Abstract
OBJECTIVE
The goal was to examine the associations of weight-for-length at birth and at 6 months with obesity at 3 years of age.
METHODS
We studied 559 children in Project Viva, an ongoing, prospective, cohort study of pregnant women and their children. We measured length and weight at birth, 6 months, and 3 years. Our main exposures were weight-for-length z score at birth adjusted for gestational age and weight-for-length z score at 6 months adjusted for weight-for-length z score at birth. We used multivariate regression analyses to predict the independent effects of birth weight-for-length z score and, separately, 6-month weight-for-length z score on BMI z score, the sum of subscapular and triceps skinfold thicknesses, and obesity (BMI for age and gender of ≥95th percentile) at age 3.
RESULTS
Mean weights at birth, 6 months, and 3 years were 3.55, 8.15, and 15.67 kg, respectively. Corresponding lengths were 49.9, 66.9, and 97.4 cm. At 3 years, 48 children (9%) were obese. After adjustment for confounding variables and birth weight-for-length z score, each increment in 6-month weight-for-length z score was associated with higher BMI z scores, higher sums of subscapular and triceps skinfold thicknesses, and increased odds of obesity at age 3. The predicted obesity prevalence among children in the highest quartiles of both birth and 6-month weight-for-length z scores was 40%, compared with 1% for children in the lowest quartiles of both. Whereas birth weight-for-length z scores were associated with higher BMI z scores, the magnitude of effect was smaller than that of weight-for-length z scores at 6 months.
CONCLUSIONS
More-rapid increases in weight for length in the first 6 months of life were associated with sharply increased risk of obesity at 3 years of age. Changes in weight status in infancy may influence risk of later obesity more than weight status at birth.
Keywords: obesity, early infancy, weight for length, birth size
During the past 30 years, the prevalence of overweight among children in the United States has increased dramatically.1,2 Rapid weight gain during the first weeks or months of infancy predicts obesity3–10 and higher blood pressure11,12 later in childhood and adulthood. Preventive interventions beginning in infancy may help avoid lifetime complications of excess weight.
More than 2 dozen studies have addressed the association between birth weight and later obesity, and almost all found that higher birth weight was associated with higher attained BMI in childhood and adulthood.7,10 Most of those studies, however, either collected gestational age data retrospectively or did not adjust for gestational age. It is important to distinguish the relative contributions of fetal growth and length of gestation, because the 2 have different determinants and sequelae13 and may suggest different causes (ie, the impact of prematurity versus nutritional or hormonal “fetal programming”).
Previous studies addressing size at birth, infant growth, and later obesity were limited by their reliance on weight measures alone.3,4,8–10 Measures of size that include length or height in addition to weight reflect adiposity better than does weight alone14 and thus may be more informative regarding future obesity risk. In addition, few studies examined possible confounders of the relationship between rapid infant growth and later obesity, including prenatal factors such as maternal prepregnancy BMI and smoking. Few studies examined whether there were differences in the relationship between rapid infant growth and later obesity for boys and girls. Patterns of growth in infancy vary between boys and girls, with boys tending to gain weight and height in infancy more rapidly than girls.15 The purpose of this study was to examine the extent to which weight-for-length (WFL) at birth and WFL from birth to 6 months of age are associated with obesity at 3 years of age.
METHODS
Study Design and Participants
Study subjects were participants in Project Viva, a prospective, observational, cohort study of gestational diet, pregnancy outcomes, and offspring health.16 Details of recruitment and retention procedures are available else-where. 16 Of the 2128 women who delivered a live infant, 1579 were eligible for 3-year follow-up assessments on the basis of having completed prenatal nutritional assessments and providing consent for their children to undergo follow-up monitoring. We collected follow-up information for 1401 children (89% of 1579 children), with in-person examinations for 1292 (82%). For this analysis, we excluded 1 participant who was missing birth weight data and 534 who were missing birth length data. The primary reason for missing length data was that we did not attempt to obtain newborn measurements for infants who were born on weekends. In addition, we excluded 164 participants who were missing 6-month WFL z scores and 34 who were missing 3-year BMI data, which yielded a cohort of 559 mother-child pairs for analysis. The human subjects committees of Harvard Pilgrim Health Care, Brigham and Women’s Hospital, and Beth Israel Deaconess Medical Center approved the study protocols.
