Summary
Background
Growing evidence implicates the involvement of extracellular nucleotides in the regulation of platelet, leukocyte, endothelial cell (EC) and vascular smooth muscle cell (VSMC) phenotype and function. Within the quiescent vasculature extracellular nucleotides are rapidly hydrolyzed by CD39, the dominant endothelial nucleoside triphosphate diphosphohydrolase (NTPDase-1). However, vascular CD39/NTPDase-1 activity is lost in EC activated by oxidative stress or pro-inflammatory mediators, and upon denudation of the endothelium following balloon injury. The consequent increase in extracellular nucleotide concentrations triggers signaling events leading to prothrombotic responses and increased VSMC proliferation.
Objectives
To investigate the effect of overexpressed CD39/NTPDase-1 in injured aorta.
Methods
Using adenoviral-mediated gene transfer we expressed CD39/NTPDase-1 in mechanically denudated rat aortas. We measured intima formation by morphometry and VSMC proliferation by the [3H]-thymidine incorporation assay.
Results
Targeted expression of CD39 in injured vessels increased NTPDase activity (from 2.91±0.31 to 22.07±6.7 nmols Pi/mg protein, four days after exposure to the adenovirus) and prevented the formation of neointima. The thickness of intimal layer in injured aortas exposed to Ad-CD39 was 26.2±3.9 µm versus 51.8±6.1 μm and 64.4±22.2 µm (P<0.001) in vessels treated with Ad-β-gal and saline, respectively. Moreover, targeted expression of CD39/NTPDase-1 caused a 70% (P<0.01) decrease in proliferation of VSMC isolated from transduced rat aortas as compared to VSMC derived from control vessels.
Conclusions
The presented data suggest that increasing CD39/NTPDase-1 activity in VSMC could represent a novel therapeutic approach for the prevention of stenosis associated with angioplasty and other occlusive vascular diseases.
Keywords: angioplasty, aorta injury, CD39, NTPDase, restenosis, smooth muscle cell proliferation
Introduction
Growing evidence indicates that extracellular nucleotides are involved in the development of diverse vascular pathologies, including atherosclerosis, transplant arteriosclerosis and restenosis associated with angioplasty [1–5]. It is well documented that extracellular nucleotides mediate vascular inflammation and thrombosis, both contributing to the pathogenesis of occlusive vascular diseases [6]. Extracellular nucleotides accumulate in tissue fluids and plasma as a consequence of cellular responses to pro-inflammatory cytokines and as a result of tissue damage and cell death. Nucleotides, such as ATP or ADP, may be released from a variety of injured or activated cells, including neurons, erythrocytes, platelets, endothelial cells and T lymphocytes [7]. Extracellular nucleotides are ligands for purinergic P2 receptors, which are classified into two groups, P2X (ion-gated) and P2Y (G-protein coupled) [8]. Blood concentrations of extracellular nucleotides, and consequently P2 receptor signaling, are modulated by ecto-nucleoside triphosphate diphosphohydrolases (NTPDases) and other ecto-nucleotidases on the cell surface, which hydrolyze nucleotides to nucleosides. Degradation of nucleotides terminates P2 receptor signaling and leads to generation of adenosine, which inhibits platelet activation and smooth muscle cell proliferation and stimulates endothelial cell proliferation and thus may be useful in the prevention of restenosis following balloon angioplasty [3, 4].
The main vascular ecto-NTPDase, CD39 (NTPDase-1; E.C. 3.6.1.5.), is an endothelial membrane-bound enzyme, which metabolizes ATP and ADP to AMP [9]. CD39/NTPDase-1 is recognized as a major thromboregulatory factor, as it hydrolyses and removes from the circulation ADP, a potent platelet agonist. Endothelial NTPDase activity is lost during EC activation [10], oxidative stress [9], heart allograft rejection [11] and endothelial denudation following balloon injury [12].
The mitogenic effect of extracellular ATP in VSMC has been known for more than a decade [13, 14]. More recent reports confirmed the role of ATP, as well as UTP and UDP, and their respective receptors, P2Y2, P2Y1, P2Y4 and P2Y6, in modulating VSMC proliferation and migration [1, 15–17]. Additional data suggest that ATP released from damaged EC and VSMC is sufficient to activate P2 receptors, initiating lesions of intimal hyperplasia [18].
The contribution of NTPDase-1 loss to enhanced VSMC migration, proliferation and vascular remodeling has not yet been clearly established. It is our hypothesis that decreased vascular NTPDase-1 activity may be responsible for nucleotide-induced proliferation of VSMC and therefore involved in neointima formation. To test this hypothesis we over expressed CD39/NTPDase-1 in balloon-injured rat aortas and verified whether increased NTPDase-1 activity could lower nucleotide levels and consequently inhibit local VSMC proliferation and prevent intimal hyperplasia.
