Abstract
Objective
To test the hypothesis that during root resorption, organic matrix proteins and cytokines from the surrounding bone and dentin are released into the gingival crevice.
Material and Methods
Subjects with mild (< 2 mm loss) and severe root resorption (> 2 mm) were identified. Control group subjects with no loss of root structure or undergoing orthodontic treatment were also identified. Gingival crevicular fluid (GCF) was collected non-invasively from the mesial and distal sides of each of the four upper incisors by using filter paper strips. The eluted GCF was used for analysis using western blot and ELISA techniques. Antibodies used were against OPN, OPG and RANKL.
Results
Western blot analysis showed differential expression of OPN, OPG and RANKL in the control and root resorbed subjects. However, processed forms of these proteins were only observed in the root resorbed subjects. Results from ELISA with OPG antibodies revealed a difference in OPG concentration between the control and root resorption groups. ELISA results with RANKL antibodies did show a statistically significant difference between the control group and the two study groups. The ratio RANKL/OPG was statistically higher in subjects with severe root resorption than in the control subjects.
Conclusions
Preliminary results confirm the presence of matrix proteins and cytokines in the GCF of root resorbed subjects. Further, OPG was locally present in excess amounts over RANKL and an increased RANKL/OPG in the study groups could be correlated with an increased bone resorption activity during orthodontic tooth movement.
Keywords: dentin matrix proteins, gingival crevicular fluid, cytokines, root resorption
Introduction
Root resorption has been a common complication associated with orthodontic treatment. It is usually mild and often clinically insignificant; however it can occur in large amounts i.e., loss of over one third of the root length, in some patients. Estimated rates of orthodontic patients with root resorption vary extensively from study to study. The range extends from as low as 26% (1) to as high as 100% (2). However, as reported by Brezniak and Wasserstein (3) it is hard to compare the results and conclusions from these studies because of the differences in methods employed in each study. There were many differences regarding teeth examined (i.e., incisors versus entire dentition) treatment time (i.e., days versus years), types of tooth movement (intrusion, full fixed appliances etc) or technique employed to detect resorption (periapical radiographs, cephalographs). Nevertheless, it seems that when teeth are evaluated histologically, root resorption is always present in varying extents regardless of length of treatment or type of treatment (4). In fact, root resorption may even be detected in normal, non- treated subjects (5).
Several factors have been identified that might be responsible for root resorption. Some of the clinically relevant risk factors were chronological age, gender, pre-existing root condition, type of tooth movement, amount and type of force and treatment duration. Most of the studies seem to support the idea that there is an increased risk for root resorption in older patients as adults tend to have more quiescent periodontal ligament (6). The presence of root resorption before treatment is usually considered a strong predisposing factor for root resorption during treatment. Much has been debated about the effect of mechanical factors on root resorption. Intermittent forces have been argued to cause less harm than continuous forces because they allow the resorbed cementum to heal and prevent further resorption (7). The magnitude of the force used has also been implicated in root resorption. Thus, information regarding the risk factors for root resorption is conflicting. Most clinicians agree that individual variation seems to be the only major factor determining patients’ susceptibility to develop severe root resorption (8, 9).
At present, root resorption can only be monitored radiographically. Several drawbacks exist with this technique, namely that standard radiographs: are technique sensitive, can detect resorption only after it has occurred in large amounts, and provide two-dimensional information. In addition, periodic progress radiographs result in additional radiation exposure to the patient.
The purpose of this study is to determine if an alternate molecular method can be employed to assess ongoing resorption in active orthodontic patients. Our working hypothesis is that: (a) during the process of root resorption, organic matrix proteins and cytokines are released into the nearby crevice; (b) differences exist between levels of these proteins in gingival crevicular fluid (GCF) of subjects undergoing orthodontic treatment with radiographic signs of root resorption and subjects not in treatment and without radiographic signs of root shortening; (c) differences exist between levels of these proteins in GCF of subjects with mild and severe root resorption evaluated by radiographs.
The GCF was first utilized by periodontists attempting to develop a diagnostic test for periodontal diseases. This fluid is an osmotically-mediated transudate. The aqueous component is derived mainly from the serum; the constituents are derived from the serum, the gingival tissues through which the fluid passes, and the bacteria in the tissue and in the crevice (10). GCF was chosen because of its ready accessibility and because its collection poses minimal risk or harm to the patients. Orthodontic forces induce the movement of periodontal ligament fluids and with them any cellular and biochemical product produced from prior mechanical perturbation. During the course of orthodontic treatment, the forces exerted produce a distortion of the periodontal ligament extracellular matrix, resulting in alterations of cellular shape and cytoskeletal configuration. Such events lead to the synthesis and presence in the deeper periodontal tissues of extracellular matrix components, tissue degrading enzymes, acids and inflammatory mediators which induce cellular proliferation and differentiation and promote wound healing and tissue remodeling (11).