Measurements
Main Exposures
We abstracted birth weight data from the medical records. We calculated gestational ages from the last menstrual period; if the estimate of gestational age from the second-trimester ultrasound assessment differed by >10 days, then we used the ultrasound determination. We determined birth WFL z scores by using US national reference data.17 Project staff members weighed infants at 6 months and 3 years of age with a digital scale (model 881; Seca, Hamburg, Germany) and measured infant length at birth and 6 months of age and infant height at 3 years of age with a Shorr measuring board (Shorr Productions, Olney, MD). We calculated age- and gender- specific WFL, weight-for-age (WFA), length-for-age (LFA), and BMI z scores by using US national reference data.18
Our main exposures were birth WFL z score adjusted for gestational age z score and WFL z score at 6 months of age adjusted for WFL z score at birth. We refer to this expression as the change in WFL z score from birth to 6 months, because it is algebraically identical to the change in WFL z score from birth to 6 months of age adjusted for the birth WFL z score. As secondary exposures, we also examined the change in WFA z score and the change in LFA z score from birth to 6 months of age.
Outcome Measures
Our main outcomes at 3 years of age were the age- and gender-specific BMI z score, the sum of subscapular and triceps skinfold thicknesses, and obesity. We measured subscapular and triceps skinfold thicknesses by using Holtain calipers (Holtain, Crosswell, United Kingdom) and calculated the sum of the 2 thicknesses. We defined obesity as BMI for age and gender of ≥95th percentile.19 Research assistants performing all measurements followed standardized techniques20 and participated in biannual in-service training to ensure measurement validity (I. J. Shorr, MPS personal oral communication, 2004–2007).
Other Measures
Mothers reported information about maternal age, height, weight, education, household income, smoking during pregnancy, duration of breastfeeding, and paternal (biological father) height and weight. At the first-trimester study visit, we asked mothers to report their weights just before they became pregnant. We calculated total weight gain by subtracting prepregnancy weight from the last prenatal weight. In analyses, we expressed gestational weight gain in categories based on the 1990 recommendations of the Institute of Medicine.21 We assessed maternal diet at both the first and second trimesters of pregnancy by using a validated, 166-item, semiquantitative, food frequency questionnaire.22 We calculated intakes of docosahexaenoic acid, eicosapentaenoic acid, α-linolenic acid, arachidonic acid, total n−3 polyunsaturated fatty acids, and total n−6 polyunsaturated fatty acids for each food frequency questionnaire, and the mean of the first and second trimester values was the assigned covariate for each woman during pregnancy. We defined hypertensive disorders during pregnancy according to published standards.23 Definitions of glucose tolerance status were described elsewhere.24 At 6 months after delivery, mothers also reported the number of hours their children slept in a 24-hour period.
Statistical Analyses
We first examined the bivariate relationships of our main exposures with other covariates and the main outcomes. After testing our assumption of linearity, we used multiple linear and logistic regression models to assess the independent effects of birth WFL z score and, separately, the change in WFL z score from birth to 6 months of age on our main outcomes. In multivariate models, we included only those covariates that were of a priori interest or confounded associations of birth WFL z score or change in WFL z score from birth to 6 months of age with child adiposity at 3 years of age. Adjustment for maternal intake of n−3 and n−6 polyunsaturated fatty acids during pregnancy, hypertensive disorders of pregnancy, and gestational diabetes status did not change the estimates; therefore, these factors were not included in the final model. Model 1 included only child age and gender. Multivariate model 2 also included maternal and child sociodemographic variables. In multivariate model 3, we also adjusted for gestational weight gain, smoking during pregnancy, maternal prepregnancy BMI, and paternal BMI. Finally, because breastfeeding and sleep duration might be confounders or intermediates, we also adjusted for breastfeeding status and child’s sleep duration at 6 months of age.
To assess effect modification according to birth size, we grouped WFL z scores at birth and at 6 months of age into quartiles, and we used parameter estimates from our multivariate model to estimate the predicted probability of obesity at 3 years of age for each of the resulting 16 categories. All models were fit separately for boys and girls; we combined results and adjusted for gender if gender-specific estimates were similar. We also tested multiplicative interaction terms in the models to determine whether birth size and, separately, gender modified the relationship between the change in WFL z score from birth to 6 months of age and obesity at 3 years of age. We performed data analyses with SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
Sample Characteristics
The mean weights at birth, 6 months, and 3 years were 3.55, 8.15, and 15.67 kg, respectively. Corresponding lengths were 49.9, 66.9, and 97.4 cm. WFL z scores (mean ± SD) were 0.47 ± 0.77 at birth and 0.70 ± 0.96 at 6 months of age. By 3 years of age, 9% of children had BMI values of ≥95th percentile for their age and gender. Other participant characteristics are shown in Table 1.
TABLE 1.
