −0.37, 0.22, P = 0.61). Maternal IGT was not associated with offspring SS+TR (0.25 mm, 95% CI −0.48, 0.99, P = 0.5) (Table 3) or BMI z-score (0.002 units, 95% CI −0.17, 0.17, P = 0.98). Maternal GDM was not associated with offspring central adiposity, measured as SS/TR ratio (0.01, 95% CI −0.04, 0.05, P = 0.8). However, IGT was marginally associated with higher SS/TR (0.03, 95% CI 0.001, 0.06, P = 0.05).
Table 3.
Associations of intrauterine exposure to IGT and GDM with child overall and central adiposity at age 3 years
| BMI z-score (units)a | SS+TR (mm)a | SS/TR (ratio)b | |
|---|---|---|---|
| Child age 3-year outcomes | Effect estimates (95% confidence limit) | ||
| Normoglycemia | 0.0 (referent) | 0.0 (referent) | 0.0 (referent) |
| IGT | 0.002 (−0.17, 0.17) | 0.25 (−0.48, 0.99) | 0.03 (0.001, 0.06) |
| GDM | −0.08 (−0.37, 0.22) | 1.31 (0.08, 2.55) | 0.01(−0.04,0.05) |
BMI, body mass index; GDM, gestational diabetes mellitus; IGT, impaired glucose tolerance; SS+TR, sum of subscapular and tricep skinfolds; SS/TR, ratio of subscapular and tricep skinfolds.
Adjusted for child sex and age; maternal education, race/ethnicity, smoking, parity, age, prepregnancy BMI, and pregnancy weight gain; paternal BMI; birth weight z-score.
SS/TR additionally adjusted for age 3 BMI z-score.
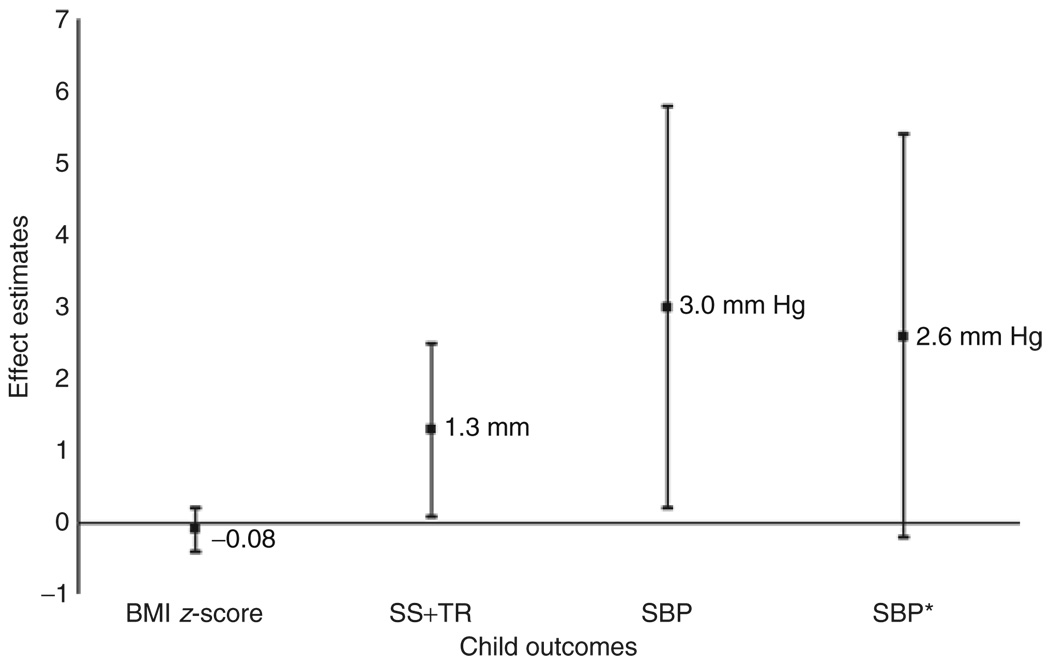
In analyses adjusted for sex, age, and measurement conditions, children exposed to GDM in utero had SBP 3.2 (95% CI 0.6, 5.7, P = 0.02) mm Hg higher than children born to normoglycemic mothers (Table 4). Further adjustment for maternal education, race/ethnicity, age, smoking, paternal BMI and hypertension, maternal prepregnancy BMI, postpartum SBP, parity, and pregnancy weight gain did not substantially change the association (3.0 mm Hg 95% CI 0.2, 5.8, P = 0.03). Further adjustment for fetal growth, attained child BMI z-score, and height—measures that might be in the pathway between intrauterine exposure and child blood pressure—did not attenuate results (3.2 mm Hg, 95% CI 0.4, 5.9, P = 0.02). However, final adjustment for sum of skin-folds (SS+TR) did attenuate estimates and significance (2.6 mm Hg, 95% CI: −0.2, 5.4, P = 0.07) (Figure 1). We did not observe any association of maternal IGT with offspring SBP (−0.7 mm Hg, 95% CI: −2.4, 0.9, P = 0.39) with additional adjustment for SS+TR.