The goal of this study is to determine the levels of OPN (osteopontin), OPG (osteoprotegerin) and RANKL (receptor activator of nuclear factor kappa B ligand) in gingival crevicular fluid (GCF) of patients who have been in treatment for at least 1 year who show radiographic evidence of root resorption.
Materials and Methods
Subject selection
Sixty subjects were selected from patients seeking treatment in the Department of Orthodontics at the University of Illinois at Chicago. Three groups were set up, one “control” and two “study” groups. The control group included 20 subjects who had not started treatment yet with no radiographic evidence of root resorption. One “study” group included 20 subjects in treatment for at least one year and with radiographic signs of mild root resorption less than 2 mm of root loss and the other group included 20 subjects with radiographic signs of severe root resorption more than 2 mm of root loss. An informed consent was obtained from each patient and the project was approved by the Institutional Review Board (IRB# 2001-0328) prior to collection of any samples.
Gingival crevicular fluid collection
Gingival crevicular fluid was collected from the mesial and distal sides of the upper central and lateral incisors by using filter paper strips (Periopaper, Oraflow, Plainview NY) inserted 1–2 mm into the gingival sulcus for 30 sec. After 1 min a second collection was performed. Care was taken to avoid mechanical injury to the soft tissue. The contents were eluted out into 1X PBS containing protease inhibitor 0.1 mM phenylmethylsulphonylfluoride and stored at −80°C until further analysis.
Analysis by SDS-PAGE
The absorbed GCF was eluted from the filters were estimated for their protein content by Bradford assay and then resolved on a 10% SDS-PAGE gel. The gels were then stained by Coomassie and silver staining techniques.
Western blotting analysis
The total proteins were transferred to nitrocellulose membranes and then processed for further analysis. Membranes were then incubated with the primary antibodies against the proteins under investigation OPN (1:250 dilution); OPG (1:1000 dilution) and RANKL (1:1000) overnight at room temperature. The membranes were then washed with 1X PBS with 0.1% Tween 20 for 10 mins. They were then incubated with the corresponding secondary antibodies conjugated to alkaline phosphatase and developed using alkaline phosphatase conjugate substrate kit (Bio-Rad, California). Recombinant proteins were used as positive controls.
Enzyme-linked immunosorbent assay
Indirect ELISA technique was used except for RANKL that was measured by a two-site ELISA (IMK-513, Imgenex, California). All samples and standards were assayed in duplicate and for each sample three dilutions were assayed. Protein values in the samples were recorded as ng/µL derived from the protein standard curve. Microtiter plates were coated overnight at room temperature with a concentrated aliquot of crevicular fluid samples serially diluted in 1X PBS saline solution. The residual binding sites were blocked with 5% non-fat dry milk in 1X PBS solution for 2 hrs at room temperature on a shaker at medium speed. Wells were then washed 3 times with 1X PBS, 0.05% Tween - 20 solution and incubated with the primary antibodies for 2 hrs at room temperature on a shaker at medium speed. The primary antibody dilution for OPG and RANKL was 1:1000. Following incubation with primary antibody, the wells were washed 3 times and incubated with secondary antibody for 1 hour at room temperature. The secondary antibody used was 1:10,000 dilution for anti-mouse IgG (OPG, RANKL). Wells were washed again 3 times prior to addition of the substrate, p-nitrophenyl phosphate (Sigma, St. Louis, MO). Following incubation at 37°C for 30 mins, the optical density was measured at 405nm with a microtiter plate reader after 45 mins. Elisa with OPG and RANKL was performed using 6 severe root resorption subjects, 8 mild root resorption subjects and 8 control group subjects.
Statistical Analysis
Statistical analysis among the groups was performed using one-way ANOVA and Scheffé test to evaluate the statistical difference between each pair of groups. Shapiro-Wilk statistics was used to assess the normal distribution of the concentration values of the dentin matrix proteins.
Results
SDS-PAGE analysis
Crevicular fluid samples from study and control groups were loaded onto the gels to confirm the presence of proteins. Coomassie blue staining revealed only few bands while silver staining, having a higher sensitivity than Coomassie blue protein dye, revealed multiple bands (12). The concentration of proteins from the study group was higher when compared with those in the control sample. The mean concentration value for the severe root resorption group was 0.89 µg/µL ± 0.32 µg for the moderate root resorption group was 0.77 µg/µL ± 0.21 µg and for the control group was 0.22 µg/µL ± 0.05 µg.