Characteristics of 559 Mother-Infant Pairs in Project Viva, According to Infants’ 6-Month WFL z Score Quartiles
| Overall | 6-mo WFL z Score Quartile | P for Trend | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| N | 559 | 139 | 140 | 140 | 140 | |
| Mean z score | −0.53 | 0.39 | 1.05 | 1.89 | ||
| Maternal characteristics | ||||||
| Maternal age at enrollment, mean ± SD, y | 32.8 ± 5.0 | 33.0 ± 4.6 | 32.3 ± 5.1 | 32.6 ± 5.2 | 33.1 ± 5.1 | .82 |
| Prepregnancy BMI, mean ± SD, kg/m2 | 24.4 ± 4.9 | 23.3 ± 4.1 | 24.3 ± 4.7 | 24.6 ± 4.6 | 25.5 ± 5.8 | .0002 |
| Gestational weight gain, mean ± SD, kg | 15.5 ± 5.4 | 15.4 ± 5.9 | 15.7 ± 4.7 | 15.5 ± 5.4 | 15.5 ± 5.7 | .99 |
| Excessive gestational weight gain (Institute of Medicine category), % (n) | 50 (276) | 49 | 52 | 51 | 47 | .48 |
| Mother smoked during index pregnancy, % (n) | 10 (53) | 6 | 11 | 11 | 11 | .97 |
| Maternal diet during pregnancy, mean ± SD, g/d | ||||||
| Docosahexaenoic acid plus eicosapentaenoic acid | 0.17 ± 0.21 | 0.19 ± 0.20 | 0.16 ± 0.13 | 0.16 ± 0.31 | 0.15 ± 0.11 | .11 |
| α-Linolenic acid | 0.97 ± 0.33 | 0.99 ± 0.36 | 0.95 ± 0.35 | 0.95 ± 0.31 | 0.98 ± 0.32 | .77 |
| Arachidonic acid | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.10 ± 0.03 | .94 |
| Total n−3 polyunsaturated fatty acids | 1.15 ± 0.40 | 1.19 ± 0.39 | 1.13 ± 0.36 | 1.12 ± 0.49 | 1.14 ± 0.36 | .26 |
| Total n−6 polyunsaturated fatty acids | 12.2 ± 2.7 | 12.3 ± 2.9 | 12.1 ± 2.7 | 12.2 ± 2.8 | 12.1 ± 2.6 | .63 |
| Glucose tolerance status, % (n) | ||||||
| Impaired glucose tolerance | 12 (67) | 13 | 11 | 14 | 10 | .41 |
| Gestational diabetes mellitus | 4 (22) | 6 | 3 | 3 | 4 | |
| Hypertensive disorder of pregnancy, % (n) | ||||||
| Chronic hypertension | 0.7 (4) | 0 | 0.7 | 0.7 | 1.5 | .08 |
| Gestational hypertension | 8 (41) | 5 | 7 | 9 | 9 | |
| Preeclampsia | 3 (15) | 4 | 1 | 1 | 4 | |
| Nulliparous, % (n) | 48 (266) | 52 | 42 | 46 | 50 | .96 |
| Annual household income of less than $70 000, % (n) | 33 (184) | 28 | 33 | 36 | 35 | .03 |
| College graduate or more, % (n) | 74 (415) | 81 | 69 | 75 | 73 | .27 |
| Paternal BMI, mean ± SD, kg/m2 | 26.3 ± 3.7 | 26.3 ± 4.2 | 25.7 ± 3.0 | 26.2 ± 3.0 | 27.0 ± 4.2 | .05 |
| Child and home environment | ||||||
| Male, % (n) | 49 (276) | 50 | 49 | 49 | 49 | .96 |
| White, % (n) | 72 (400) | 68 | 65 | 85 | 69 | .18 |
| Birth characteristics | ||||||
| Birth weight, mean ± SD, kg | 3.55 ± 0.49 | 3.55 ± 0.50 | 3.48 ± 0.46 | 3.55 ± 0.46 | 3.64 ± 0.54 | .06 |
| Birth WFL z score, mean ± SD | 0.47 ± 0.77 | 0.32 ± 0.80 | 0.34 ± 0.71 | 0.51 ± 0.70 | 0.73 ± 0.79 | <.0001 |
| Gestational age at birth, mean ± SD, wk | 39.8 ± 1.4 | 39.8 ± 1.36 | 39.7 ± 1.39 | 39.8 ± 1.44 | 39.7 ± 1.37 | .94 |
| Birth weight-for-gestational age z score (fetal growth), mean ± SD | 0.26 ± 0.93 | 0.23 ± 0.95 | 0.12 ± 0.87 | 0.26 ± 0.88 | 0.43 ± 1.01 | .04 |
| Infant feeding at 6 mo, % (n) | ||||||
| Any breast milk feeding at 6 mo | 54 (301) | 66 | 55 | 47 | 47 | .0006 |
| Introduction of solids at <4 mo | 14 (74) | 10 | 19 | 10 | 17 | .44 |
| Sleep duration at 6 mo, mean ± SD, h/d | 12.2 ± 1.8 | 12.1 ± 1.9 | 12.5 ± 1.9 | 12.2 ± 2.7 | 12.1 ± 1.7 | .48 |
| Age 3 anthropometric characteristics | ||||||
| BMI z score, mean ± SD | 0.44 ± 1.0 | −0.15 ± 1.0 | 0.13 ± 0.9 | 0.60 ± 0.8 | 1.16 ± 0.9 | <.0001 |
| Sum of subscapular and triceps skinfold thicknesses, mean ± SD, mm | 16.6 ± 4.2 | 15.4 ± 3.5 | 15.9 ± 3.9 | 17.0 ± 4.3 | 18.4 ± 4.3 | <.0001 |
| BMI of ≥95th percentile, % (n) | 9 (48) | 1 | 3 | 9 | 21 | <.0001 |