Material and Methods
Adenoviral vectors
A replication defective recombinant adenovirus vector type 5 encoding human CD39/NTPDase-1 cDNA (Ad-CD39) and the control recombinant β-galactosidase adenovirus (Ad-β-gal) were generated as described [19]. Virus stocks were stored in 20–50 µl aliquots in PBS with 10% glycerol at − 80°C until used.
Aorta injury model and in vivo gene transfer
The animal experimentation protocol was reviewed and approved by the Ethical Committee of the Medical University of Warsaw. F344 male rats weighting 350 g were anesthetized with intraperitoneal injection of urethane (1 ml/100 g body weight). Vessel injury was induced by balloon angioplasty of abdominal aorta, using a 2-F arterial embolectomy cathether (Baxter Healthcare Corporation, Irvine, Ca, USA). After all lumbar arteries and the left iliac artery had been clamped, 0.1 ml of solution containing 1010 pfu/ml Ad-CD39, Ad-β-gal or saline was injected to the aortic lumen through the right iliac artery. An increased pressure was applied to expand the aortic diameter by 50%. Twenty minutes after perfusion the flow was restored and vessel contents released into the circulation.
RNA isolation and RT-PCR
Four days after balloon injury, total RNA was isolated [20] from rat aortas of control and Ad-CD39-transduced animals. Reverse transcription (RT) of RNA and PCR amplification of human CD39 cDNA fragment 1339–1672 (GenBank accession no. S73813) were carried out as described previously [21]. The following primers were used: sense - GCAAGGCTATCATTTCAC, and antisense - CACCACTGCGATGGAGGAAAT. The annealing temperature was 52.4°C.
Immunohistochemistry of rat aortas
Rat aortas of control and Ad-CD39-transduced animals were harvested four days after balloon injury, and snap-frozen in pre-cooled isopentanefor immunohistochemical staining. Five-µm cryostat sections were fixed in ice-cold acetone containing 5% (v/v) formalin for 3 min and rinsed in phosphate-buffered saline (PBS). After initial blocking with 7% horse serum diluted in PBS for 30 min at room temperature, tissues were incubated overnight at 4°C with primary mouse anti-human CD39 antibody (1 µg/ml; Zymed Laboratories, San Francisco, CA), rinsed with PBS, and then blocked with 3% H2O2 in methanol for 5 min to deplete endogenous peroxidase. Sections were incubated with biotinylated secondary antibody (horse anti-mouse, 2 µg/ml; Vector Laboratories, Burlingame, CA) for 1 h at room temperature and then incubated with Avidin-Biotin-HRP Complex (Dako) for 30 min. Staining was performed with DAB-peroxidase substrate kit (Vector).
Aorta preparations and NTPDase activity measurement
At day 0 before the procedure and at days 0, 2, 4, 8 and 12 following the balloon injury and transduction with Ad-CD39 or Ad-β-gal, 3–4 animals in each group were euthanized, and rat aortas were isolated. Tissues were washed three times with 20 mM Tris-HCl, pH 8.0, containing 50 mM NaCl (Tris-saline buffer) at 4°C, disrupted in a Potter homogenizer in Tris-saline buffer, containing 0.1 mM phenylmethylsulfonyl fluoride and aprotinin (0.02 KIU/ml), and centrifuged at 800×g for 15 min at 4°C. Supernatants were used for the measurement of NTPDase activity.
NTPDase activity of Ca2+/Mg2+-dependent CD39/NTPDase-1 was determined by measuring inorganic phosphate release from ATP, as previously described [9]. The effect of alkaline phosphatase was eliminated by its inhibition with 5 mM tetramisole (Sigma, St. Louis, MO). Aortic cell lysates were incubated with 200 µM ATP, and free phosphate release was determined over time. Malachite green was added to stop the reaction, and absorbance was measured at 610 nm to determine phosphate generation against the standard curve of KH2PO4 [22]. Protein was measured according to the Bradford method [23].
Evaluation of intima formation
At day 16 following the viral transduction, samples of aortic tissues were collected (at least 6 animals in each group) and stained with hematoxylin-eosine and orcein. Digital morphometry was performed using Nicon E400 microscope (Nicon Corporation, Japan) and LUCIA® 3.5 software (Laboratory Imaging S.R.O., Prague, Czech Republic).
