Abstract
Antibodies specific for Vibrio cholerae lipopolysaccaride (LPS) are common in humans recovering from cholera, and constitute a primary component of the vibriocidal response, a serum complement-mediated bacteriocidal response correlated with protection against cholera. In order to determine whether transcutaneous immunization (TCI) with a V. cholerae neoglycoconjugate (CHO-BSA) comprised of a synthetic terminal hexasaccharide of the O-specific polysaccharide of V. cholerae O1 (Ogawa) conjugated with bovine serum albumin (BSA) could induce anti-V. cholerae LPS and vibriocidal responses, we applied CHO-BSA transcutaneously in the presence or absence of the immune adjuvant cholera toxin (CT) to mice. Transcutaneously applied neoglycoconjugate elicited prominent V. cholerae specific LPS IgG responses in the presence of CT, but not IgM or IgA responses. CT applied on the skin induced strong IgG and IgA serum responses. TCI with neoglycoconjugate did not elicit detectable vibriocidal responses, protection in a mouse challenge assay, or stool anti-V. cholerae IgA responses, irrespective of the presence or absence of CT. Our results suggest that transcutaneously applied synthetic V. cholerae neoglycoconjugate is safe and immunogenic, but predominantly induces systemic LPS responses of the IgG isotype.
1. Introduction
Vibrio cholerae is a non-invasive organism that colonizes the human intestine and produces cholera toxin (CT), an ADP-ribosylating protein that causes a severe secretory diarrhea in infected humans. Strains of V. cholerae can be differentiated serologically by the O-specific polysaccharide of the lipopolysaccharide (LPS) component of the outer membrane. The vast majority of strains that induce epidemic cholera belong to serogroups O1 or O139. V. cholerae O1 is divided into two biotypes, classical and El Tor, which differ clinically and biochemically. Based on O-antigen differences, each O1 biotype can be further subdivided into three serotypes: Ogawa, Inaba, and Hikojima. During outbreaks or sustained transmission, V. cholerae O1 may switch between Ogawa and Inaba serotypes [1]. The Hikojima serotype is rare and thought to be unstable.
V. cholerae LPS is immunogenic in humans following wild type disease, inducing significant increases in V. cholerae O1 LPS IgG, IgM, and IgA serum antibody responses, as well as antibody secreting cell responses [2-6]. Wild type disease also induces intestinal secretory IgA responses, and immunity against V. cholerae O1 LPS of the IgA and IgM (but not IgG) isotypes is associated with protection from cholera in humans [3,4,7,8]. Passive immunization with anti-V. cholerae LPS antibodies protects against V. cholerae challenge in both mice [9,10] and rabbits [11], and immunization with purified V. cholerae LPS confers protection against challenge in rabbits [12] and humans [5,13,14]. V. cholerae specific LPS antibodies of the IgM and IgG isotypes constitute a primary component of the vibriocidal response [7], a serum complement-mediated bacteriocidal response correlate with protection against cholera [15].
V. cholerae O1 LPS is comprised of a lipid component, core polysaccharide, and an O-specific polysaccharide (O-SP). The O-SP in V. cholerae O1 consists of (1->2)-alpha-linked 4-amino-4,6-dideoxy-D-mannose (perosamine), in which the amino group is acylated with 3-deoxy-L-glycero-tetronic acid [16]. The Inaba O-SP has a terminal sugar characterized by a 2-O-hydroxyl group; in the Ogawa O-SP, the hydroxyl group is replaced by a 2-O-methyl group [17]. The differences in the terminal sugars of the Inaba and Ogawa are thought to define their respective serotypes.
Unfortunately, a cholera subunit vaccine based on parenteral immunization with V. cholerae LPS has a number of real and potential shortcomings, including difficulties in manufacturing, a high reactogenicity profile following vaccine administration, and the requirement for needle-based administration. To decrease reactogenicity, detoxified versions of LPS consisting largely of the O-polysaccharide with an altered and decreased lipid component have been developed; however, polysaccharide-based vaccines often induce low level and short term immunity via T cell independent pathways [18]. Efforts to circumvent this issue have involved coupling detoxified LPS and polysaccharides to protein carriers such as CT [19,20] or tetanus toxoid [21], and administration of such vaccines is immunogenic in animals and humans [19-21]. A different approach involves the construction of synthetic neoglycoconjugates, in which different lengths of perosamine polymers of V. cholerae O-SP are chemically linked to a protein carrier [16,18,22-25]. Ogawa and Inaba neoglycoconjugates are immunogenic in mice, and intra-peritoneal vaccination of mice with a synthesized hexasaccharide derived from the O-SP component of V. cholerae O1 Ogawa LPS bound to BSA was protective in a V. cholerae neonatal mouse challenge model [18,22]. We were thus interested in evaluating whether needle-free transcutaneous immunization (TCI) with an Ogawa V. cholerae O1 neoglycoconjugate would induce immunity in mice [26,27].
2. Materials and Methods
2.1. Bacterial strains and media
V. cholerae O1 El Tor Ogawa strain X25049 was used to prepare LPS for immunogen preparation and immunological assays, and wild type classical V. cholerae O1 classical Ogawa strain O395 was used in vibriocidal assays and mouse challenge models, as described below [28]. Prior to use in challenge studies, organisms were grown for 12 hours at 37°C with aeration in Luria-Bertani broth containing streptomycin (100 μg/ml).