In bivariate analyses, children with higher 6-month WFL z scores were more likely to have mothers with higher prepregnancy BMI values and to have higher birth WFL and were less likely to be breastfed (Table 1). Maternal intake of n−3 and n−6 polyunsaturated fatty acids during pregnancy, glucose tolerance status, and hypertensive disorders of pregnancy were not associated with 6-month WFL z scores.
WFL at Birth
In age-, gender-, and gestational age-adjusted multivariate models, we observed a linear association between birth WFL z scores (in quartiles) and BMI z scores at 3 years of age. Compared with children in the lowest quartile of birth WFL z scores, those in the highest quartile had higher BMI z scores at 3 years of age (β = 0.51 [95% confidence interval [CI]: 0.28–0.75]). In multivariate models adjusted for confounding variables, each 1-unit increase in birth WFL z scores (continuous) was associated with slightly higher BMI z scores (β = 0.17 [95% CI: 0.06–0.28]) and with increased odds of obesity (odds ratio [OR]: 1.58 [95% CI: 0.99–2.53]) at 3 years of age. Each 1-unit increase in birth WFL z scores was not associated with the sums of subscapular and triceps skin-fold thicknesses at 3 years of age (β = 0.04 [95% CI: −0.42 to 0.56]).
Change in WFL From Birth to 6 Months
In multivariate models adjusted for confounding variables and birth WFL z scores, we observed a direct association of 6-month WFL z scores, expressed in quartiles, with BMI z scores, sums of subscapular and triceps skin-fold thicknesses, and obesity at 3 years of age (Table 2). Table 3 shows the association of our 3-year anthropometric outcomes with infants’ WFL z scores at 6 months, expressed as a 1-unit increase rather than in quartiles. In models adjusted for confounding variables and birth WFL z scores, each increment in 6-month WFL z scores was associated with higher BMI z scores (β = 0.51 [95% CI: 0.43–0.59]), greater sums of subscapular and triceps skinfold thicknesses (β = 1.30 [95% CI: 0.93–1.67]), and increased odds of obesity (OR: 6.84 [95% CI: 3.84–12.19]) at 3 years of age (Table 3). Adjustment for maternal and child socioeconomic characteristics, as well as breastfeeding status and infant sleep duration at 6 months of age, did not change the unadjusted estimates substantially (Table 3).
TABLE 2.
Associations of Quartiles of 6-Month WFL z Score Adjusted for WFL z Score at Birth With Risk of Clinical Outcomes at 3 Years of Age
| Clinical Outcomes at 3 y | Quartile of 6-mo WFL z Score Adjusted for WFL z Score at Birtha | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| β (95% CI) | ||||
| BMI z score | ||||
| Age and gender adjusted | 0.00 (reference) | 0.28 (0.07–0.49) | 0.75 (0.54–0.96) | 1.29 (1.08–1.50) |
| Multivariate modelb | 0.00 (reference) | 0.21 (−0.01 to 0.42) | 0.68 (0.46–0.89) | 1.16 (0.94–1.37) |
| Sum of subscapular and triceps skinfold thicknesses | ||||
| Age and gender adjusted | 0.00 (reference) | 0.63 (−0.28 to 1.55) | 1.73 (0.81–2.65) | 3.14 (2.20–4.08) |
| Multivariate model | 0.00 (reference) | 0.51 (−0.45 to 1.47) | 1.35 (0.38–2.32) | 2.95 (1.96–3.94) |
| OR (95% CI) | ||||
| BMI of ≥95th percentilec | ||||
| Age and gender adjusted | 1.00 (reference) | 1.99 (0.36–11.12) | 7.54 (1.63–34.92) | 28.37 (6.42–125.43) |
| Multivariate model | 1.00 (reference) | 1.86 (0.29–12.03) | 8.31 (1.44–47.92) | 33.17 (6.19–177.68) |
Results are from multivariate analyses of data for infants participating in Project Viva.