Western blot analysis
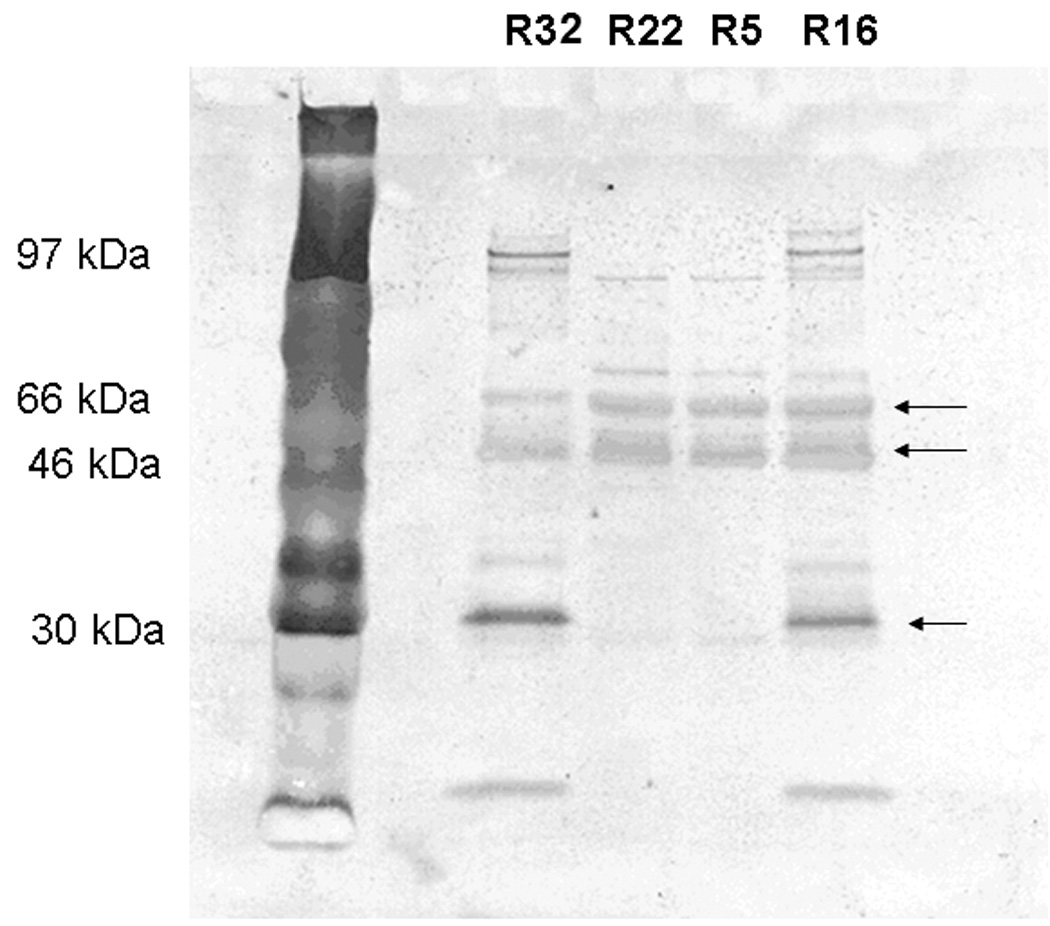
Western blot analysis was performed to detect osteoprotegerin, RANKL and osteopontin in the control and study groups. Immunoblotting against OPN detected one band at 54 kDa in the control group sample (Fig. 1A) and two bands at 54 kDa and 66 kDa (Fig. 1B) and several degraded fragments in the study group samples. Immunoblotting against OPG demonstrated the presence of one band at 55 kDa in both control and study groups and the presence of another band which might be a cleavage product of OFG (Fig. 2A). This 30 kDa peptide was present only in the mild and severe root resorption groups (Fig. 2A). Immunoblotting with antibodies against RANKL identified a band at 45 kDa in both the control and study group (Fig. 2B)
Figure 1.