2.2. Vaccine antigens
V. cholerae Ogawa neoglycoconjugate was comprised of a synthesized hexasaccharide derived from the O-SP component of V. cholerae O1 Ogawa lipopolysaccharide (CHO) bound through a linker to BSA in a molar ratio of 5 hexasaccharides: 1 protein (CHO-BSA; Figure 1) [16,18]. To produce the conjugate, hexasaccharide squarate (3.78 mg, 0.0021 mM) was added to a solution of BSA (Sigma A-4503, purified [29]; 20 mg, 0.0003 mM) and borate buffer pH 9.00 (0.5 M, 0.53 ml). The reaction mixture was gently stirred and the reaction was periodically monitored by SELDI TOF-MS, which, after 8 h, showed the carbohydrate-protein ratio to be 5.0 [30]. Borate buffer pH 7.00 was added to terminate the reaction, and the mixture was transferred to a centrifugal filter device (10 k, Amicon Ultra, Millipore), and dialyzed against 10 mM ammonium carbonate solution (8 times) to remove low molecular mass material. The retentate was lyophilized and resuspended in TEAN (30 mM Tris, 1mM EDTA, 3mM NaN3, 200mM NaCl), pH7.5 prior to use in immunization regimens [18,30].
Figure 1.
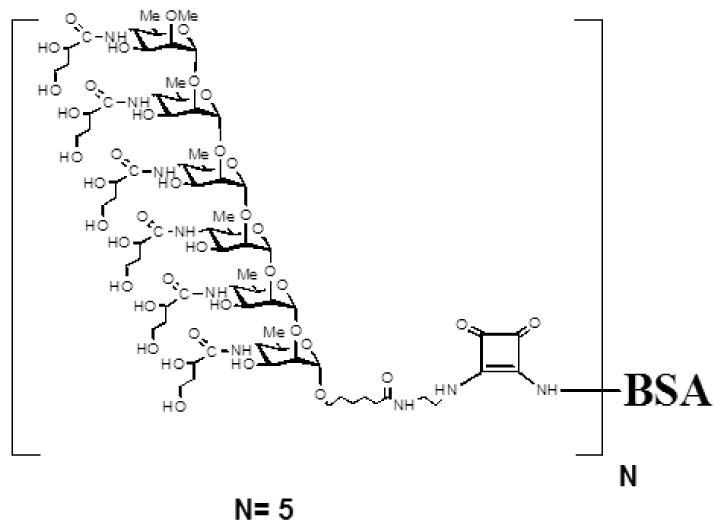
Structure of O-specific Ogawa synthetic hexasaccharide BSA conjugate. Molar ratio, 5 hexasaccharide molecules: 1 BSA. BSA, bovine serum albumen; Me, methyl.
We also immunized mice with purified LPS derived from V. cholerae O1 Ogawa strain X25049, which was first prepared, purified, and lyophilized as previously described [6,28], and resuspended in TEAN. Cholera toxin (CT; List Biological Laboratories, Campbell, CA) was used as an immunogen and immunoadjuvant.
2.3. Immunization of mice
We transcutaneously immunized cohorts of 3-12 adult BALB/c mice (Charles River Laboratories) with CT (25 μg), CHO-BSA (10 μg of perosamine weight), or both CT (25 μg) and CHO-BSA (10 μg of perosamine weight). We subcutaneously immunized a fourth group of mice with CHO-BSA (2.5 μg of perosamine weight) and CT (10 μg), and transcutaneously immunized a fifth group of mice with purified Ogawa LPS (10 μg) and CT (25 μg). Mice were immunized on days 0, 14, and 28, and prior to collection of blood for use in the neonatal challenge assay, mice received a booster immunization on day 42 after which blood was collected on day 56. Transcutaneous immunizations were performed as previously described [26,31,32]. Briefly, we shaved the dorsum of mice using a clipper with a no. 40 blade (Wahl Clipper Corp., Sterling, IL); then rested the mice for 24 hr. We then anesthetized mice with an intraperitoneal injection of 0.017 ml/g of a 2.5% solution in normal saline of 0.25 gm of 2-2-2 tribromoethanol (Sigma) mixed with 0.25 ml of tertiary amyl alcohol (Sigma) to prevent grooming following immunization. In order to enhance absorption of reagents into the skin, the shaved area was hydrated for 5 min with sterile water, gently brushed with emery paper (to remove the outer layers of stratum corneum), and then hydrated a second time for 5 min. We then blotted the region dry and immediately applied the vaccine antigens to approximately 1cm2 of shaved hydrated skin surface area. The mice were then rested and observed for 1 hr. The shaved region was then covered with a patch consisting of tape and gauze soaked in sterile phosphate buffered saline (PBS). After 24 hr, the patch was removed and the region was washed thoroughly with 1 L of warm water. The use of animals complied fully with relevant governmental and institutional requirements, guidelines, and policies.
2.4. Immunological sampling
We collected blood samples via tail bleeds on days 0, 14, 28, 42, and 56, and collected stool on day 56. Samples were collected, processed, aliquoted, and stored as previously described [26,31,33-35].