Table 4.
Associations of intrauterine exposure to IGT and GDM with child SBP at age 3 years
| Normoglycemia | IGT | GDM | |
|---|---|---|---|
| Effect estimates in mm Hg (95% confidence interval) | |||
| Model 1: child sex, age, measurement conditionsa | 0.0 (referent) | −0.4 (−2.0, 1.2) | 3.2 (0.6,5.7) |
| Model 2: +maternal education, race/ethnicity, smoking, parity, age, postpartum BP, prepregnancy BMI, pregnancy weight gain; paternal hypertension and BMI |
0.0 (referent) | −0.6 (−2.3, 1.0) | 3.0 (0.2, 5.8) |
| Model 3: +birth weight z-score and child height at age 3 years | 0.0 (referent) | −0.7 (−2.4, 1.0) | 3.0 (0.2, 5.7) |
| Model 4: +child (SS+TR) | 0.0 (referent) | −0.7 (−2.4, 0.9) | 2.6 (−0.2, 5.4) |
BMI, body mass index; GDM, gestational diabetes mellitus; IGT, impaired glucose tolerance; SBP, systolic blood pressure; SS+TR, sum of subscapular and tricep skinfolds.
Measurement conditions were infant state, extremity, cuff size, body position, and measurement sequence number.
Figure 1.
Associations of intrauterine exposure to gestational diabetes mellitus with body mass index (BMI) z-score, sum of subscapular and triceps skinfolds (SS+TR), and systolic blood pressure (SBP) at age 3 years, among 1,238 mother-child pairs in Project Viva. Estimates (95% confidence interval) adjusted for child sex and age; maternal education, race/ethnicity, smoking, parity, age, prepregnancy BMI, and pregnancy weight gain; paternal BMI; and birth weight z-score. SBP estimates are additionally adjusted for measurement conditions, maternal blood pressure, paternal hypertension, age 3 child height, and, in an an additional model*, the SS+TR.
For each increase of 10 mg/dl in maternal blood glucose following glucose challenge testing, we observed no effect on offspring BMI z-score (−0.01 units, 95% CI: −0.03, 0.01, P = 0.5), SS/TR (0.003, 95% CI: −0.001, 0.006, P = 0.13), SS+TR (0.03 mm, 95% CI: −0.07, 0.12, P = 0.6) or SBP (0.02 mm Hg, 95% CI: −0.19, 0.23, P = 0.88) in fully adjusted models.
Discussion
In this study of more than 1,000 children, children with intrauterine exposure to gestational diabetes had increased overall adiposity and higher SBP at age 3 years. GDM was associated with child adiposity as represented by the sum of skinfolds but not by BMI z-score. The association of GDM with child SBP was independent of birth weight and attained BMI. However, after adjustment for skinfold thickness, this association was attenuated and no longer significant, highlighting a possible mediation of the effect of GDM on SBP by the effects of GDM on adiposity.