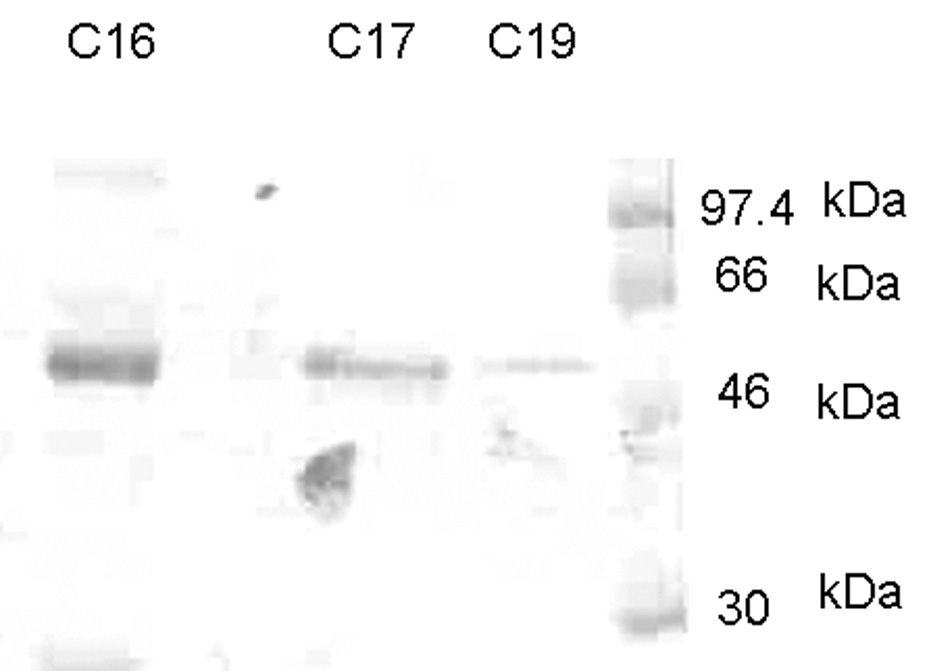
Western blot analysis using anti-OPN antibody. C16, C17 and C19 are samples from control subjects. A single band at ~54 kDa is observed. Figure 1B shows Western blot analysis from patients undergoing root resorption using anti-OPN antibody. R5 & R22 are mild root resorption group sample. R16 &R32 are from severe root resorption group samples. Arrows indicate two major bands at ~66 & ~54 kDa and smaller fragments of ~30kDa and less.
Figure 2.
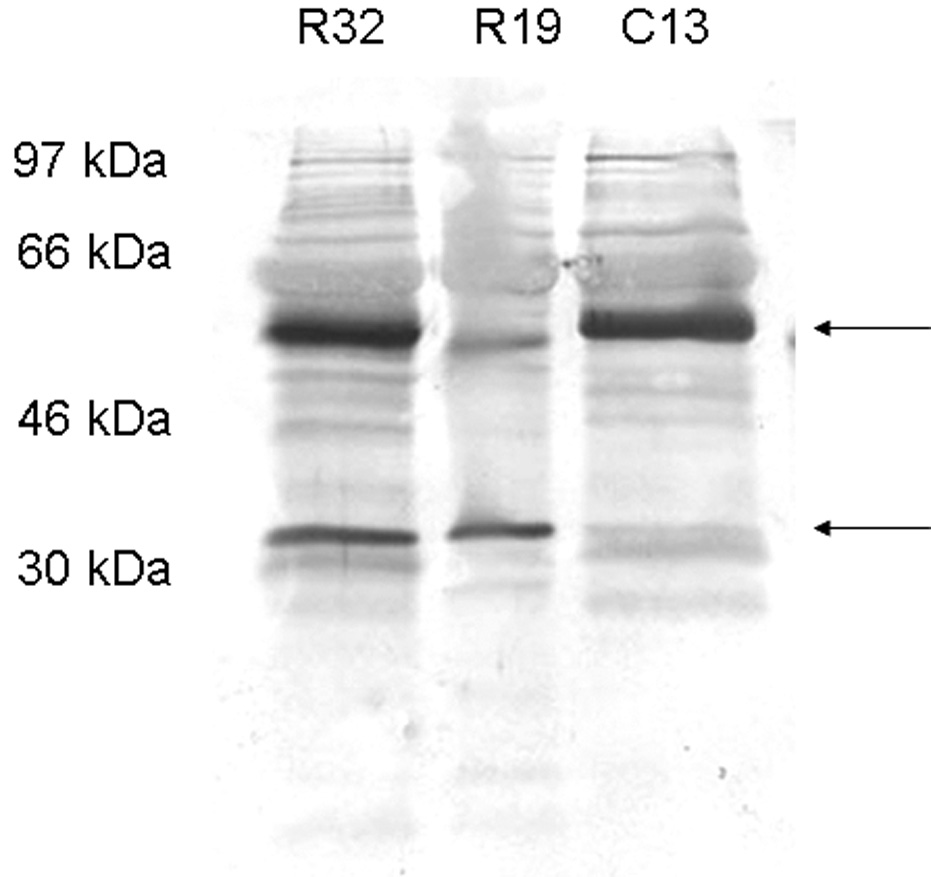
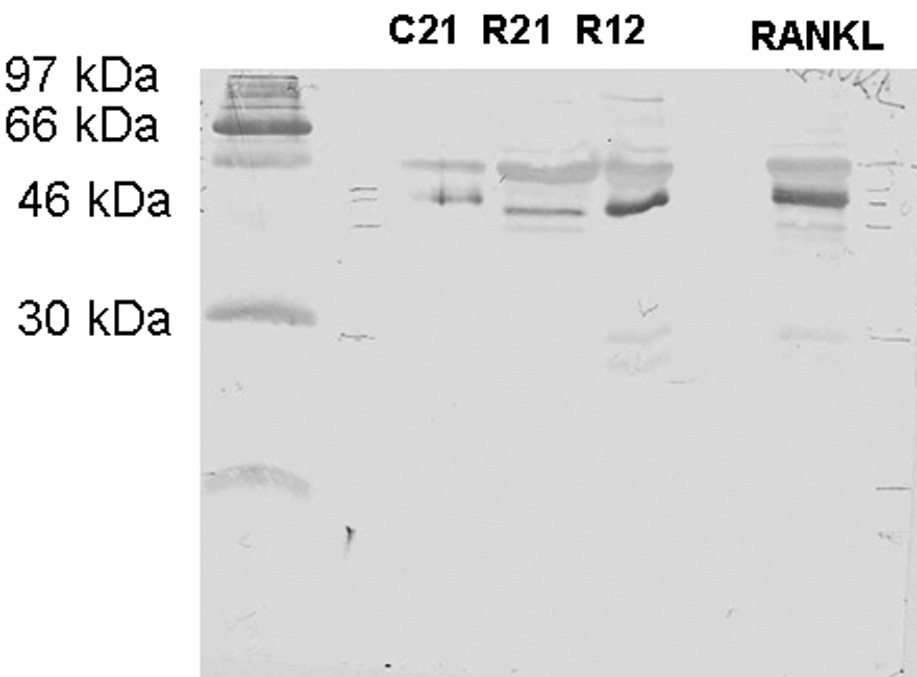
Figure 2A shows Western blot analysis using anti-OPG antibody. R19 is mild root resorption sample, R30 severe root resorption sample and C13 control group sample. Two major bands are detected at 46–54 kDa and 30 kDa in the study group samples. Figure 2B shows western blot analysis using anti-RANKL antibody. R12 is