Aortic smooth muscle cell isolation and cell proliferation assay
VSMC from aortas of control and adenovirus-treated animals were isolated by collagenase digestion and cultured in medium F-12 supplemented with 50 µg/ml L-ascorbic acid, 50 µg/ml streptomycin, 50 IU/ml penicillin and 10% fetal calf serum (F-12/10% FCS; GIBCO, Rockville, MD) as described [24]. Proliferation of VSMC was assessed by incorporation of [3H]-thymidine. For the assay, cells were seeded in 96-well flat-bottom plates, 5 × 103 cells per well, and cultured for 24 h in medium containing 5% FCS and 48 h in a regular culture medium. [3H]-thymidine (2 µCi/ml; Amersham Pharmacia Biotech, Little Chalfont, U.K.) was added 18 h before the termination of the cell culture, and radioactivity of the samples was measured.
Statistical Analysis
Results are presented as mean ± standard deviation (SD). Data were analyzed by one-way analyses of variance (ANOVA) followed by the post hoc Tukey multiple range test. Differences between groups were rated significant at a probability error (P) of < 0.05.
Results
Detection of human CD39/NTPDase-1 mRNA in the rat aorta following Ad-CD39 transduction
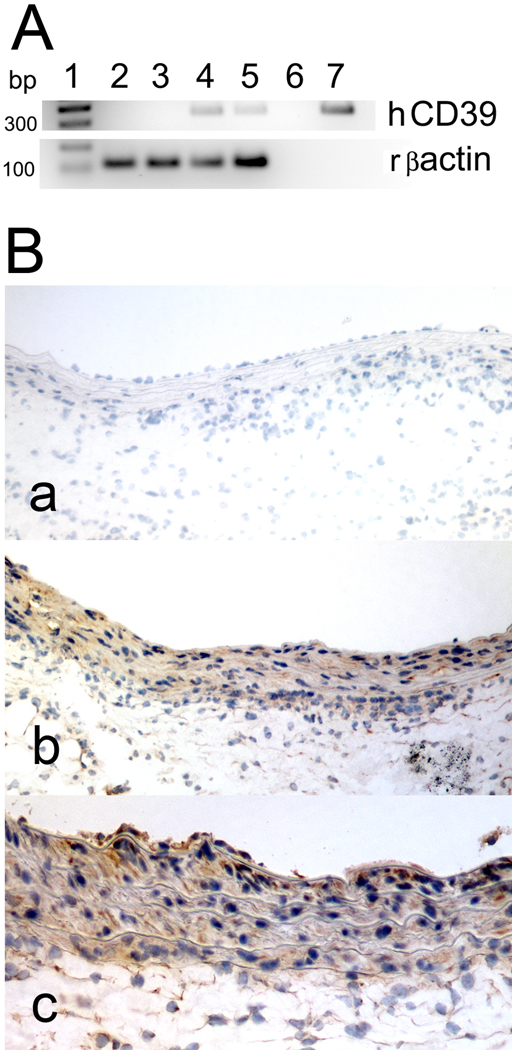
The expression of human CD39/NTPDase-1 in rat aortas was analyzed by RT-PCR. The expected fragment of human CD39 (334 bp) was detected in Ad-CD39 transduced aortas, but not in control rat aortas (Fig. 1A). mRNA for a control gene, rat β-actin, has been detected in all analyzed aortas.
Fig. 1. Transduction of rat aortas with Ad-CD39 induces expression of human CD39/NTPDase-1.
A. Agarose gel (1.8%) analysis of RT-PCR products, human CD39/NTPDase and rat β-actin, obtained from mRNA isolated from control (1, 2) and Ad-CD39-transduced (3, 4) rat aortas. 5 – negative control; 6 – positive control (human umbilical vein EC); 1 – 100-base pair ladder. B. Immunohistochemistry of control (a) and Ad-CD39-transduced aortas (b, c) using antibody to human CD39. Magnification 200× (a, b) and 400× (c). Results represent stainings of at least 2 aortas.
Detection of human CD39/NTPDase-1 protein in the rat aorta following Ad-CD39 transduction
The expression of human CD39/NTPDase-1 in rat aortas was analyzed by immunohistochemistry, using an antibody to human CD39/NTPDase-1. The staining for CD39/NTPDase-1 was detected in Ad-CD39-transduced aortas, but not in control rat aortas (Fig. 1B).
NTPDase activity in rat aorta after Ad-CD39 transduction
To confirm that Ad-CD39 transduction leads to the expression of an active protein, we measured NTPDase activity in aortic tissue. Exposure of the injured aortas to Ad-CD39 effectively increased NTPDase activity as compared to saline-treated animals (Table 1). Maximal, a 7.6-fold increase in CD39 activity was observed 4 days after the procedure and returned to initial values by day 8.
Table 1.