All trend P values are <.0001.
The multivariate model adjusted for child’s gender, age, and race/ethnicity; mother’s age, prepregnancy BMI, gestational weight gain, smoking history, education, parity, and household income; and father’s BMI.
The comparison group was children with BMI values in the 5th to <85th percentiles.
TABLE 3.
Changes in 3-Year Obesity-Related Outcomes for Each 1-Unit Increment in WFL z Score at 6 Months of Age Adjusted for WFL z Score at Birth
| Model | β (95% CI) | OR (95% CI), BMI of ≥95th Percentilea | |
|---|---|---|---|
| BMI z Score | Sum of Subscapular and Triceps Skinfold Thicknesses | ||
| Model 1: child’s age and gender | 0.54 (0.46–0.62) | 1.38 (1.03–1.72) | 5.52 (3.48–8.75) |
| Model 2: model 1 plus maternal age, education, income, and parity and child’s race/ethnicity | 0.54 (0.46–0.62) | 1.36 (1.02–1.71) | 5.85 (3.59–9.52) |
| Model 3: model 2 plus gestational weight gain, maternal smoking, maternal prepregnancy BMI, and paternal BMI | 0.51 (0.43–0.59) | 1.30 (0.93–1.67) | 6.84 (3.84–12.19) |
Results are from multivariate analyses of data for 559 mother-child pairs participating in Project Viva.
The comparison group was children with BMI values in the 5th to <85th percentiles
We also examined the change in WFA z scores and the change in LFA z scores from birth to 6 months of age. Compared with the WFL results, we observed slightly smaller magnitudes of effect for WFA. In the final multivariate models (model 3), each increment in 6-month WFA z scores (adjusted for birth WFA z score) was associated with higher BMI z scores (β = 0.47 [95% CI: 0.40–0.53]), higher sums of subscapular and triceps skinfold thicknesses (β = 1.06 [95% CI: 0.77–1.35]), and increased odds of obesity (OR: 5.54 [95% CI: 3.77–8.16]) at 3 years of age. In both gender-specific and combined analyses, changes in LFA from birth to 6 months were not associated with our anthropometric outcomes at age 3; each increment in LFA z scores at 6 months was associated with a 0.01 (95% CI: −0.11 to 0.14) higher BMI z score at 3 years of age.
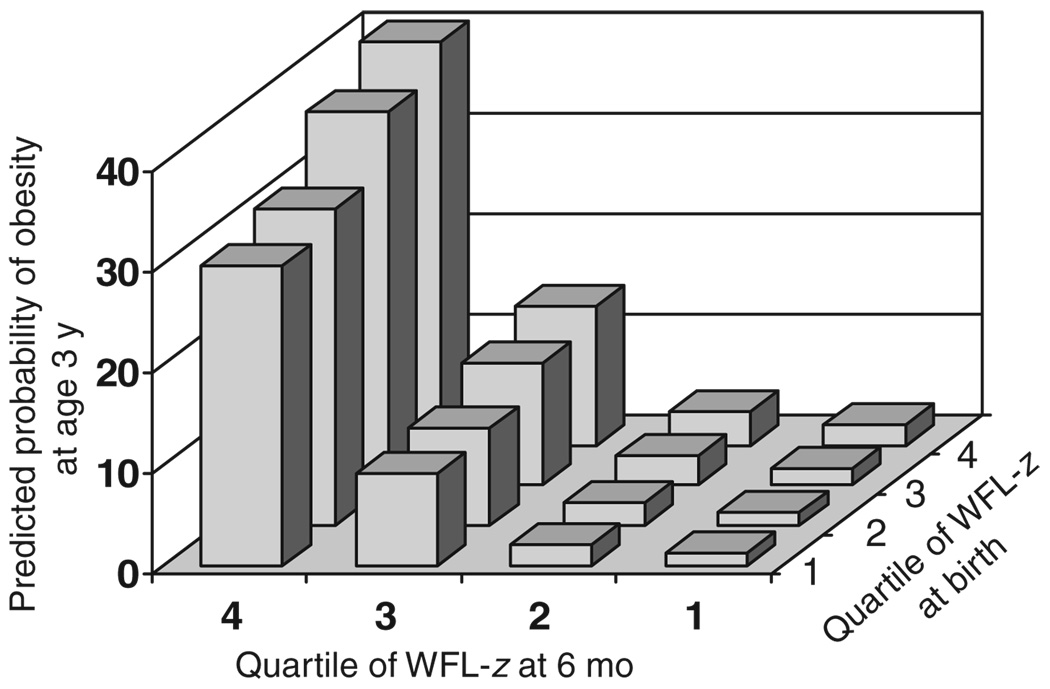
In Fig 1, we show the covariate-adjusted predicted probability of 3-year obesity according to WFL z score quartiles at birth and at 6 months of age. Predicted obesity prevalence among children in the highest quartiles of both birth and 6-month WFL z scores was 40%, compared with 1% among children in the lowest quartiles of both. WFL at 6 months was more strongly related to risk of obesity than was WFL at birth (Fig 1). The association between WFL at 6 months and obesity at 3 years did not vary substantially according to birth size (P for interaction = .92).