2.5. Detection of specific antibody responses in serum and stool
To detect antibody responses to LPS, we coated microtiter plates overnight at room temperature with 250 ng of LPS in PBS per well, and subsequently blocked plates for 40 minutes at 37°C with PBS-1% ovalbumin (Sigma). We added 100 μl of diluted sera (using the following dilutions by measured isotype: IgM, 1:200; IgG, 1:50; IgA, 1:50) in PBS-0.1% ovalbumin- 0.05% Tween 20 (Sigma), and incubated plates at 37°C for 1.5 hr. Following washing of plates with PBS-0.05% Tween 20 (PBS-T), to detect IgG, IgA, or IgM responses, we added 100 μl of sheep anti-mouse IgG conjugated to HRP (1:1000, Amersham Biosciences, Little Chalfont, Buckinghamshire, England), goat anti-mouse IgA conjugated to HRP (1:1000, Southern Biotech, Birmingham, AL), or goat anti-mouse IgM conjugated to HRP (1:1000 Southern Biotech, Birmingham, AL), respectively, to each well. We incubated plates at 37°C for 1.5 hr, washed them with PBS-T, and developed with a 0.55 mg/ml solution of 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS; Sigma) with 0.03% H2O2 (Sigma). We determined the optical density at 405 nm with a Vmax microplate kinetic reader (Molecular Devices Corp., Sunnyvale, CA). Plates were read for 5 min at 14s intervals, and the maximum slope for an optical density change of 0.2 U was reported as millioptical density units per minute (mOD/min), as previously described [26,31]. To compare values across plates, results were divided by a pooled plate control and reported as ELISA units.
To detect antibody responses to CT, we coated microtiter plates sequentially with 1 μg of type III ganglioside (Sigma) in 50 mM carbonate buffer (pH 9.6) per well overnight at room temperature, followed by 100 ng CT per well in PBS overnight at room temperature. Following blocking of plates for 4-6 hr at room temperature with PBS-1% ovalbumin, we added dilutions of sera (IgG, 1:6000; IgA, 1:50) in PBS-0.1% ovalbumin-0.05% Tween 20, and incubated plates overnight at room temperature. Following washing of the plates, to detect IgG and IgA responses, we added sheep anti-mouse IgG conjugated to HRP or goat anti-mouse IgA conjugated to HRP, respectively, and developed as described above.
To detect LPS specific IgA antibody in feces, we first prepared fecal extracts and measured total fecal IgA in samples as previously described [26,31]. Briefly, we added duplicate serial twofold dilutions of fecal samples (1:10 to 1:320) in PBS-T to wells previously coated with rat monoclonal anti-mouse IgA antibody C10-3 (Pharmingen, San Diego, CA) at a dilution of 1:1,000 [26,31]. We then added a 1:1,000 dilution of goat anti-mouse IgA HRP-linked antibody to wells and developed the plates as described above. Comparisons were made to a mouse IgA standard (Kappa TEPC 15; Sigma). To detect specific anti-CT or anti-LPS antibodies in feces, we added 75 ng of total fecal IgA in PBS-T to wells in enzyme-linked immunosorbent assays identical to those used to measure serum anti-CT or anti-LPS responses, and we processed plates as described above.
2.6. Measurement of serum vibriocidal responses
We measured serum vibriocidal antibody titers in a micro-assay as previously described [33,36]. We inactivated the endogenous complement activity of mouse sera by heating sera to 56°C for 1 hr. We then added 50 μl aliquots of serial twofold dilutions of heat-inactivated sera in PBS (1:25 to 1:25,600) to wells of sterile 96-well tissue culture plates containing 50 μl per well of a 108-CFU/ml culture of V. cholerae O395 in PBS and 22% guinea pig complement (EMD Biosciences, San Diego, CA). We then incubated the plates for 1 hr at 37°C, added 150 μl of brain heart infusion broth (Becton Dickinson, Sparks, MD) to each well, incubated the plates for an additional 2 hr at 37°C, and then measured the optical density of each well at 600 nm. We calculated the vibriocidal titer as the dilution of serum causing 50% reduction in optical density compared with that of wells containing no serum [37,38].
2.7 The infant mouse challenge model
To evaluate protective efficacy, we used a V. cholerae infant mouse LD50 challenge model [18,26]. In brief, we removed 3 to 5 day old CD-1 suckling mice from their non-immunized mothers at least 3 hr prior to inoculation. We then administered to pups a 50 μl inoculum comprised of 25 μl of LB containing 106 or 107 CFU of wild type V. cholerae O395 and 25 μl of day 56 pooled serum from immunized mice (n = 15 mice/cohort). Following oral challenge, we kept neonates separate from dams at 30°C, and monitored animals every 3 to 6 hr for 48 hr, after which, surviving animals were euthanized.
2.8. Statistics and Graphs
We compared data from different test groups using two-tailed Mann Whitney U tests. Within each group, comparisons of data from different time points to baseline data (day 0) were carried out using one-tailed Wilcoxon Signed Ranks tests. Survival curves were analyzed by log rank testing. We used Statistical Package for Social Sciences version 12.0 for Windows (SPSS, Chicago, IL), and plotted data using Microsoft Excel 2002 and Prism5.3.