NTPDase activity in Ad-CD39-transduced and saline-treated aortas (nmol Pi/mg protein). Results are presented as mean±SD from 3–4 animals per group.
| Day | 0 (before BI) | 0 (after BI) | 2 | 4 | 8 | 12 |
|---|---|---|---|---|---|---|
| Ad-CD39 | 2.91±0.31 | 1.82±0.44 | 11.81±3.81 | 22.07±6.73 | 2.77±1.46 | 3.03±1.37 |
| Saline | 2.91±0.31 | 1.96±0.46 | 5.62±1.37 | 5.80±2.78 | 2.67±1.25 | 2.14±0.82 |
BI – balloon injury
Effects of Ad-CD39 transduction on neointima formation
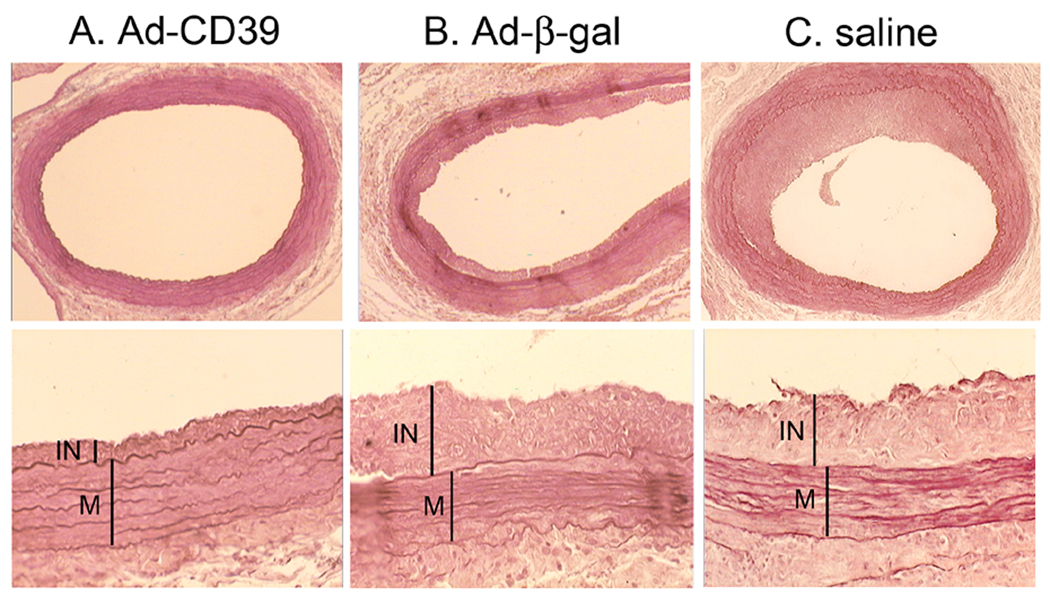
We then evaluated the effects of targeted expression of CD39/NTPDase-1 on the development of intimal hyperplasia in balloon-injured rat aortas. Sixteen days after balloon injury and viral transduction, abdominal aortas were isolated and stained with hematoxylin-eosine and orcein and evaluated histologically (Fig. 2 and Table 2).
Fig. 2. CD39/NTPDase-1 inhibits intimal hyperplasia.
Images of injured aorta following Ad-CD39 (A) and Ad-β-gal (B) transductions or saline injection (C). Isolated aortas were stained with hematoxylin-eosine and orcein, and evaluated histologically. IN – intima, M – media.
Table 2.
Histological characteristic of injured aorta following Ad-CD39 transduction. Results are presented as mean±SD from 6–10 animals.
| Ad-CD39 (n=10) | Ad-β-Gal (n=6) | Saline (n=9) | |
|---|---|---|---|
| Intimal layer: | |||
| Thickness (µm) | 26.2±3.9 *** | 51.8±6.1 | 64.4±22.2 |
| Area (mm2) | 0.075±0.013 *** | 0.184±0.020 | 0.212±0.079 |
| Media layer: | |||
| Thickness (µm) | 120.6±14.8 | 111.4±11.5 | 108.6±16.6 |
| Area (mm2) | 0.438±0.093 | 0.458±0.077 | 0.431±0.060 |
| Intima/Media area | 0.17±0.03*** | 0.40±0.04 | 0.49±0.18 |
P<0.001 comparing to the saline group
The results presented in Table 2 indicate that neointima formation in the Ad-CD39-treated animals was considerably decreased as compared to aortas from control animals. There was no statistically significant difference between saline and Ad-β-gal groups. The recorded thickness of intimal layer was decreased almost 2-fold for Ad-CD39 group as compared to Ad-β-gal and saline controls (P<0.001 for Ad-CD39 versus saline group). Ad-CD39 transduction did not affect the thickness of media, however the intima over media ratio was significantly greater in both control groups as opposed to Ad-CD39-transduced aortas (P<0.001 for Ad-CD39 versus saline group).