FIGURE 1.
Predicted probability of obesity (BMI of ≥95th percentile) at 3 years of age according to quartile of WFL z score at birth and at 6 months of age, with adjustment for maternal smoking status, gestational weight gain, education, household income, parity, age, and prepregnancy BMI, paternal BMI, and child age, gender, and race/ethnicity.
In gender-specific analyses, mean ± SD WFL z scores at birth were 0.38 ± 0.81 for boys and 0.57 ± 0.72 for girls. At 6 months, WFL z scores were 0.78 ± 1.01 for boys and 0.63 ± 0.90 for girls. At 3 years of age, 11% of boys and 7% of girls were obese. Despite these differences in exposure and outcome frequency, gender-stratified multivariate analyses of effects of infant gains in adiposity on 3-year outcomes showed no differences between boys and girls. For example, in multivariate models adjusted for confounders and birth WFL z scores, the odds of obesity at 3 years of age were 7.62 (95% CI: 3.44–16.92) for boys and 7.53 (95% CI: 2.14–26.48) for girls for each increment in 6-month WFL z scores (P for interaction term = .66).
DISCUSSION
In this prospective cohort study, more-rapid increases in WFL in the first 6 months of life were associated with sharply increased risk of obesity and adiposity (measured as the sum of skinfold thicknesses) at 3 years of age. We found the predicted probability of obesity at age 3 to be 40% among infants in the highest quartiles of both birth and 6-month WFL. These effects were independent of a number of potential confounders, including socioeconomic status and maternal smoking, gestational weight gain, and prepregnancy BMI. Birth WFL was only minimally associated with our anthropometric outcomes, which suggests that early interventions to prevent rapid increase in weight status in the first months of life may help reduce children’s risk of obesity later in childhood. Furthermore, the observed associations between rapid increases in weight status in the first 6 months of life and later obesity did not vary according to gender.
Our study was unique in 3 ways. First, we had research- level measures of length at birth and 6 months; most previous studies in this area were limited by their reliance on weight alone. Second, we were able to compare the effect of birth WFL adjusted for gestational age (“fetal growth”), as opposed to simply birth weight, with changes in WFL from birth to 6 months of age. Third, we were able to adjust for several prenatal and postnatal confounders, some of which were not available for previous longitudinal birth cohorts (eg, paternal BMI). Our findings are consistent with previous studies of both contemporary9,10 and historical3 cohorts and confirm the findings of 2 systematic reviews of infant growth and obesity that concluded that infants at the highest end of the weight or BMI distribution and infants who grew most rapidly (usually measured as weight gain) were more likely to be obese later in life.7,8 In the review by Baird et al,7 relative risks of later obesity ranged from 1.17 to 5.70 among infants with more-rapid weight gain in the first year of life. Our observed magnitudes of effect were larger than previously reported values, possibly because we were able to use measures of length in addition to weight, which together reflect adiposity better than weight alone. It is also possible that the relatively close measurement of exposures and outcomes contributed to our observed magnitudes of effect.
In contrast to a previous study that examined changes in length in infancy,6 we found that changes in LFA from birth to 6 months were not associated with later obesity. Changes in WFA and changes in WFL from birth to 6 months were associated with later obesity and higher adiposity, which suggests that it is rapid weight gain in infancy that puts children at risk.
