Abstract
Posttraumatic stress disorder (PTSD) is characterized mainly by symptoms of re-experiencing, avoidance and hyperarousal as a consequence of catastrophic and traumatic events that are distinguished from ordinary stressful life events. Although extensive research has already been done, the etiology of PTSD remains unclear. Research on the impact of trauma on neurobiological systems can be expected to inform the development of treatments that are directed specifically to symptoms of PTSD. During the past 25 years there has been a dramatic increase in the knowledge about noradrenergic and serotonergic mechanisms in stress response, PTSD and more recently in resilience and this knowledge has justified the use of antidepressants with monoaminergic mechanisms of action for patients with PTSD. Nevertheless, available treatments of PTSD are only to some extent effective and enhanced understanding of the neurobiology of PTSD may lead to the development of improved treatments for these patients. In the present review, we aim to close existing gaps between basic research in psychopathology, neurobiology and treatment development with the ultimate goal to translate basic research into clinically relevant findings which may directly benefit patients with PTSD.
Keywords: Stress, resilience, PTSD, serotonin, norepinephrine, neuropeptides
Introduction
The Concept of PTSD and Resilience
The definition of posttraumatic stress disorder (PTSD) in DSM-IV (American Psychiatric Association, 1994) links a specific syndrome characterized mainly by symptoms of re-experiencing, avoidance and hyperarousal with catastrophic and traumatic events that are distinguished from ordinary stressful life events. Epidemiological surveys in the United States have documented that the probability of developing PTSD following traumatic exposure is approximately 10% (Breslau et al., 1998; Kessler et al., 1995); women are more likely than men to develop PTSD once trauma occurs (Breslau et al., 1999; Norris, 1992; Stein et al., 1997; Stein et al., 2000). The increased morbidity (Hoge et al., 2007; Kubzansky et al., 2007), disability (Schnurr et al., 2006; Zatzick et al., 1997) and mortality (Boscarino, 2006) associated with PTSD call for increased efforts to develop more informative models for testing pathophysiologic and treatment hypotheses.
To date, there exists an important gap in trauma research because whereas available research has made important contributions to understand risk factors for negative mental health consequences of traumatic stress exposure, the identification of characteristics associated with resilience to the impact of traumatic stress exposure could inform studies of preventive and treatment procedures for people with or at risk of trauma exposure (Rutter, 1985). Resilience, in contrast to recovery from symptomatic PTSD, is defined as the absence of psychopathology by DSM-IV criteria in adults who were exposed to extreme life stressors (Bonanno et al., 2007; DuMont et al., 2007; Tiet et al., 1998). A relatively large body of research has focused on the identification of psychosocial factors associated with the capability of trauma-exposed individuals to successfully adapt to extreme stress exposure. These studies have shown that lower lifetime trauma load (Breslau et al., 2008), male gender (Brewin et al., 2000), the use of adaptive coping strategies, e.g. emotional expression or the ability to elicit social support, optimism, cognitive flexibility, mastery, religion, and purpose in life, and the lower use of avoidant coping strategies, e.g. denial are associated with resilience (Alim et al., 2008; Yehuda et al., 2006b). Comparatively few studies have examined neurobiological mechanisms that confer resilience and thereby allow successful adaptation to extreme stress exposure without developing psychopathology. From a neurobiological perspective, preclinical and clinical studies have provided strong evidence that neuropeptide Y, and the monoamines serotonin (5-HT) and norepinephrine (NE) play an important role in models of resilience.
Given that current prevention and treatment strategies for PTSD are non-optimal, additional research is needed to investigate basic mechanisms underlying the adaptive and maladaptive responses to severe stress in order to decrease the devastating impact of these disorders on public health. PTSD is increasingly understood to involve central neurotransmitter imbalances and neuroanatomical disruptions (Figure 1.), along with potential dysregulation of immune, autonomic, endocrine function, and cardiovascular function. In this paper emphasis is placed on recent advances in PTSD research, and discussion on future directions that might catalyze discovery of innovative treatments.
Figure 1.