mild root resorption group sample; R21 is severe root resorption group sample; C21 is control group sample. A band is detected at 46 kDa.
ELISA
Results from ELISA performed with OPG antibodies reveal a difference in OPG concentration between the control and the root resorption groups and a small difference between the two study groups (Fig. 3A). One way ANOVA test did not show a statistically significant difference among the groups and this could be due to the insufficient number of samples used (observed power =0.416). ELISA results with RANKL antibodies reveal a difference in RANKL concentration between the control group and the root resorption groups (Fig. 3B) and a small difference between the study groups. One way ANOVA and Scheffé test did show a statistically significant difference between the control group and the two study groups but not between the mild and severe root resorption groups as shown in Figure 3B. Also the ratio between RANKL/OPG is statistically higher in subjects with severe root resorption than in the control subjects (Fig. 3C).
Figure 3.
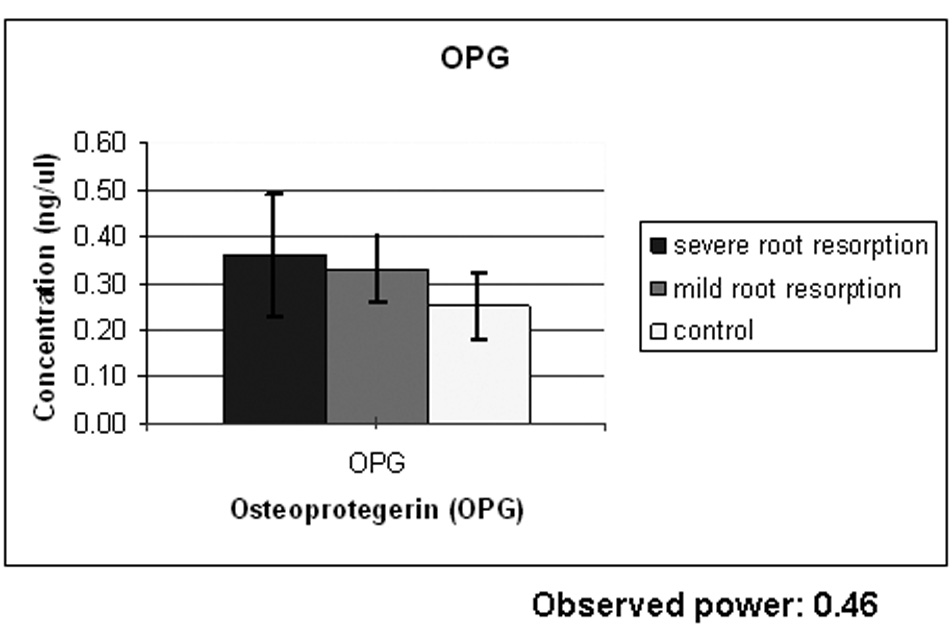
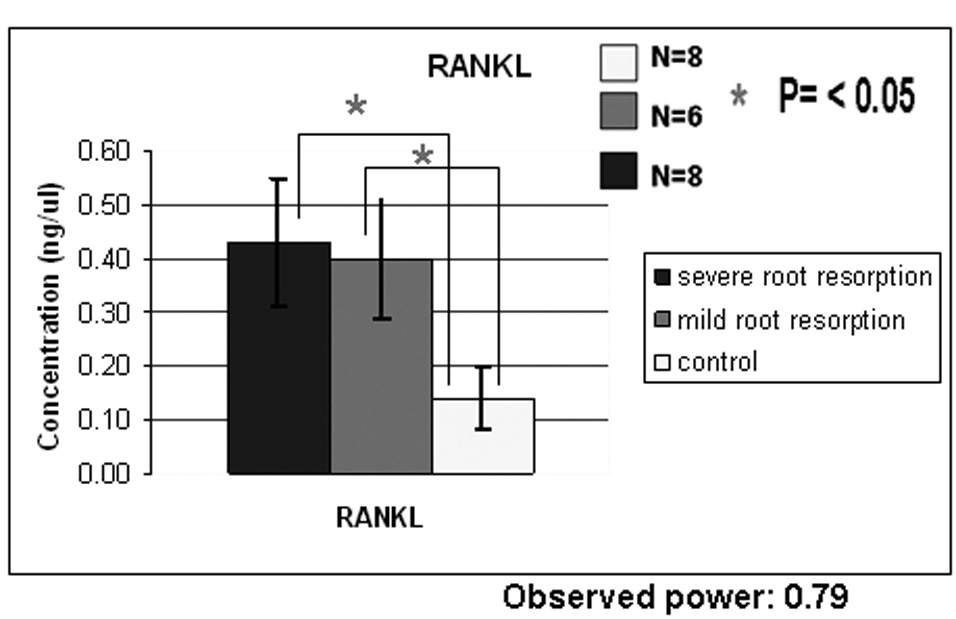
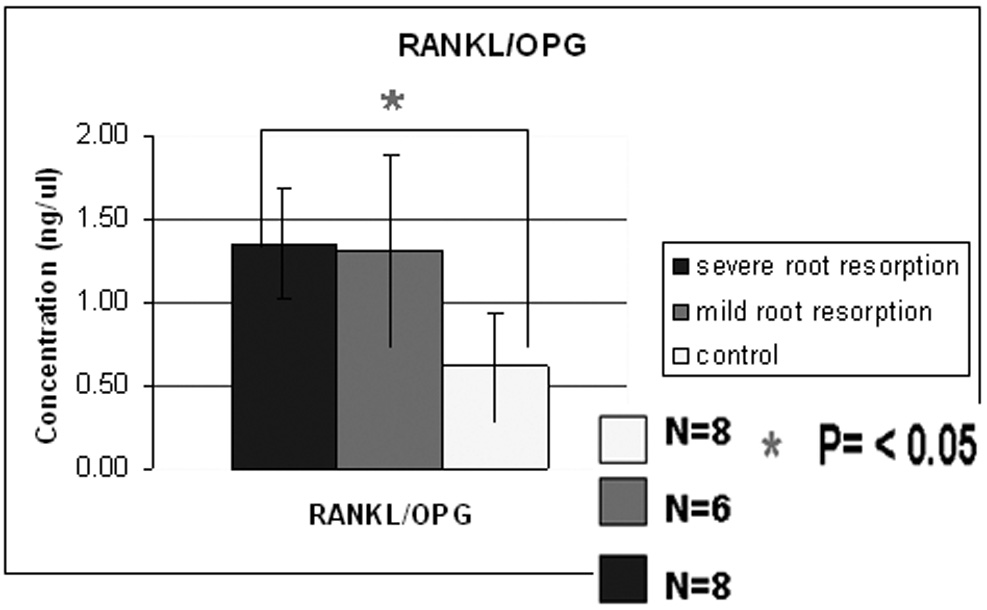
Figure 3A shows ELISA chart demonstrating the concentration (ng/µl) of OPG in the gingival crevicular fluid of control, severe and mild root resorption group subjects. Data are expressed as the mean ± SEM, P<0.05. Figure 3B shows ELISA chart demonstrating the concentration (ng/µl) of RANKL in the gingival crevicular fluid of control, mild and severe root resorption group subjects. Data are expressed as the mean ± SEM, P< 0.05. Figure 3C shows ELISA chart demonstrating the ratio between RANKL/OPG in the GCF of control, mild and severe root resorption groups.