In this study, we were able to examine a wide range of factors that might confound the relationship between changes in WFL in infancy and later obesity. Parental body habitus, maternal smoking during pregnancy, and gestational weight gain all confounded the relationship between changes in WFL in infancy and later obesity. Although previous studies found that gestational diabetes and glucose tolerance were associated with offspring obesity, especially for older children and adolescents,25 we did not find maternal glucose tolerance to be associated with 6-month WFL or to be a confounder of the relationship between changes in WFL in infancy and later obesity. In addition, although previous studies raised the possibility that increased maternal intake of n−3 polyunsaturated fatty acids during pregnancy might be associated with reduced adiposity in children,26–29 we did not find this factor to be a confounder.
Although breastfeeding was independently associated with a lower prevalence of obesity at age 3,30 the mode of infant feeding was not a confounder in this study. We were not able to assess fully whether breastfeeding was an intermediate of the relationship between changes in WFL in infancy and later obesity. It is possible that the quality of the infant diet after weaning also may mediate the relationship between infant weight gain and later obesity. Finally, we were not able to examine social and behavioral interactions regarding infant feeding. Hodges et al31 suggested that overfeeding and low caregiver responsiveness to child feeding cues might contribute to early infant weight gain and later obesity. Future studies should examine whether the mode of infant feeding, the quality of the infant diet after weaning, and overfeeding because of lack of parental responsiveness to infants’ satiety cues might explain the association of infant weight gain with later obesity.
When our study results are being interpreted, several limitations should be considered. First, although mothers in the study had diverse racial/ethnic backgrounds, their educational and income levels were relatively high. Our results may not be generalizable to more socioeconomically disadvantaged populations. Second, most of our measures were from self-reports, including prepregnancy weight, smoking, breastfeeding, and infant sleep, and loss to follow-up monitoring was not random. These factors might have introduced bias. Finally, our main outcome was obesity at age 3. Obesity at this age does not predict adult consequences as well as obesity later in childhood32 but can presage serious adverse health consequences in childhood itself.33
CONCLUSIONS
There is growing evidence that rapid changes in weight status during infancy may substantially increase individuals’ risk of obesity later in life. Our findings confirmed that rapid gains in WFL in the first 6 months of life were associated with sharply increased risk of later obesity. Additional studies are needed to identify the modifiable determinants of gain in adiposity in the early weeks or months of life that also underlie long-term risks of obesity-related sequelae.
The mounting evidence regarding infancy as a critical period in obesity prevention may support efforts of health professionals and public health researchers in formulating policies and interventions to reduce rapid infant weight gain. Given the increasing prevalence of childhood obesity, prevention efforts should assume new urgency in the 21st century.
What’s Known on this Subject
Rapid weight gain during the first weeks or months of infancy predicts obesity and higher blood pressure later in childhood and adulthood.
What This Study Adds
Previous studies addressing infant size and later obesity were limited by reliance on weight measures alone. We found that more-rapid increases in WFL in the first 6 months of life were associated with increased risk of obesity at 3 years.
ACKNOWLEDGMENTS
This study was supported in part by grants from the National Institutes of Health (grants HD34568, HL64925, and HL68041). Dr Taveras was supported in part by the Physician Faculty Scholars Program of the Robert Wood Johnson Foundation.
Abbreviations
- WFL
weight-for-length
- OR
odds ratio
- CI
confidence interval
- WFA
weight-for-age
- LFA
length-for-age
Footnotes
This work was presented at the Developmental Origins of Health and Disease annual meeting; November 7–10, 2007; Perth, Australia.
The authors have indicated they have no financial relationships relevant to this article to disclose.