3. Results
3.1. Measurement of immune responses
In order to determine whether transcutaneous immunization with CHO-BSA and CT would elicit systemic anti-LPS humoral immune responses, we compared anti-LPS antibody responses in sera collected on days 0, 14, 28 and 42 from mice given zero, one, two, or three transcutaneous immunizations, respectively, of CHO-BSA and CT, or CT alone. Following two immunizations, we found mice transcutaneously immunized with CHO-BSA and CT had significant serum anti-LPS IgG responses compared to responses of mice receiving CT alone (day 28, p<0.01; Figure 2A), and that anti-LPS IgG responses increased following a third immunization (day 42, p<0.01). We found no significant anti-LPS IgG response in mice transcutaneously immunized with CHO-BSA in the absence of CT. Anti-LPS IgG responses were highest in animals receiving subcutaneously administered CHO-BSA and CT, although responses were not significantly different from those observed in mice transcutaneously immunized with CHO-BSA and CT in this study. We detected no appreciable anti-LPS response following transcutaneous immunization with LPS and CT. We detected no appreciable serum IgM (Figure 2B), serum IgA (Figure 3A), or stool IgA anti-LPS responses or vibriocidal responses (data not shown) in any vaccinated cohort.
Figure 2.
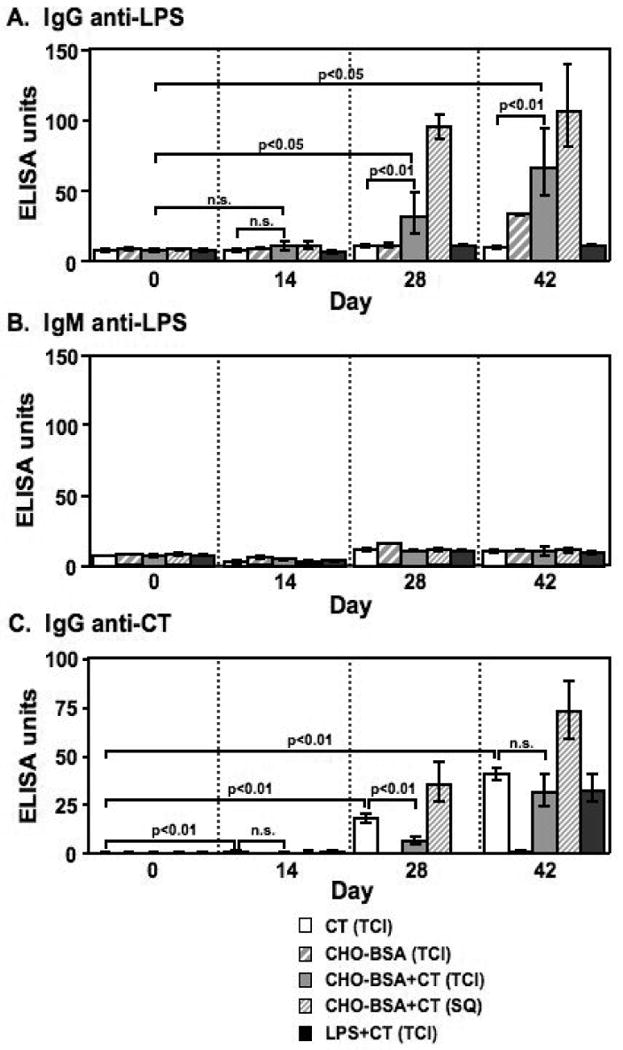
Serum (A) anti-LPS IgG, (B) anti-LPS IgM and (C) anti-CT IgG responses in mice transcutaneously immunized (TCI) with CT, CHO-BSA, CHO-BSA and CT, or LPS and CT, or subcutaneously immunized (SQ) with CHO-BSA and CT. Geometric mean and standard error of the mean are reported for each group.
Figure 3.
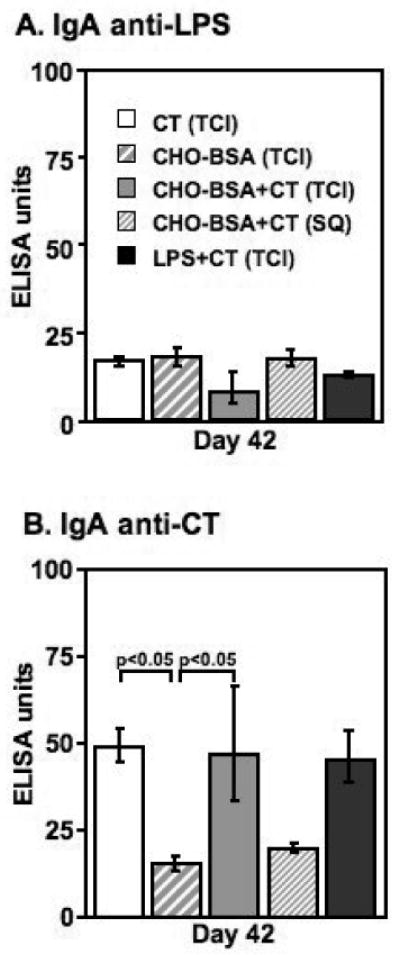
Serum day 42 (A) anti-LPS IgA, and (B) anti-CT IgA responses in mice transcutaneously immunized (TCI) with CT, CHO-BSA, CHO-BSA and CT, or LPS and CT, or subcutaneously immunized (SQ) with CHO-BSA and CT. Geometric mean and standard error of the mean are reported for each group.