These results suggest that BMI alone may not be a sensitive enough measure of adiposity to detect the milder effects of exposure to GDM in younger children. BMI is easily measured and thus useful in clinical settings, in large epidemiological studies, or when outcomes are by self-report, but BMI is only a surrogate measure of adiposity as it incorporates lean body mass as well as fat mass. As compared with BMI, skinfold thickness is an estimate more strongly correlated with adiposity measured with gold-standard methods such as dual-energy X-ray absorptiometry (DXA) or densitometry.27–29
Maternal diabetes results in fetal hyperglycemia which, in turn, induces fetal hyperinsulinemia. Fetal hyperinsulinemia during critical periods of fetal development might induce insulin and leptin resistance and fat cell overgrowth, which may increase risk of obesity in postnatal life.4–6 Our results suggest the mechanism of the effect of GDM on childhood adiposity is not entirely mediated through increased birth weight, but that GDM may have delayed influence that strengthens with time.
We add to a small body of literature suggesting that exposure to gestational diabetes increases offspring blood pressure. The previous few studies have been small, and studied older children. Cho et al.7 found an 8 mm Hg higher SBP in a multiethnic population of 99 offspring of diabetic mothers aged 10–16 years, compared to 80 offspring of nondiabetic mothers. Rostand et al.9 adjusted their estimates for child height and maternal socioeconomic factors and found that SBP was 8 mm Hg higher among black and white children in a small population of offspring of diabetic mothers (n = 10), compared to the those born to nondiabetic mothers (n = 252). Investigators in these two studies did not account for any measure of attained adiposity. Bunt et al. adjusted for percent body fat and gender and found an 11 mm Hg higher SBP in 22 Pima Indian children aged 7–11 years born to mothers with DM, compared to 20 offspring of nondiabetic Pima mothers.8
In this study, we adjusted for a wider variety of potential confounders than these previous studies. After adjustment for paternal hypertension and maternal blood pressure, maternal family history of diabetes, parity, smoking history, and socioeconomic factors, intrauterine exposure to GDM remained associated with higher offspring blood pressure. Compared to previous studies, we noted a smaller effect of GDM on offspring blood pressure, with CIs that crossed 0 after adjustment for the sum of skinfold thickness. The other previous studies did not adjust for validated measures of adiposity which may be the reason for their stronger direct effects. One additional possibility may be that our study focused on younger children. The effect of gestational diabetes on offspring blood pressure may start out small and then strengthen over time, similar to associations with adiposity.9 However, children with higher blood pressures, even within the normal range, tend to maintain higher blood pressures over time and are at increased risk of adult hypertension.30–32
Alternatively, glycemic control may have been better in our population compared to those in previous studies, which did not detail the level of glycemic control the mothers were able to maintain during their pregnancies. Tight glycemic control after diagnosis of GDM may ameliorate childhood risk for childhood overweight.33 We speculate that the treatment of GDM may ameliorate the risk of higher blood pressure as well. It is also possible that tight glycemic control among these mothers who were diagnosed and treated for GDM accounted for the lack of association between GDM and fetal growth in the study population, whereas IGT was associated with increased fetal growth.
The physiologic mechanisms by which maternal diabetes during pregnancy might influence offspring blood pressure are not known. The association is likely mediated at least in part by increases in adiposity. In our population, adjustment for the sum of skinfold thickness attenuated the association of GDM and SBP. In addition, animal and human studies suggest a direct influence of hyperglycemia or hyperinsulinemia on the kidneys leading to altered blood pressure regulation. This kidney effect has been seen in high-risk ethnicities; among 503 Pima adults, the odds of having elevated urinary albumin excretion was nearly four times greater among those exposed to diabetes in utero compared with unexposed individuals.34 Pregnant rats experimentally exposed to hyperglycemia, even with minor elevations in serum glucose, had offspring with up to 1/3 fewer nephrons.35 In addition to elevated glucose levels, women with GDM have altered lipid profiles including higher triglyceride and low-density lipoprotein cholesterol, and lower high-density lipoprotein cholesterol, which may influence offspring blood pressure through other mechanisms.7
Our study population was larger than in previous studies, and we accounted for a greater number of potential confounding and pathway variables. Other notable strengths included clinical assessment of glucose tolerance according to current guidelines and research standard outcome measurements. Our results may not be generalizable to all American mothers, as participants were generally well educated and somewhat older than in many populations. However, the prevalence of GDM in our Project Viva cohort was 4.3%, similar to the estimated national prevalence of 2–5%,36 and predictors of GDM in Project Viva were similar to those reported in other study populations.37,38 Child anthropometry and blood pressure measures were also similar to those measured in reference populations.20,25 We collected all blood pressure measurements on a single study day, which may not reflect true baseline blood pressure. However, such imprecision in outcome measurement would likely bias results toward the null.
