Abstract
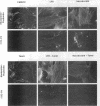
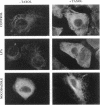
Fibronectin (FN) matrix assembly is a cell-dependent process mediated by cell surface-binding sites for the 70-kDa amino-terminal region of FN. We have shown recently that lysophosphatidic acid (LPA) is a stimulator of FN matrix assembly. Disruption of microtubules has been shown to mimic some of the intracellular effects of LPA including the formation of actin stress fibers and myosin light chain phosphorylation. We compared the effects of microtubule disruption and LPA on FN binding and actin cytoskeleton organization. The disruption of microtubules by nocodazole or vinblastine increased FN binding to adherent cells. The modulation of binding sites was rapid, dynamic, and reversible. Enhanced binding was due to increases in both the number and affinity of binding sites. These effects are similar to the effects of LPA on FN binding. Binding induced by nocodazole was inhibited by the microtubule-stabilizing agent Taxol but not by pretreatment with a concentration of phospholipase B that totally abolished the stimulatory effect of LPA. Fluorescence microscopy revealed a close correlation among actin stress fiber formation, cell contraction, and FN binding. Blockage of the small GTP binding protein Rho or actin-myosin interactions inhibited the effects of both nocodazole and LPA on FN binding. These observations demonstrate that Rho-dependent actin stress fiber formation and cell contraction induce increased FN binding and represent a rapid labile way that cells can modulate FN matrix assembly.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre K. M., McCormick R. J., Schwarzbauer J. E. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J Biol Chem. 1994 Nov 11;269(45):27863–27868. [PubMed] [Google Scholar]
- Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989 Aug;109(2):863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
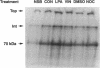
- Aktories K., Just I. In vitro ADP-ribosylation of Rho by bacterial ADP-ribosyltransferases. Methods Enzymol. 1995;256:184–195. doi: 10.1016/0076-6879(95)56023-8. [DOI] [PubMed] [Google Scholar]
- Allen-Hoffmann B. L., Mosher D. F. Matrix assembly sites for exogenous fibronectin are decreased on human fibroblasts after treatment with agents which increase intracellular cAMP. J Biol Chem. 1987 Oct 15;262(29):14361–14365. [PubMed] [Google Scholar]
- Allio A. E., McKeown-Longo P. J. Extracellular matrix assembly of cell-derived and plasma-derived fibronectins by substrate-attached fibroblasts. J Cell Physiol. 1988 Jun;135(3):459–466. doi: 10.1002/jcp.1041350313. [DOI] [PubMed] [Google Scholar]
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem. 1996 Aug 23;271(34):20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Barry E. L., Mosher D. F. Factor XIII cross-linking of fibronectin at cellular matrix assembly sites. J Biol Chem. 1988 Jul 25;263(21):10464–10469. [PubMed] [Google Scholar]
- Barry E. L., Mosher D. F. Factor XIIIa-mediated cross-linking of fibronectin in fibroblast cell layers. Cross-linking of cellular and plasma fibronectin and of amino-terminal fibronectin fragments. J Biol Chem. 1989 Mar 5;264(7):4179–4185. [PubMed] [Google Scholar]
- Bershadsky A., Chausovsky A., Becker E., Lyubimova A., Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996 Oct 1;6(10):1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 activity specific for phosphatidic acid. A possible mechanism for the production of arachidonic acid in platelets. J Biol Chem. 1981 Jun 10;256(11):5399–5403. [PubMed] [Google Scholar]
- Boucaut J. C., Darribère T., Poole T. J., Aoyama H., Yamada K. M., Thiery J. P. Biologically active synthetic peptides as probes of embryonic development: a competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol. 1984 Nov;99(5):1822–1830. doi: 10.1083/jcb.99.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl A. M., Johnson N. L., Dhanasekaran N., Johnson G. L. G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J Biol Chem. 1995 Oct 20;270(42):24631–24634. doi: 10.1074/jbc.270.42.24631. [DOI] [PubMed] [Google Scholar]
- Checovich W. J., Mosher D. F. Lysophosphatidic acid enhances fibronectin binding to adherent cells. Arterioscler Thromb. 1993 Nov;13(11):1662–1667. doi: 10.1161/01.atv.13.11.1662. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M., Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996 Jun;133(6):1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer L. P., Mitchison T. J. Myosin is involved in postmitotic cell spreading. J Cell Biol. 1995 Oct;131(1):179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L., Carney D. H. Evidence that microtubule depolymerization early in the cell cycle is sufficient to initiate DNA synthesis. Cell. 1981 Jan;23(1):61–71. doi: 10.1016/0092-8674(81)90270-1. [DOI] [PubMed] [Google Scholar]