In comparison, we detected low level but significant anti-CT IgG responses in serum following a single transcutaneous immunization with CT (p<0.01; Figure 2C), and anti-CT IgG responses increased with subsequent boosters. Significant serum anti-CT responses were observed in all mice immunized with CT irrespective of route or cohort, including mice transcutaneously immunized with holo-LPS and CT (p<0.05). We also observed serum anti-CT IgA responses in all groups transcutaneously immunized with CT (p<0.05), but not in cohorts of mice subcutaneously immunized with CT (Figure 3B). We detected no significant anti-CT IgA responses in feces (data not shown).
3.1. Neonatal mouse challenge assay
We detected no difference in survival between mice challenged with wild type V. cholerae O395 mixed with sera collected from mice transcutaneously immunized with CHO-BSA and CT, compared to survival following challenge with wild type V. cholerae O395 mixed with sera from mice transcutaneously immunized with CT alone (Figure 4).
Figure 4.
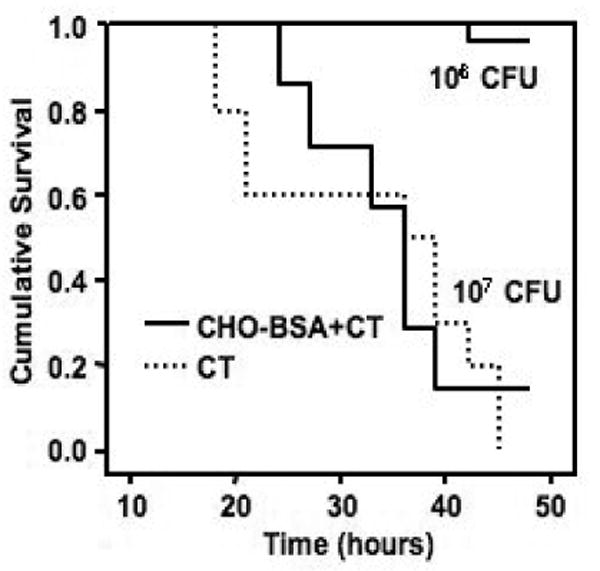
Survival likelihoods of neonatal CD-1 mice following oral challenge with wild-type O1 Ogawa V. cholerae O395. Three- to five-day-old pups (cohort size 15) were gavaged with 50 μl of a preparation containing 106 or 107 CFU of V. cholerae O395 mixed 1:1 with pooled day 56 serum from mice transcutaneously immunized with neoglycoconjugate (CHO-BSA) and immunoadjuvantative cholera toxin (CT) or CT alone. Challenged mice were kept at 30°C and monitored for death every 3 to 6 h starting 18 h after oral challenge till 48 h after oral challenge. Survival curves were compared by log rank testing.
4. Discussion
Parenterally administered vaccines that primarily elicit anti-LPS responses (such as the killed whole cell typhoid and cholera vaccines, which are no longer manufactured) can be effective, but often require repetitive dosing in immunologically naive individuals. Parenteral administration of such vaccines is also often associated with high adverse event profiles (including moderate or severe local reactions at the inoculation site), and often induces relatively short lived immunity with immune responses often decreasing substantially within 6 months of vaccination. LPS specificity is largely directed to the O-specific polysaccharide component of LPS; however, polysaccharides, which are T cell independent antigens, are themselves often poorly immunogenic in humans. The coupling of polysaccharides to protein carriers increases T cell activation, markedly improves the magnitude and duration of immune responses following immunization, and is the basis of a number of anti-bacterial vaccines now in wide use in humans, including the Haemophilus influenzae b, pneumococcal, and meningococcal conjugate vaccines [39]. These organisms differ from V. cholerae O1, the predominant cause of cholera globally, in that a component of their polysaccharides can be relatively easily produced for manufacturing purposes through the induction of a polysaccharide capsule during in vitro growth, and capsular polysaccharide does not contain the endotoxic lipid A component of LPS. To overcome this problem, polysaccharide components may be chemically synthesized prior to conjugation to a protein carrier. Such an approach has previously been applied to V. cholerae, and intra-peritoneal immunization of mice with a glycoconjugate comprised of a synthetic hexasaccharide of Ogawa O1 perosamines attached to BSA was found to induce serum anti-LPS and vibriocidal antibody responses, and protection in a challenge assay [18].
Transcutaneous immunization has been shown to elicit prominent IgG antibody responses, and weaker, but still significant mucosal immune responses to highly immunogenic proteins such as CT [27,40], and other antigens, when co-administered with an immunoadjuvant [26,31,32,41]. For instance, transcutaneous immunization with TcpA, an important virulence factor in V. cholerae, gives rise to systemic and mucosal anti-TcpA immune responses that are protective against V. cholerae challenge in mice [26]. Previous evaluation of the immunogenicity of transdermally applied polysaccharides, although less well studied, has also been successful: transcutaneous application of a Haemophilus influenzae b conjugate vaccine has been shown to induce in animals an anti-Hib polysaccharide immune response that is protective [42]. We therefore evaluated whether a cholera synthetic glycoconjugate could similarly induce protective immunity against cholera following transcutaneous immunization. Although we were able to induce anti-LPS IgG, anti-CT IgG, and anti-CT IgA serum responses, we were not able to detect anti-LPS IgA following transcutaneous immunization. We were also unable to detect anti-LPS IgM responses following immunization, which may in part be due to the ability of protein conjugation to alter T cell independence of polysaccharide processing to T cell dependence and isotype maturation [18]. This lack of induction of IgM may in part explain our inability to detect a vibriocidal response, since anti-LPS IgM is thought to be a primary component of the vibriocidal antibody response [7].