In summary, we found that intrauterine exposure to GDM was associated with a small increase in adiposity at age 3 years. We also observed a direct association of GDM with SBP that may be mediated, at least in part, by increased adiposity. With the prevalence of GDM on the rise, an increasing number of children in the United States are born following intrauterine exposure. Adverse effects of GDM on children may start earlier than previously thought and may strengthen as the child grows. Greater adiposity and higher blood pressure in these children may herald increased cardiovascular risk throughout the life span.
Acknowledgment
This project was supported by grants from the National Institutes of Health (HD 34568, OK STET 64925, HL), and by the March of Dimes Birth Defects Foundation.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29:1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 2.Aerts L, Holemans K, Van Assche FA. Maternal diabetes during pregnancy: consequences for the offspring. Diabetes Metab Rev. 1990;6:147–167. doi: 10.1002/dmr.5610060303. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen J, Bojsen-Moller B, Poulsen H. Blood sugar in newborn infants of diabetic mothers. Acta Endocrinol. 1954;15:33–52. doi: 10.1530/acta.0.0150033. [DOI] [PubMed] [Google Scholar]
- 4.Pettitt DJ, Knowler WC. Long-term effects of the intrauterine environment, birth weight, and breast-feeding in Pima Indians. Diabetes Care. 1998;21 Suppl 2:B138–B141. [PubMed] [Google Scholar]
- 5.Silverman BL, Rizzo T, Green OC, Cho NH, Winter RJ, Ogata ES, Richards GE, Metzger BE. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40 Suppl 2:121–125. doi: 10.2337/diab.40.2.s121. [DOI] [PubMed] [Google Scholar]
- 6.Gillman MW, Rifas-Shiman SL, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111:e221–e226. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 7.Cho NH, Silverman BL, Rizzo TA, Metzger BE. Correlations between the intrauterine metabolic environment and blood pressure in adolescent offspring of diabetic mothers. J Pediatr. 2000;136:587–592. doi: 10.1067/mpd.2000.105129. [DOI] [PubMed] [Google Scholar]
- 8.Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin A(1)c and systolic blood pressure in pima Indian children. J Clin Endocrinol Metab. 2005;90:3225–3229. doi: 10.1210/jc.2005-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rostand SG, Cliver SP, Goldenberg RL. Racial disparities in the association of foetal growth retardation to childhood blood pressure. Nephrol Dial Transplant. 2005;20:1592–1597. doi: 10.1093/ndt/gfh833. [DOI] [PubMed] [Google Scholar]
- 10.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 11.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 12.World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. [PubMed] [Google Scholar]
- 13.Gestational Diabetes Mellitus. Diabetes Care. 2004;27:88S–90S. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;8:1–27. [PubMed] [Google Scholar]
- 15.Goran MI, Kaskoun M, Shuman WP. Intra-abdominal adipose tissue in young children. Int J Obes Relat Metab Disord. 1995;19:279–283. [PubMed] [Google Scholar]
- 16.Shorr I. How to Weigh and Measure Children. New York: United Nations; 1986. [Google Scholar]
- 17.Mueller WH, Martorell R. Reliability and accuracy of measurement. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. pp. 83–86. [Google Scholar]
- 18.Gillman MW, Cook NR. Blood pressure measurement in childhood epidemiological studies. Circulation. 1995;92:1049–1057. doi: 10.1161/01.cir.92.4.1049. [DOI] [PubMed] [Google Scholar]