Aortic smooth muscle cell proliferation
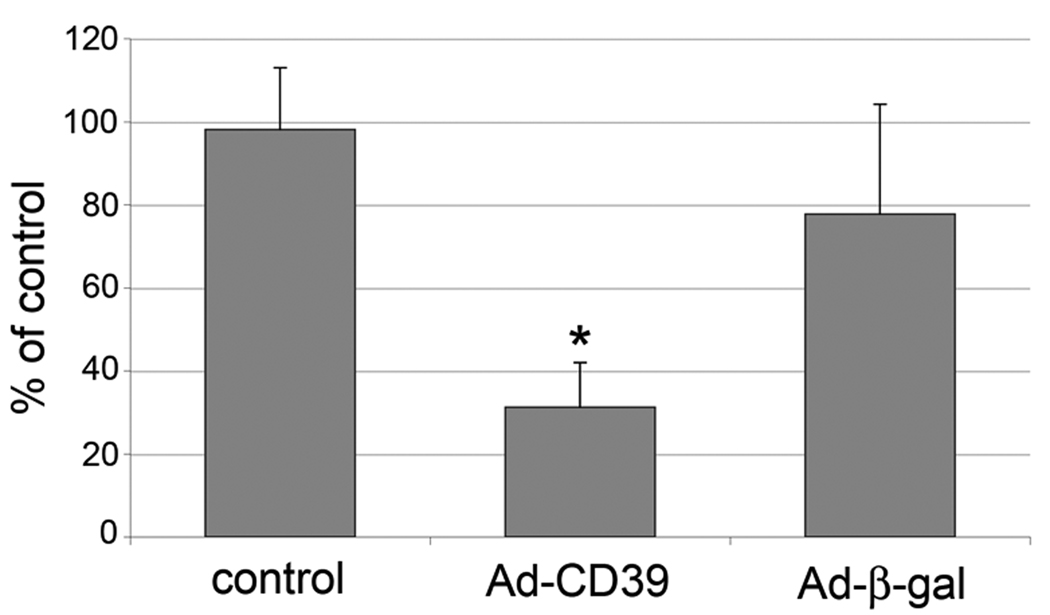
The observed protective effect of CD39/NTPDase-1 on restenosis associated with balloon injury of rat aorta, prompted us to evaluate the impact of CD39/NTPDase-1 activity upon VSMC proliferation evaluated ex vivo. There was no statistically significant difference in cell proliferation between saline and Ad-β-gal groups. However, proliferation of VSMC isolated from rat aortas transduced with Ad-CD39 was decreased by 70% (P<0.01), comparing to saline treated aortas as measured by the [3H]-thymidine incorporation assay (Fig. 3).
Fig. 3. CD39/NTPDase-1 decreases proliferation of VSMC.
VSMC were isolated from injured aortas of control (saline-treated) and Ad-CD39 or Ad-β-gal-transduced animals followed by analysis of cell proliferation using [3H]-thymidine incorporation method. Results are presented as mean±SD from two experiments in triplicates. * - P < 0.01 comparing to the control.
Discussion
This study demonstrates that adenovirus-mediated expression of CD39/NTPDase-1 augments vascular NTPDase activity and prevents intimal hyperplasia following a mechanical injury to rat aortas. It also indicates that changes in extracellular nucleotide concentrations related to altered CD39/NTPDase-1 activity might play an essential role in the regulation of VSMC proliferation and vascular remodeling.
The pathogenesis of intimal lesions, such as those found in atherosclerosis and post-angioplasty restenosis has not been fully elucidated, but the role of heightened VSMC proliferation in intimal thickening is well established [25].
Therefore, extracellular nucleotides and their respective P2 receptors, whose role in VSMC proliferation is well documented, may indeed be involved in the process of neointima formation [18]. Of relevance, P2Y2 receptors have been linked to the development of intimal hyperplasia in a model of carotid balloon injury in the rat [17, 26].
Adventitial fibroblasts constitute another cell population involved in the formation of vascular lesions [27]. Medial injury triggers both intense proliferation and extracellular matrix synthesis by these cells [28]. Limited, but persuasive data show that activated fibroblasts migrate from the adventitia to the intima [29]. Extracellular nucleotides have been reported to stimulate fibroblast proliferation [30], and at least one published report has confirmed the role of P2 receptors in ATP induced adventitial fibroblast growth [30].