In this study, transcutaneous immunization with CHO-BSA was not associated with protection in a challenge assay despite the induction of serum anti-LPS IgG. In humans, serum IgG with LPS specificity is not associated with protection from cholera, although serum IgA with LPS specificity and vibriocidal antibodies (comprised in part by complement-fixing anti-LPS IgM) is associated with protection. This suggests, that while LPS may be immunogenic, the type of antibody response may be critical to mediating protection, with responses that reflect mucosal immunity and those that bind complement perhaps being the most important. Indeed, since V. cholerae is a non-invasive organism that does not cross the intestinal epithelial surface, the serum vibriocidal response may in fact be a surrogate marker for as yet poorly understood mucosal immune responses.
It is unclear why intra-peritoneal immunization with a similar vaccine previously resulted in protective immune responses in mice, but trafficking and antigenic handling by intra-peritoneal macrophages and lymphocytes differ from those associated with subcutaneous and dermal structures, and immunoadjuvants used in the two studies were also different (RIBI in the previous study [18], CT in this study). Although we did not perform dose ranging studies in this experiment, the amount of antigen used in our vaccine preparation (approximately 100 μg total antigen, containing 10 μg of sugar) is equivalent to doses previously found to be effective for sugar and protein antigens when administered transcutaneously, and would probably represent the maximum for viable manufacturing purposes [18,26,31,42].
We recognize that the synthetic hexasaccharide conjugate included in our vaccine preparation represents only a small antigenic fragment of the V. cholerae O-SP. It was for this reason that we also evaluated immune responses following transcutaneous application of the intact LPS antigen. Interestingly, we were unable to detect any anti-LPS responses (including IgG) using transcutaneously applied holo-LPS, although we were able to detect prominent IgG and IgA responses to co-administered CT. Whether the lack of anti-LPS responses in our study reflected a lack of penetration of antigen to dermal Langerhan's cells or poor immunogenicity is currently unclear.
Although we were unable to induce protective immune responses with a synthetic V. cholerae LPS glycoconjugate administered transcutaneously in this study, our ability to induce anti-LPS responses in immunologically naïve mice with TCI raises the possibility that transcutaneous immunization with glycoconjugate could be used to boost pre-existing anti-LPS and vibriocidal responses in previously primed subjects [34,43]. In addition, analysis of immune cell trafficking and antigenic handling could give future insights into the potential role of transcutaneous immunization using polysaccharide-conjugate vaccines against cholera.
Acknowledgments
This work was supported by grants from the National Institutes of Health, including the National Institute of Allergy & Infectious Diseases (AI067342 [ETR], AI40725 [ETR]. AI058935 [SBC]), and a Training Grant in Vaccine Development from the Fogarty International Center (TW05572 [AS, FQ]). We are grateful to William Wade for helpful input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Koelle K, Pascual M, Yunus M. Serotype cycles in cholera dynamics. Proc Biol Sci. 2006 Nov 22;273(1603):2879–86. doi: 10.1098/rspb.2006.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svennerholm AM, Jertborn M, Gothefors L, Karim AM, Sack DA, Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984 Jun;149(6):884–93. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- 3.Harris JB, Khan AI, LaRocque RC, et al. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infect Immun. 2005 Nov;73(11):7422–7. doi: 10.1128/IAI.73.11.7422-7427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris JB, LaRocque RC, Chowdhury F, et al. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis. 2008;2(4):e221. doi: 10.1371/journal.pntd.0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed A, Bhattacharjee AK, Mosley WH. Characteristics of the serum vibriocidal and agglutinating antibodies in cholera cases and in normal residents of the endemic and non-endemic cholera areas. J Immunol. 1970 Aug;105(2):431–41. [PubMed] [Google Scholar]
- 6.Qadri F, Wenneras C, Albert MJ, et al. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect Immun. 1997 Sep;65(9):3571–6. doi: 10.1128/iai.65.9.3571-3576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Losonsky GA, Lim Y, Motamedi P, et al. Vibriocidal antibody responses in North American volunteers exposed to wild-type or vaccine Vibrio cholerae O139: specificity and relevance to immunity. Clin Diagn Lab Immunol. 1997 May;4(3):264–9. doi: 10.1128/cdli.4.3.264-269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass RI, Svennerholm AM, Khan MR, Huda S, Huq MI, Holmgren J. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J Infect Dis. 1985 Feb;151(2):236–42. doi: 10.1093/infdis/151.2.236. [DOI] [PubMed] [Google Scholar]