Discussion
During the process of root resorption, organic matrix proteins and cytokines are released into the gingival crevice. The objective of this study is to study if matrix proteins such as osteopontin and cytokines such as osteoprotegrin (OPG) and receptor activator of nuclear factor –κB ligand (RANKL) could be used as biological markers for root resorption related to orthodontic treatment. Results from this study demonstrate that differences exist between levels of these proteins in GCF of subjects with mild and severe root resorption evaluated by radiographs.
The western blot results for osteopontin (OPN) show a single band at 54 kDa in the control group while 2 predominant polypeptides at 54 and 66 kDa and several smaller peptides were detected in the study group samples. Osteopontin is a major glycosylated protein in the bone and dentin matrix and is produced by osteoblasts, odontoblasts, osteoclasts and macrophages. Presence of OPN in GCF is likely to be derived from the neighboring tissues such as alveolar bone, cementum, dentin, macrophages in periodontal tissues, blood and salivary glands. The degraded fragments of OPN in GCF of the study groups may be derived from enzymatic activity during bone resorption. Cysteine proteases produced by osteoclasts, macrophages and fibroblasts are detected in GCF and this and other proteases released during degradation of the extracellular matrix of bone and dentin might be responsible for proteolytic cleavage of OPN (13). These results are in agreement with the study of Nakamura et al (14) on markers of alveolar bone resorption in periodontal disease.
Odontoclasts which are multinucleated cells resorb three dental hard tissues namely; cementum, dentin and enamel (15). These cells have morphological and functional characteristics similar to those of bone resorbing osteoclasts. Osteoclasts and odontoclasts precursors originate from hematopoietic cells in the bone marrow. Osteoclast formation from hematopoietic precursors is induced by cytokines such as macrophage colony-stimulating factor (M-CSF) and Receptor activator of nuclear factor kappa B (RANK), a membrane-bound ligand expressed by bone marrow stromal cells. RANK and its ligand RANKL have been localized in odontoblasts, pulp fibroblasts, periodontal ligament fibroblasts and in single odontoclasts, the latter finding suggesting an autocrine/paracrine role. Osteoclastogenesis is modulated by an inhibitor, osteoprotegrin (OPG). OPG is a soluble (decoy) receptor for RANKL and a member of tumor necrosis factor (TNF) receptor superfamily that inhibits osteoclastogenesis by competing with the binding of RANKL to RANKL receptor. Both RANKL and OPG act as positive and negative regulators of osteoclastogenesis, respectively, thus controlling the bone remodeling process. In certain resorptive bone diseases the RANKL/OPG mRNA ratio increased resorptive processes (16).
In the current study the mean OPG concentration value was higher in the subjects undergoing orthodontic treatment (0.36 ng/µL) than in the controls (0.25 ng/µL). ANOVA analysis did not show any statistically significant difference among the groups and this could be due to the insufficient number of samples used (observed power=0.416). These results suggest that more bone remodeling with bone turnover is occurring during orthodontic tooth movement.
Concentrations of RANKL in GCF were significantly higher in subjects with mild and severe root resorption than in the controls. The ratio RANKL/OPG showed that RANKL concentration is higher than OPG concentration in the study groups but lower in the control group. Since OPG inhibits osteoclast differentiation by competing with the binding of RANKL to the RANKL receptor, the alteration of RANKL and OPG in the GCF may reflect the comprehensive biological responses that occur during orthodontic tooth movement. Our results suggest that OPG is locally present in excess amounts over RANKL under physiologic conditions and an increased RANKL/OPG ratio in the study groups could be correlated with an increased bone resorption activity during orthodontic tooth movement (17).
Further studies are required to evaluate RANKL/OPG ratio during orthodontic tooth movement and root resorption with an increase in number of subjects for statistical analysis.
Clinical Relevance
Orthodontic root resorption is generally thought of as a side effect to the cellular activity involved in the removal of necrotic tissue in the periodontal ligament space. Upon initial force application, the tooth moves a small distance until the periodontal ligament fibers are compressed. Compression of the fibers results in the tooth standing still until undermining resorption from the marrow space removes the hyalinized tissue. Early detection of small root resorptions is essential for identifying teeth at risk of severe resorption. Identifying biological markers for developing a screen test would be highly valuable to monitor root resorption.