The important role of extracellular nucleotides in VSMC proliferative responses emphasizes the significance of nucleotide hydrolyzing enzymes (NTPDases), which, by decreasing nucleotide concentrations, may modulate P2 receptor signaling. Endothelial CD39/NTPDase-1 is the major source of vascular NTPDase activity [31–33]. The loss of NTPDase-1 by endothelial denudation after balloon angioplasty significantly abrogates the ability of the vessel to hydrolyze extracellular nucleotides, hence leads to the increase in local concentrations of ATP and ADP [12]. An induction of prothrombotic phenotype, permissive for platelet activation and recruitment is the immediate consequence of such a disturbance in nucleotide metabolism [10, 34, 35]. Indeed, thrombosis has been the most extensively studied side effect of NTPDase-1 loss. In this report we demonstrate that an increase in nucleotide concentration due to decreased NTPDase-1 expression, also affects purinergic signaling in VSMC, leading to their enhanced proliferation and consequently, to the development of intimal lesions.
Several attempts to reconstitute the NTPDase-1 activity have been reported. Earlier data confirmed that adenoviral-mediated gene transfer of CD39 augments NTPDase activity in balloon injured rabbit arteries [12]. Accordingly, adenovirus mediated gene transfer of human placental NTPDase to VSMC suppressed platelet aggregation in vitro, and restrained thrombus formation [36]. Previously published report from our group has shown that the use of Ad-CD39 can prolong cardiac survival in a xenograft model [19].
In this work, we verified whether targeted overexpression of CD39/NTPDase-1 in injured aortas could reduce the process of restenosis. Adenoviral-mediated gene transfer to rat aortas immediately following balloon injury (i.e., to medial VSMC) led to a transient but significant increase in NTPDase-1 activity with a maximum reached four days following the gene transfer. Temporary augmentation of enzymatic activity of NTPDase-1 in injured aortas was sufficient to achieve a long lasting biological effect, i.e., a prevention of intimal hyperplasia and a protection from restenosis. We suggest that this beneficial effect is related to decreased proliferation of VSMC derived from Ad-CD39-transduced aortas, as opposed to controls. To our knowledge, this is the first demonstration showing that overexpression of CD39/NTPDase-1 in VSMC is a valid therapeutic approach to prevent intimal hyperplasia and restenosis following balloon angioplasty.
The vasculoprotective effects achieved by upregulation of CD39/NTPDase-1 expression are still incompletely characterized. They could obviously relate to decreasing local levels of nucleotides (ATP and ADP) and/or to increasing adenosine levels. Adenosine, a downstream product of extracellular nucleotide metabolism, is a well-known modulator of vascular remodeling. This nucleoside not only inhibits VSMC proliferation but also may induce VSMC apoptosis [37, 38], therefore the observed decrease in intima thickness can be also associated with adenosine function. Furthermore, adenosine is a very powerful inhibitor of platelet reactivity.
In conclusion, we have shown a successful adenoviral-mediated CD39/NTPDase-1 gene transfer, which resulted in a decline of VSMC proliferation rate and a prevention of restenosis after angioplasty. We propose that the reduction of the intimal hyperplasia observed after CD39/NTPDase-1 overexpression is associated with a decrease in extracellular nucleotide concentration and therefore diminished P2 receptor signaling, which is involved in the regulation of VSMC growth. Combining anti-thrombotic function of CD39/NTPDase-1 with its anti-proliferative effect on VSMC may be highly beneficial for therapy and the prevention of vascular intimal disease, which still remains a serious challenge in clinical practice.
Acknowledgments
This work was supported in part by NIH (HL66167 to E.K.; HL57307, HL63972 to S.C.R). We thank Dr. C. Ferran for critical reading of the manuscript and Dr. C. da Silva for statistical analysis of data.