- 9.Apter FM, Michetti P, Winner LS, III, Mack JA, Mekalanos JJ, Neutra MR. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993 Dec;61(12):5279–85. doi: 10.1128/iai.61.12.5279-5285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winner L, III, Mack J, Weltzin R, Mekalanos JJ, Kraehenbuhl JP, Neutra MR. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991 Mar;59(3):977–82. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonson G, Osek J, Svennerholm AM, Holmgren J. Immune mechanisms and protective antigens of Vibrio cholerae serogroup O139 as a basis for vaccine development. Infect Immun. 1996 Sep;64(9):3778–85. doi: 10.1128/iai.64.9.3778-3785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svennerholm AM, Holmgren J. Synergistic protective effect in rabbits of immunization with Vibrio cholerae lipopolysaccharide and toxin/toxoid. Infect Immun. 1976 Mar;13(3):735–40. doi: 10.1128/iai.13.3.735-740.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosley WH, Benenson AS, Barui R. A serological survey for cholera antibodies in rural east Pakistan. 2. A comparison of antibody titres in the immunized and control population of a cholera-vaccine field-trial area and the relation of antibody titre to cholera case rate. Bull World Health Organ. 1968;38(3):335–46. [PMC free article] [PubMed] [Google Scholar]
- 14.Mosley WH, Benenson AS, Barui R. A serological survey for cholear antibodies in rural east Pakistan. 1. The distribution of antibody in the control population of a cholera-vaccine field-trial area and the relation of antibody titre to the pattern of endemic cholera. Bull World Health Organ. 1968;38(3):327–34. [PMC free article] [PubMed] [Google Scholar]
- 15.Saha D, LaRocque RC, Khan AI, et al. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J Infect Dis. 2004 Jun 15;189(12):2318–22. doi: 10.1086/421275. [DOI] [PubMed] [Google Scholar]
- 16.Chernyak A, Karavanov A, Ogawa Y, Kovac P. Conjugating oligosaccharides to proteins by squaric acid diester chemistry: rapid monitoring of the progress of conjugation, and recovery of the unused ligand. Carbohydr Res. 2001 Feb 28;330(4):479–86. doi: 10.1016/s0008-6215(01)00018-0. [DOI] [PubMed] [Google Scholar]
- 17.Hisatsune K, Kondo S, Isshiki Y, Iguchi T, Haishima Y. Occurrence of 2-O-methyl-N-(3-deoxy-L-glycero-tetronyl)-D-perosamine (4-amino-4,6-dideoxy-D-manno-pyranose) in lipopolysaccharide from Ogawa but not from Inaba O forms of O1 Vibrio cholerae. Biochem Biophys Res Commun. 1993 Jan 15;190(1):302–7. doi: 10.1006/bbrc.1993.1046. [DOI] [PubMed] [Google Scholar]
- 18.Chernyak A, Kondo S, Wade TK, et al. Induction of protective immunity by synthetic Vibrio cholerae hexasaccharide derived from V. cholerae O1 Ogawa lipopolysaccharide bound to a protein carrier. J Infect Dis. 2002 Apr 1;185(7):950–62. doi: 10.1086/339583. [DOI] [PubMed] [Google Scholar]
- 19.Gupta RK, Taylor DN, Bryla DA, Robbins JB, Szu SC. Phase 1 evaluation of Vibrio cholerae O1, serotype Inaba, polysaccharide-cholera toxin conjugates in adult volunteers. Infect Immun. 1998 Jul;66(7):3095–9. doi: 10.1128/iai.66.7.3095-3099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta RK, Szu SC, Finkelstein RA, Robbins JB. Synthesis, characterization, and some immunological properties of conjugates composed of the detoxified lipopolysaccharide of Vibrio cholerae O1 serotype Inaba bound to cholera toxin. Infect Immun. 1992 Aug;60(8):3201–8. doi: 10.1128/iai.60.8.3201-3208.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boutonnier A, Villeneuve S, Nato F, Dassy B, Fournier JM. Preparation, immunogenicity, and protective efficacy, in a murine model, of a conjugate vaccine composed of the polysaccharide moiety of the lipopolysaccharide of Vibrio cholerae O139 bound to tetanus toxoid. Infect Immun. 2001 May;69(5):3488–93. doi: 10.1128/IAI.69.5.3488-3493.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meeks MD, Saksena R, Ma X, et al. Synthetic fragments of Vibrio cholerae O1 Inaba O-specific polysaccharide bound to a protein carrier are immunogenic in mice but do not induce protective antibodies. Infect Immun. 2004 Jul;72(7):4090–101. doi: 10.1128/IAI.72.7.4090-4101.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saksena R, Ma X, Wade TK, Kovac P, Wade WF. Length of the linker and the interval between immunizations influences the efficacy of Vibrio cholerae O1, Ogawa hexasaccharide neoglycoconjugates. FEMS Immunol Med Microbiol. 2006 Jun;47(1):116–28. doi: 10.1111/j.1574-695X.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 24.Saksena R, Ma X, Wade TK, Kovac P, Wade WF. Effect of saccharide length on the immunogenicity of neoglycoconjugates from synthetic fragments of the O-SP of Vibrio cholerae O1, serotype Ogawa. Carbohydr Res. 2005 Oct 17;340(14):2256–69. doi: 10.1016/j.carres.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Wade TK, Saksena R, Shiloach J, Kovac P, Wade WF. Immunogenicity of synthetic saccharide fragments of Vibrio cholerae O1 (Ogawa and Inaba) bound to Exotoxin A. FEMS Immunol Med Microbiol. 2006 Nov;48(2):237–51. doi: 10.1111/j.1574-695X.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 26.Rollenhagen JE, Kalsy A, Cerda F, et al. Transcutaneous immunization with toxin-coregulated pilin A induces protective immunity against Vibrio cholerae O1 El Tor challenge in mice. Infect Immun. 2006 Oct;74(10):5834–9. doi: 10.1128/IAI.00438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glenn GM, Scharton-Kersten T, Vassell R, Mallett CP, Hale TL, Alving CR. Transcutaneous immunization with cholera toxin protects mice against lethal mucosal toxin challenge. J Immunol. 1998 Oct 1;161(7):3211–4. [PubMed] [Google Scholar]