- Danowski B. A. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989 Jun;93(Pt 2):255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Fourcade O., Simon M. F., Viodé C., Rugani N., Leballe F., Ragab A., Fournié B., Sarda L., Chap H. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 1995 Mar 24;80(6):919–927. doi: 10.1016/0092-8674(95)90295-3. [DOI] [PubMed] [Google Scholar]
- Gailit J., Clark R. A. Wound repair in the context of extracellular matrix. Curr Opin Cell Biol. 1994 Oct;6(5):717–725. doi: 10.1016/0955-0674(94)90099-x. [DOI] [PubMed] [Google Scholar]
- George E. L., Georges-Labouesse E. N., Patel-King R. S., Rayburn H., Hynes R. O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993 Dec;119(4):1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., Robinson P. Identification of the molecular species of lysophosphatidic acid produced when platelets are stimulated by thrombin. Biochim Biophys Acta. 1989 Feb 20;1001(3):282–285. doi: 10.1016/0005-2760(89)90112-4. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Guo Z., Liliom K., Fischer D. J., Bathurst I. C., Tomei L. D., Kiefer M. C., Tigyi G. Molecular cloning of a high-affinity receptor for the growth factor-like lipid mediator lysophosphatidic acid from Xenopus oocytes. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14367–14372. doi: 10.1073/pnas.93.25.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht J. H., Weiner J. A., Post S. R., Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996 Nov;135(4):1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H., Takemori S. Butanedione monoxime suppresses contraction and ATPase activity of rabbit skeletal muscle. J Biochem. 1989 Apr;105(4):638–643. doi: 10.1093/oxfordjournals.jbchem.a122717. [DOI] [PubMed] [Google Scholar]
- Hocking D. C., Smith R. K., McKeown-Longo P. J. A novel role for the integrin-binding III-10 module in fibronectin matrix assembly. J Cell Biol. 1996 Apr;133(2):431–444. doi: 10.1083/jcb.133.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking D. C., Sottile J., McKeown-Longo P. J. Fibronectin's III-1 module contains a conformation-dependent binding site for the amino-terminal region of fibronectin. J Biol Chem. 1994 Jul 22;269(29):19183–19187. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jalink K., van Corven E. J., Hengeveld T., Morii N., Narumiya S., Moolenaar W. H. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994 Aug;126(3):801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K., van Corven E. J., Moolenaar W. H. Lysophosphatidic acid, but not phosphatidic acid, is a potent Ca2(+)-mobilizing stimulus for fibroblasts. Evidence for an extracellular site of action. J Biol Chem. 1990 Jul 25;265(21):12232–12239. [PubMed] [Google Scholar]
- Kajstura J., Bereiter-Hahn J. Disruption of microtubules induces formation of actin fibrils in density-inhibited 3T3 cells. Cell Biol Int. 1993 Nov;17(11):1023–1031. doi: 10.1006/cbir.1993.1032. [DOI] [PubMed] [Google Scholar]
- Kapeller R., Chakrabarti R., Cantley L., Fay F., Corvera S. Internalization of activated platelet-derived growth factor receptor-phosphatidylinositol-3' kinase complexes: potential interactions with the microtubule cytoskeleton. Mol Cell Biol. 1993 Oct;13(10):6052–6063. doi: 10.1128/mcb.13.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996 Jul 12;273(5272):245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kolodney M. S., Elson E. L. Contraction due to microtubule disruption is associated with increased phosphorylation of myosin regulatory light chain. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10252–10256. doi: 10.1073/pnas.92.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai N., Morii N., Fujisawa K., Nemoto Y., Narumiya S. ADP-ribosylation of rho p21 inhibits lysophosphatidic acid-induced protein tyrosine phosphorylation and phosphatidylinositol 3-kinase activation in cultured Swiss 3T3 cells. J Biol Chem. 1993 Nov 25;268(33):24535–24538. [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M., Cuatrecasas P. The phosphatidylinositol cycle and the regulation of arachidonic acid production. Nature. 1981 Jul 23;292(5821):367–369. doi: 10.1038/292367a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C. W., Smith C. G., Woods A., Rees D. A. Mechanisms of cellular adhesion. II. The interplay between adhesion, the cytoskeleton and morphology in substrate-attached cells. Exp Cell Res. 1977 Dec;110(2):427–437. doi: 10.1016/0014-4827(77)90309-3. [DOI] [PubMed] [Google Scholar]
- Matsui T., Amano M., Yamamoto T., Chihara K., Nakafuku M., Ito M., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996 May 1;15(9):2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Mautner V., Hynes R. O. Surface distribution of LETS protein in relation to the cytoskeleton of normal and transformed cells. J Cell Biol. 1977 Dec;75(3):743–768. doi: 10.1083/jcb.75.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo P. J., Mosher D. F. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol. 1983 Aug;97(2):466–472. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo P. J., Mosher D. F. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J Cell Biol. 1985 Feb;100(2):364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo P. J., Mosher D. F. Mechanism of formation of disulfide-bonded multimers of plasma fibronectin in cell layers of cultured human fibroblasts. J Biol Chem. 1984 Oct 10;259(19):12210–12215. [PubMed] [Google Scholar]