- 19.Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13:2021–2028. doi: 10.1038/oby.2005.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oken E, Kleinman KP, Rich-Edwards JW, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 22.Bo S, Menato G, Bardelli C, Lezo A, Signorile A, Repetti E, Massobrio M, Pagano G. Low socioeconomic status as a risk factor for gestational diabetes. Diabetes Metab. 2002;28:139–140. [PubMed] [Google Scholar]
- 23.Thorpe LE, Berger D, Ellis JA, Bettegowda VR, Brown G, Matte T, Bassett M, Frieden TR. Trends and racial/ethnic disparities in gestational diabetes among pregnant women in New York City, 1990–2001. Am J Public Health. 2005;95:1536–1539. doi: 10.2105/AJPH.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 25.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 26.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 27.Freedman DS, Wang J, Ogden CL, Thornton JC, Mei Z, Pierson RN, Dietz WH, Horlick M. The prediction of body fatness by BMI and skinfold thicknesses among children and adolescents. Ann Human Biol. 2007;34:183–194. doi: 10.1080/03014460601116860. [DOI] [PubMed] [Google Scholar]
- 28.Mei Z, Grummer-Strawn LM, Wang J, Thornton JC, Freedman DS, Pierson RN, Jr, Dietz WH, Horlick M. Do skinfold measurements provide additional information to body mass index in the assessment of body fatness among children and adolescents? Pediatrics. 2007;119:e1306–e1313. doi: 10.1542/peds.2006-2546. [DOI] [PubMed] [Google Scholar]
- 29.Steinberger J, Jacobs DR, Raatz S, Moran A, Hong CP, Sinaiko AR. Comparison of body fatness measurements by BMI and skinfolds vs dual energy X-ray absorptiometry and their relation to cardiovascular risk factors in adolescents. Int J Obes (Lond) 2005;29:1346–1352. doi: 10.1038/sj.ijo.0803026. [Erratum appears in. Int J Obes (Lond) 2006; 30:1170] [DOI] [PubMed] [Google Scholar]
- 30.Webber LS, Cresanta JL, Croft JB, Srinivasan SR, Berenson GS. Transition of cardiovascular risk factors from adolescence to young adulthood—the Bogalusa Heart Study: II. Alteration in anthropometric, blood pressure, and serum lipoprotein variables. J Chronic Dis. 1986;39:91–103. doi: 10.1016/0021-9681(86)90065-2. [DOI] [PubMed] [Google Scholar]
- 31.Wattigney WA, Webber LS, Srinivasan SR, Berenson GS. The emergence of clinically abnormal levels of cardiovascular disease risk factor variables among young adults: the Bogalusa Heart Study. Prev Med. 1995;24:617–626. doi: 10.1006/pmed.1995.1097. [DOI] [PubMed] [Google Scholar]
- 32.Gillman MW, Cook NR, Rosner B, Evans DA, Keough ME, Taylor JO, Hennekens CH. Identifying children at high risk for the development of essential hypertension. J Pediatr. 1993;122:837–846. doi: 10.1016/s0022-3476(09)90005-1. [DOI] [PubMed] [Google Scholar]
- 33.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles M-A, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–2292. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 34.Nelson RG, Morgenstern H, Bennett PH. Intrauterine diabetes exposure and the risk of renal disease in diabetic Pima Indians. Diabetes. 1998;47:1489–1493. doi: 10.2337/diabetes.47.9.1489. [DOI] [PubMed] [Google Scholar]
- 35.Amri K, Freund N, Vilar J, Merlet-Benichou C, Lelievre-Pegorier M. Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes. 1999;48:2240–2245. doi: 10.2337/diabetes.48.11.2240. [DOI] [PubMed] [Google Scholar]
- 36.American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol. 2001;98:525–538. [PubMed] [Google Scholar]
- 37.Radesky JS, Oken E, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Gillman MW. Diet during early pregnancy and development of gestational diabetes. Paediatr Perinat Epidemiology. 2008;22:47–59. doi: 10.1111/j.1365-3016.2007.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, Stampfer MJ, Speizer FE, Spiegelman D, Manson JE. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278:1078–1083. [PubMed] [Google Scholar]