Reprints Information about ordering reprints can be found online http://www.pediatrics.org/misc/reprints.shtml
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Troiano RP, Briefel RR, Kuczmarski RJ, Flegal KM, Johnson CL. Prevalence of overweight among preschool children in the United States, 1971 through 1994. Pediatrics. 1997;99(4) doi: 10.1542/peds.99.4.e1. Available at www.pediatrics.org/cgi/content/full/99/4/e1. [DOI] [PubMed] [Google Scholar]
- 3.Stettler N, Zemel BS, Kumanyika SK, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109(2):194–199. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 4.Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adult-hood in a cohort of African Americans. Am J Clin Nutr. 2003;77(6):1374–1378. doi: 10.1093/ajcn/77.6.1374. [DOI] [PubMed] [Google Scholar]
- 5.Eid EE. Follow-up study of physical growth of children who had excessive weight gain in first six months of life. Br Med J. 1970;2(5701):74–76. doi: 10.1136/bmj.2.5701.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunnarsdottir I, Thorsdottir I. Relationship between growth and feeding in infancy and body mass index at the age of 6 years. Int J Obes Relat Metab Disord. 2003;27(12):1523–1527. doi: 10.1038/sj.ijo.0802438. [DOI] [PubMed] [Google Scholar]
- 7.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life: a systematic review. Obes Rev. 2005;6(2):143–154. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 9.Dennison BA, Edmunds LS, Stratton HH, Pruzek RM. Rapid infant weight gain predicts childhood overweight. Obesity (Silver Spring) 2006;14(3):491–499. doi: 10.1038/oby.2006.64. [DOI] [PubMed] [Google Scholar]
- 10.Hui LL, Schooling CM, Leung SS, et al. Birth weight, infant growth, and childhood body mass index: Hong Kong’s children of 1997 birth cohort. Arch Pediatr Adolesc Med. 2008;162(3):212–218. doi: 10.1001/archpediatrics.2007.62. [DOI] [PubMed] [Google Scholar]
- 11.Belfort MB, Rifas-Shiman SL, Rich-Edwards J, Kleinman KP, Gillman MW. Size at birth, infant growth, and blood pressure at three years of age. J Pediatr. 2007;151(6):670–674. doi: 10.1016/j.jpeds.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18(7):815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kramer MS. Intrauterine growth and gestational duration determinants. Pediatrics. 1987;80(4):502–511. [PubMed] [Google Scholar]
- 14.Benn RT. Some mathematical properties of weight-for-height indices used as measures of adiposity. Br J Prev Soc Med. 1971;25(1):42–50. doi: 10.1136/jech.25.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tate AR, Dezateux C, Cole TJ. Is infant growth changing? Int J Obes (Lond) 2006;30(7):1094–1096. doi: 10.1038/sj.ijo.0803310. [DOI] [PubMed] [Google Scholar]
- 16.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144(2):240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 17.Oken E, Kleinman KP, Rich-Edwards JW, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. [Accessed June 19, 2003];2000 CDC growth charts: United States. Available at www.cdc.gov/nccdphp/dnpa/growthcharts/sas.htm. [PubMed]
- 19.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 20.Shorr IJ. How to Weigh and Measure Children. New York, NY: United Nations; 1986. [Google Scholar]
- 21.Institute of Medicine. Nutrition During Pregnancy. Washington, DC: National Academy Press; 1990. [Google Scholar]
- 22.Rifas-Shiman SL, Fawzi W, Rich-Edwards JW, Willett WC, Gillman MW. Validity of a semi-quantitative food frequency questionnaire (SFFQ) during early pregnancy. Pediatr Perinat Epidemiol. 2000;14(4):A25–A26. doi: 10.1016/j.annepidem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Roberts JM, Pearson GD, Cutler JA, Lindheimer MD. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertens Pregnancy. 2003;22(2):109–127. doi: 10.1081/PRG-120016792. [DOI] [PubMed] [Google Scholar]
- 24.Oken E, Ning Y, Rifas-Shiman SL, Radesky JS, Rich-Edwards JW, Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol. 2006;108(5):1200–1207. doi: 10.1097/01.AOG.0000241088.60745.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49(12):2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 26.McGregor JA, Allen KG, Harris MA, et al. The omega-3 story: nutritional prevention of preterm birth and other adverse pregnancy outcomes. Obstet Gynecol Surv. 2001;56(5 suppl 1):S1–S13. doi: 10.1097/00006254-200105001-00001. [DOI] [PubMed] [Google Scholar]
- 27.Elias SL, Innis SM. Infant plasma trans, n−6, and n−3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am J Clin Nutr. 2001;73(4):807–814. doi: 10.1093/ajcn/73.4.807. [DOI] [PubMed] [Google Scholar]
- 28.Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n−3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol. 2004;160(8):774–783. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madsen L, Petersen RK, Kristiansen K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim Biophys Acta. 2005;1740(2):266–286. doi: 10.1016/j.bbadis.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Taveras EM, Rifas-Shiman SL, Scanlon KS, Grummer-Strawn LM, Sherry B, Gillman MW. To what extent is the protective effect of breastfeeding on future overweight explained by decreased maternal feeding restriction? Pediatrics. 2006;118(6):2341–2348. doi: 10.1542/peds.2006-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodges EA, Hughes SO, Hopkinson J, Fisher JO. Maternal decisions about the initiation and termination of infant feeding. Appetite. 2008;50(2–3):333–339. doi: 10.1016/j.appet.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH., Jr Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 33.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360(9331):473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]