References
- 1.Hou M, Moller S, Edvinsson L, Erlinge D. Cytokines induce upregulation of vascular P2Y(2) receptors and increased mitogenic responses to UTP and ATP. Arterioscler Thromb Vasc Biol. 2000;20:2064–2069. doi: 10.1161/01.atv.20.9.2064. [DOI] [PubMed] [Google Scholar]
- 2.Erlinge D. Extracellular ATP: a growth factor for vascular smooth muscle cells. Gen Pharmacol. 1998;31:1–8. doi: 10.1016/s0306-3623(97)00420-5. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- 4.Di Virgilio F, Solini A. P2 receptors: new potential players in atherosclerosis. Br J Pharmacol. 2002;135:831–842. doi: 10.1038/sj.bjp.0704524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imai M, Takigami K, Guckelberger O, Lin Y, Sevigny J, Kaczmarek E, Goepfert C, Enjyoji K, Bach FH, Rosenberg RD, Robson SC. CD39/vascular ATP diphosphohydrolase modulates xenograft survival. Transplant Proc. 2000;32:969. doi: 10.1016/s0041-1345(00)01065-4. [DOI] [PubMed] [Google Scholar]
- 6.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 7.Luthje J. Origin, metabolism and function of extracellular adenine nucleotides in the blood. Klin Wochenschr. 1989;67:317–327. doi: 10.1007/BF01741386. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G. Introduction: P2 receptors. Curr Top Med Chem. 2004;4:793–803. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- 9.Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, Bach FH, Robson SC. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 10.Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M, Hancock WW, Bach FH. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997;185:153–163. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai M, Takigami K, Guckelberger O, Enjyoji K, Smith RN, Lin Y, Csizmadia E, Sevigny J, Rosenberg RD, Bach FH, Robson SC. Modulation of nucleoside [correction of nucleotide] triphosphate diphosphohydrolase-1 (NTPDase-1)cd39 in xenograft rejection. Mol Med. 1999;5:743–752. [PMC free article] [PubMed] [Google Scholar]
- 12.Gangadharan SP, Imai M, Rhynhart KK, Sevigny J, Robson SC, Conte MS. Targeting platelet aggregation: CD39 gene transfer augments nucleoside triphosphate diphosphohydrolase activity in injured rabbit arteries. Surgery. 2001;130:296–303. doi: 10.1067/msy.2001.116032. [DOI] [PubMed] [Google Scholar]
- 13.Wang DJ, Huang NN, Heppel LA. Extracellular ATP and ADP stimulate proliferation of porcine aortic smooth muscle cells. J Cell Physiol. 1992;153:221–233. doi: 10.1002/jcp.1041530202. [DOI] [PubMed] [Google Scholar]
- 14.Malam-Souley R, Campan M, Gadeau AP, Desgranges C. Exogenous ATP induces a limited cell cycle progression of arterial smooth muscle cells. Am J Physiol. 1993;264:C783–C788. doi: 10.1152/ajpcell.1993.264.4.C783. [DOI] [PubMed] [Google Scholar]
- 15.Hou M, Harden TK, Kuhn CM, Baldetorp B, Lazarowski E, Pendergast W, Moller S, Edvinsson L, Erlinge D. UDP acts as a growth factor for vascular smooth muscle cells by activation of P2Y(6) receptors. Am J Physiol Heart Circ Physiol. 2002;282:H784–H792. doi: 10.1152/ajpheart.00997.2000. [DOI] [PubMed] [Google Scholar]
- 16.Pillois X, Chaulet H, Belloc I, Dupuch F, Desgranges C, Gadeau AP. Nucleotide receptors involved in UTP-induced rat arterial smooth muscle cell migration. Circ Res. 2002;90:678–681. doi: 10.1161/01.res.0000013700.98464.8e. [DOI] [PubMed] [Google Scholar]
- 17.Seye CI, Kong Q, Erb L, Garrad RC, Krugh B, Wang M, Turner JT, Sturek M, Gonzalez FA, Weisman GA. Functional P2Y2 nucleotide receptors mediate uridine 5'-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106:2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- 18.Pulvirenti TJ, Yin JL, Chaufour X, McLachlan C, Hambly BD, Bennett MR, Barden JA. P2X (purinergic) receptor redistribution in rabbit aorta following injury to endothelial cells and cholesterol feeding. J Neurocytol. 2000;29:623–631. doi: 10.1023/a:1010828302936. [DOI] [PubMed] [Google Scholar]
- 19.Imai M, Takigami K, Guckelberger O, Kaczmarek E, Csizmadia E, Bach FH, Robson SC. Recombinant adenoviral mediated CD39 gene transfer prolongs cardiac xenograft survival. Transplantation. 2000;70:864–870. doi: 10.1097/00007890-200009270-00003. [DOI] [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 21.Koziak K, Sevigny J, Robson SC, Siegel JB, Kaczmarek E. Analysis of CD39/ATP diphosphohydrolase (ATPDase) expression in endothelial cells, platelets and leukocytes. Thromb Haemost. 1999;82:1538–1544. [PubMed] [Google Scholar]