Acknowledgements
This work was supported by NIH-NIDCR grant DE 11657 and the Department of Orthodontics. We acknowledge the scientific input by Dr.Yi-Chen Liu and Dr. Laura Balducci.
References
- 1.Kennedy DB, Joondeph DR, Osterberg SK, Little RM. The effect of extraction and orthodontic treatment on dentoalveolar support. Am J Orthod. 1983;84:183–190. doi: 10.1016/0002-9416(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 2.Harry MR, Sims MR. Root resorption in bicuspid intrusion: a scanning electromicroscopic study. Angle Orthod. 1982;52:235–258. doi: 10.1043/0003-3219(1982)052<0235:RRIBI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: Part II.Literature review. Am J Orthod Dentofacial Orthop. 1993b;103:138–146. doi: 10.1016/S0889-5406(05)81763-9. [DOI] [PubMed] [Google Scholar]
- 4.Owman-Moll P, Kurol J, Lundgren D. Repair of orthodontically induced root resorption in adolescents. Angle Orthod. 1995;95:403–408. doi: 10.1043/0003-3219(1995)065<0403:ROOIRR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Bosshardt DD, Schroeder HE. How repair cementum becomes attached to the resorbed roots of human permanent teeth. Acta. Anat. 1994;150:253–266. doi: 10.1159/000147628. [DOI] [PubMed] [Google Scholar]
- 6.Reitan K. Initial tissue behavior during apical root resorption. Angle Orthod. 1974;44:68–82. doi: 10.1043/0003-3219(1974)044<0068:ITBDAR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Acar A, Canyurek U, Kocaaga M, Erverdi N. Continuous vs. Discontinuous force application and root resorption. Angle Orthod. 1999;69:159–164. doi: 10.1043/0003-3219(1999)069<0159:CVDFAA>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Linge L, Linge BO. Patient characteristics and treatment variables associated with apical root resorption during orthodontic treatment. Am J Orthod Dentofacial Orthop. 1991;99:35–43. doi: 10.1016/S0889-5406(05)81678-6. [DOI] [PubMed] [Google Scholar]
- 9.Taithongchai R, Sookkorn K, Killiany DM. Facial and dentoalveolar structure and the prediction of apical root shortening. Am J Orthod Dentofacial Orthop. 1996;110:296–302. doi: 10.1016/s0889-5406(96)80014-x. [DOI] [PubMed] [Google Scholar]
- 10.Lamster IB, Hartley LJ, Vogel RI. Development of a biochemical profile for gingival crevicular fluid. J Periodontol. 1985;56:13–21. doi: 10.1902/jop.1985.56.11s.13. [DOI] [PubMed] [Google Scholar]
- 11.Kavadia-Tsatala S, Kaklamanos EG, Tsalikis L. Effects of orthodontic treatment on gingival crevicular fluid flow rate and composition: clinical implications and applications. Int J Adult Orthodon Orthognath Surg. 2002;17:191–205. [PubMed] [Google Scholar]
- 12.Balducci L, Ramachandran A, Hao Jianjun, Narayanan K, Evans C, George A. Biological markers for evaluation of root resorption. Arch Oral Biology. 2007;52:203–208. doi: 10.1016/j.archoralbio.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mogi M, Otogoto J. Expression of cathepsin K in gingival crevicular fluid of patients with periodontitis. Arch Oral Biol. 2007;52:894–898. doi: 10.1016/j.archoralbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura KJ, Asahara Y, Kohri K, Nagata T. Osteopontin in gingival crevicular fluid. J Periodontal Res. 2001;36:328–333. doi: 10.1034/j.1600-0765.2001.360509.x. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki T, Motegi N, Suzuki H, Watanabe S, Tadokoro K, Yanagisawa T, et al. Dentin resorption mediated by odontoclasts in physiological root resorption of human deciduous teeth. Am J Anat. 2005;183:303–315. doi: 10.1002/aja.1001830404. [DOI] [PubMed] [Google Scholar]
- 16.Grimaud E, Soubigou L, Couillaud S, Coipeau P, Moreau A, Passuti N, et al. Receptor activator of nuclear factor κB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am J Pathol. 2003;163:2021–2031. doi: 10.1016/s0002-9440(10)63560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Low E, Zoellner H, Kharbanda P, Darendeliler MA. Expression of mRNA for osteoprotegrin and receptor activator of nuclear factor kappa beta ligand (RANKL) during root resorption induced by the application of heavy orthodontic forces on rat molars. Am J Orthod Dentofacial Orthop. 2005;128:497–503. doi: 10.1016/j.ajodo.2004.03.038. [DOI] [PubMed] [Google Scholar]