- McKillop D. F., Fortune N. S., Ranatunga K. W., Geeves M. A. The influence of 2,3-butanedione 2-monoxime (BDM) on the interaction between actin and myosin in solution and in skinned muscle fibres. J Muscle Res Cell Motil. 1994 Jun;15(3):309–318. doi: 10.1007/BF00123483. [DOI] [PubMed] [Google Scholar]
- Miura Y., Kikuchi A., Musha T., Kuroda S., Yaku H., Sasaki T., Takai Y. Regulation of morphology by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in Swiss 3T3 cells. J Biol Chem. 1993 Jan 5;268(1):510–515. [PubMed] [Google Scholar]
- Moolenaar W. H., Kranenburg O., Postma F. R., Zondag G. C. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr Opin Cell Biol. 1997 Apr;9(2):168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Vaheri A. Thrombin stimulates the production and release of a major surface-associated glycoprotein (fibronectin) in cultures of human fibroblasts. Exp Cell Res. 1978 Mar 15;112(2):323–334. doi: 10.1016/0014-4827(78)90215-x. [DOI] [PubMed] [Google Scholar]
- Narumiya S., Sekine A., Fujiwara M. Substrate for botulinum ADP-ribosyltransferase, Gb, has an amino acid sequence homologous to a putative rho gene product. J Biol Chem. 1988 Nov 25;263(33):17255–17257. [PubMed] [Google Scholar]
- Peters D. M., Mosher D. F. Localization of cell surface sites involved in fibronectin fibrillogenesis. J Cell Biol. 1987 Jan;104(1):121–130. doi: 10.1083/jcb.104.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Wind M. L., Hewitt A. T., Kleinman H. K. Effect of collagen and cell shape on binding of fibronectin to cells. Arch Biochem Biophys. 1981 Jan;206(1):205–212. doi: 10.1016/0003-9861(81)90082-5. [DOI] [PubMed] [Google Scholar]
- Reszka A. A., Seger R., Diltz C. D., Krebs E. G., Fischer E. H. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8881–8885. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Roman J., LaChance R. M., Broekelmann T. J., Kennedy C. J., Wayner E. A., Carter W. G., McDonald J. A. The fibronectin receptor is organized by extracellular matrix fibronectin: implications for oncogenic transformation and for cell recognition of fibronectin matrices. J Cell Biol. 1989 Jun;108(6):2529–2543. doi: 10.1083/jcb.108.6.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury S., Rasenick M. M. Tubulin-G protein association stabilizes GTP binding and activates GTPase: cytoskeletal participation in neuronal signal transduction. Biochemistry. 1994 Aug 16;33(32):9800–9805. doi: 10.1021/bi00198a052. [DOI] [PubMed] [Google Scholar]
- Shinohara Y., Nishida E., Sakai H. Initiation of DNA synthesis by microtubule disruption in quiescent rat 3Y1 cells. Eur J Biochem. 1989 Aug 1;183(2):275–280. doi: 10.1111/j.1432-1033.1989.tb14924.x. [DOI] [PubMed] [Google Scholar]
- Somers C. E., Mosher D. F. Protein kinase C modulation of fibronectin matrix assembly. J Biol Chem. 1993 Oct 25;268(30):22277–22280. [PubMed] [Google Scholar]
- Sontag E., Nunbhakdi-Craig V., Bloom G. S., Mumby M. C. A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J Cell Biol. 1995 Mar;128(6):1131–1144. doi: 10.1083/jcb.128.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. Decanoyl lysophosphatidic acid induces platelet aggregation through an extracellular action. Evidence against a second messenger role for lysophosphatidic acid. Biochem J. 1985 Nov 15;232(1):61–66. doi: 10.1042/bj2320061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Bauer J. S., Juliano R. L., McDonald J. A. The alpha 5 beta 1 integrin fibronectin receptor, but not the alpha 5 cytoplasmic domain, functions in an early and essential step in fibronectin matrix assembly. J Biol Chem. 1993 Oct 15;268(29):21883–21888. [PubMed] [Google Scholar]
- Wu C., Keivens V. M., O'Toole T. E., McDonald J. A., Ginsberg M. H. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995 Dec 1;83(5):715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Yan K., Greene E., Belga F., Rasenick M. M. Synaptic membrane G proteins are complexed with tubulin in situ. J Neurochem. 1996 Apr;66(4):1489–1495. doi: 10.1046/j.1471-4159.1996.66041489.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Checovich W. J., Peters D. M., Albrecht R. M., Mosher D. F. Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J Cell Biol. 1994 Dec;127(5):1447–1459. doi: 10.1083/jcb.127.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Mosher D. F. Cross-linking of the NH2-terminal region of fibronectin to molecules of large apparent molecular mass. Characterization of fibronectin assembly sites induced by the treatment of fibroblasts with lysophosphatidic acid. J Biol Chem. 1996 Dec 27;271(52):33284–33292. doi: 10.1074/jbc.271.52.33284. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., Hordijk P. L., Medema R. H., Bos J. L., Moolenaar W. H. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1257–1261. doi: 10.1073/pnas.90.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]