- 22.Geladopoulos TP, Sotiroudis TG, Evangelopoulos AE. A malachite green colorimetric assay for protein phosphatase activity. Anal Biochem. 1991;192:112–126. doi: 10.1016/0003-2697(91)90194-x. [DOI] [PubMed] [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 24.Religa P, Kazi M, Thyberg J, Gaciong Z, Swedenborg J, Hedin U. Fucoidan inhibits smooth muscle cell proliferation and reduces mitogen-activated protein kinase activity. Eur J Vasc Endovasc Surg. 2000;20:419–426. doi: 10.1053/ejvs.2000.1220. [DOI] [PubMed] [Google Scholar]
- 25.Ferns GA, Reidy MA, Ross R. Balloon catheter de-endothelialization of the nude rat carotid. Response to injury in the absence of functional T lymphocytes. Am J Pathol. 1991;138:1045–1057. [PMC free article] [PubMed] [Google Scholar]
- 26.Seye CI, Gadeau AP, Daret D, Dupuch F, Alzieu P, Capron L, Desgranges C. Overexpression of P2Y2 purinoceptor in intimal lesions of the rat aorta. Arterioscler Thromb Vasc Biol. 1997;17:3602–3610. doi: 10.1161/01.atv.17.12.3602. [DOI] [PubMed] [Google Scholar]
- 27.Zalewski A, Shi Y, Johnson AG. Diverse origin of intimal cells: smooth muscle cells, myofibroblasts, fibroblasts, and beyond? Circ Res. 2002;91:652–655. doi: 10.1161/01.res.0000038996.97287.9a. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y, O'Brien JE, Ala-Kokko L, Chung W, Mannion JD, Zalewski A. Origin of Extracellular Matrix Synthesis During Coronary Repair. Circulation. 1997;95:997–1006. doi: 10.1161/01.cir.95.4.997. [DOI] [PubMed] [Google Scholar]
- 29.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996;93:2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- 30.Gerasimovskaya EV, Ahmad S, White CW, Jones PL, Carpenter TC, Stenmark KR. Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J Biol Chem. 2002;277:44638–44650. doi: 10.1074/jbc.M203012200. [DOI] [PubMed] [Google Scholar]
- 31.Cote YP, Picher M, St-Jean P, Beliveau R, Potier M, Beaudoin AR. Identification and localization of ATP-diphosphohydrolase (apyrase) in bovine aorta: relevance to vascular tone and platelet aggregation. Biochim Biophys Acta. 1991;1078:187–191. doi: 10.1016/0167-4838(91)99008-g. [DOI] [PubMed] [Google Scholar]
- 32.Sevigny J, Levesque FP, Grondin G, Beaudoin AR. Purification of the blood vessel ATP diphosphohydrolase, identification and localisation by immunological techniques. Biochim Biophys Acta. 1997;1334:73–88. doi: 10.1016/s0304-4165(96)00079-7. [DOI] [PubMed] [Google Scholar]
- 33.Cote YP, Filep JG, Battistini B, Gauvreau J, Sirois P, Beaudoin AR. Characterization of ATP-diphosphohydrolase activities in the intima and media of the bovine aorta: evidence for a regulatory role in platelet activation in vitro. Biochim Biophys Acta. 1992;1139:133–142. doi: 10.1016/0925-4439(92)90092-2. [DOI] [PubMed] [Google Scholar]
- 34.Candinas D, Koyamada N, Miyatake T, Siegel J, Hancock WW, Bach FH, Robson SC. Loss of rat glomerular ATP diphosphohydrolase activity during reperfusion injury is associated with oxidative stress reactions. Thromb Haemost. 1996;76:807–812. [PubMed] [Google Scholar]
- 35.Bakker WW, Poelstra K, Timmerman W, Hardonk MJ, Koiter TR, Schuiling GA. Experimental endotoxemia in pregnancy: in situ glomerular microthrombus formation associated with impaired glomerular adenosine diphosphatase activity. J Lab Clin Med. 1989;114:531–537. [PubMed] [Google Scholar]
- 36.Furukoji E, Matsumoto M, Yamashita A, Yagi H, Sakurai Y, Marutsuka K, Hatakeyama K, Morishita K, Fujimura Y, Tamura S, Asada Y. Adenovirus-mediated transfer of human placental ectonucleoside triphosphate diphosphohydrolase to vascular smooth muscle cells suppresses platelet aggregation in vitro and arterial thrombus formation in vivo. Circulation. 2005;111:808–815. doi: 10.1161/01.CIR.0000155239.46511.79. [DOI] [PubMed] [Google Scholar]
- 37.Martin PL. Adenosine agonists for the prevention of restenosis? IDrugs. 1999;2:44–51. [PubMed] [Google Scholar]
- 38.Peyot ML, Gadeau AP, Dandre F, Belloc I, Dupuch F, Desgranges C. Extracellular adenosine induces apoptosis of human arterial smooth muscle cells via A(2b)-purinoceptor. Circ Res. 2000;86:76–85. doi: 10.1161/01.res.86.1.76. [DOI] [PubMed] [Google Scholar]