- 28.Jonson G, Svennerholm AM, Holmgren J. Vibrio cholerae expresses cell surface antigens during intestinal infection which are not expressed during in vitro culture. Infect Immun. 1989 Jun;57(6):1809–15. doi: 10.1128/iai.57.6.1809-1815.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen RF. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–81. [PubMed] [Google Scholar]
- 30.Hou SJ, Saksena R, Kovac P. Preparation of glycoconjugates by dialkyl squarate chemistry revisited. Carbohydr Res. 2008 Feb 4;343(2):196–210. doi: 10.1016/j.carres.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghose C, Kalsy A, Sheikh A, et al. Transcutaneous immunization with Clostridium difficile toxoid A induces systemic and mucosal immune responses and toxin A-neutralizing antibodies in mice. Infect Immun. 2007 Jun;75(6):2826–32. doi: 10.1128/IAI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharton-Kersten T, Yu J, Vassell R, O'Hagan D, Alving CR, Glenn GM. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins, subunits, and unrelated adjuvants. Infect Immun. 2000 Sep;68(9):5306–13. doi: 10.1128/iai.68.9.5306-5313.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crean TI, John M, Calderwood SB, Ryan ET. Optimizing the germfree mouse model for in vivo evaluation of oral Vibrio cholerae vaccine and vector strains. Infect Immun. 2000 Feb;68(2):977–81. doi: 10.1128/iai.68.2.977-981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.John M, Bridges EA, Miller AO, Calderwood SB, Ryan ET. Comparison of mucosal and systemic humoral immune responses after transcutaneous and oral immunization strategies. Vaccine. 2002 Jun 21;20(2122):2720–6. doi: 10.1016/s0264-410x(02)00208-6. [DOI] [PubMed] [Google Scholar]
- 35.John M, Crean TI, Calderwood SB, Ryan ET. In vitro and in vivo analyses of constitutive and in vivo-induced promoters in attenuated vaccine and vector strains of Vibrio cholerae. Infect Immun. 2000 Mar;68(3):1171–5. doi: 10.1128/iai.68.3.1171-1175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butterton JR, Ryan ET, Shahin RA, Calderwood SB. Development of a germfree mouse model of Vibrio cholerae infection. Infect Immun. 1996 Oct;64(10):4373–7. doi: 10.1128/iai.64.10.4373-4377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan ET, Butterton JR, Zhang T, Baker MA, Stanley SL, Jr, Calderwood SB. Oral immunization with attenuated vaccine strains of Vibrio cholerae expressing a dodecapeptide repeat of the serine-rich Entamoeba histolytica protein fused to the cholera toxin B subunit induces systemic and mucosal antiamebic and anti-V. cholerae antibody responses in mice. Infect Immun. 1997 Aug;65(8):3118–25. doi: 10.1128/iai.65.8.3118-3125.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan ET, Butterton JR, Smith RN, Carroll PA, Crean TI, Calderwood SB. Protective immunity against Clostridium difficile toxin A induced by oral immunization with a live, attenuated Vibrio cholerae vector strain. Infect Immun. 1997 Jul;65(7):2941–9. doi: 10.1128/iai.65.7.2941-2949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claesson BA, Schneerson R, Lagergard T, et al. Persistence of serum antibodies elicited by Haemophilus influenzae type b-tetanus toxoid conjugate vaccine in infants vaccinated at 3, 5 and 12 months of age. Pediatr Infect Dis J. 1991 Aug;10(8):560–4. doi: 10.1097/00006454-199108000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Glenn GM, Rao M, Matyas GR, Alving CR. Skin immunization made possible by cholera toxin. Nature. 1998 Feb 26;391(6670):851. doi: 10.1038/36014. [DOI] [PubMed] [Google Scholar]
- 41.Glenn GM, Scharton-Kersten T, Vassell R, Matyas GR, Alving CR. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins as antigens and adjuvants. Infect Immun. 1999 Mar;67(3):1100–6. doi: 10.1128/iai.67.3.1100-1106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mawas F, Peyre M, Beignon AS, et al. Successful induction of protective antibody responses against Haemophilus influenzae type b and diphtheria after transcutaneous immunization with the glycoconjugate polyribosyl ribitol phosphate-cross-reacting material 197 vaccine. J Infect Dis. 2004 Sep 15;190(6):1177–82. doi: 10.1086/423327. [DOI] [PubMed] [Google Scholar]
- 43.Wade TK, Wade WF. Variable gene family usage of protective and non-protective anti-Vibrio cholerae O1 LPS antibody heavy chains. Microbiol Immunol. 2008 Dec;52(12):611–20. doi: 10.1111/j.1348-0421.2008.00078.x. [DOI] [PubMed] [Google Scholar]