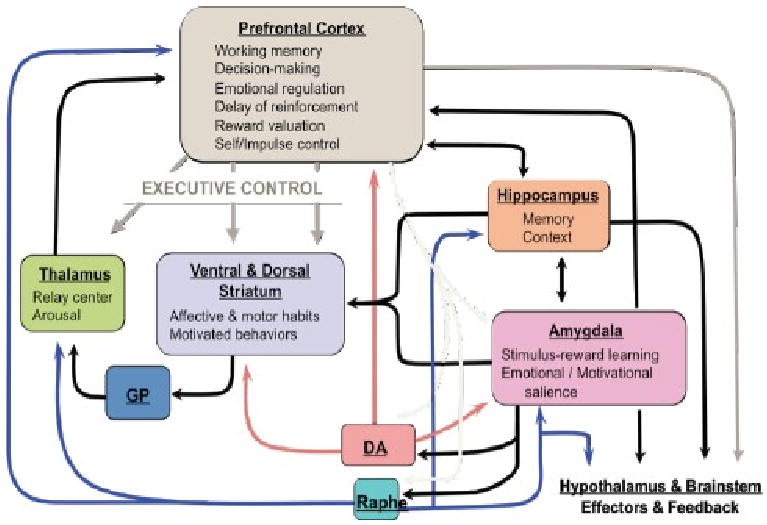
Acute and repeated stressors disrupt frontal-cortical control over limbic-striatal circuits which constitute the brain stress circuit, increase mesolimbic dopaminergic transmission and increase prefrontal cortex (PFC) norepinephrine (NE) and serotonin (5-HT) transmission. The prevailing neurocircuitry model of PTSD which has been developed from theoretical considerations, research in animals and expanded to human imaging studies emphasizes the role of the amygdala, as well as its interactions with the ventral/medial prefrontal cortex (vmPFC), hippocampus and anterior cingulate cortex. The model hypothesizes hyperresponsivity of the amygdala to threat-related stimuli and deficient ventro-medial PFC function but also evidence for generalized hypervigilance in PTSD.
Current Treatment Challenges
There have been significant advances in the pharmacotherapy of patients with PTSD, and certain medications, e.g. selective serotonin reuptake inhibitors are considered first-line treatment for adult PTSD. Nevertheless, residual symptoms after treatment are more the rule than the exception and there is concern that further research will conclude that chronicity leads to progressive treatment resistance. This has led to a shift with new emphasis on treating both acute and residual symptoms of PTSD more aggressively, with a close eye being kept on functional impairment. About 40% of patients with PTSD do not meet typical response criteria to an initial course of antidepressants, and furthermore the majority of patients are not symptom free with monotherapy (Stein et al., 2006). In fact, remission rates for sertraline, the only FDA approved antidepressant to treat PTSD are about 25% (Davidson, 2004) and therefore there is a need for additional research about how to enhance effectiveness of the existing treatment strategies for PTSD (Dieperink et al., 2005). Also, there exists a paucity of long-term trials, data on treatment effectiveness in wider clinical practice, and data on treatment-resistant patients.
This highlights the importance of defining novel targets for treatment of people with PTSD, an area of intensive research efforts around the world. Consequently, this report links the endophenotype of PTSD with the neurobiology of the disorder as well as mechanisms of resilience with a particular emphasis on monoaminergic mechanisms to help move translational research forward with the goal to identify novel targets for drug development.
Neurochemistry of PTSD: The Role of Norepinephrine
Clinical evidence suggests an important role for NE in PTSD. Given the prominence of hyperadrenergic symptoms in PTSD (e.g. hyperarousal, reexperiencing, anxiety, tachycardia, increased diastolic blood pressure, diaphoresis), which characterize patients with PTSD, the noradrenergic/locus coeruleus (LC) system and its varied pathways have been the focus of many neurobiological investigations in PTSD over the past 25 years. There is now considerable evidence that abnormal regulation of brain NE systems is observed in patients with PTSD. In particular, NE activity in the cell bodies of the LC and projections to the amygdala, hippocampus and prefrontal cortex (PFC) are thought to be important in fear and stress responses (O'Donnell et al., 2004; Shin et al., 2006). Pharmacological challenge studies with yohimbine in humans (Bremner et al., 1997; Southwick et al., 1993; Southwick et al., 1997), animal studies (Arnsten, 1998; Arnsten et al., 1998) and neuropsychological studies in patients with PTSD (Clark et al., 2003; Galletly et al., 2001; Stein et al., 2002; Vasterling et al., 2002) provide additional evidence for the importance of NE in PTSD.
Norepinephrine Transporter
Chronic depolarization of sympathetic neurons induces NE transporter (NET) expression through increasing catecholamines (Habecker et al., 2006). Pre-clinical studies show that the endogenous substrates dopamine and NE stimulate NET expression in the central and peripheral nervous systems (Arnsten et al., 1999; Arnsten and Li, 2005; Avery et al., 2000; Lee et al., 1983; Li et al., 1999; Li et al., 1994; Mao et al., 1999; Swann et al., 1985; Weinshenker et al., 2002) and may serve as a model of NET regulation during pathophysiology. This is important because deficits in NE transmission are implicated in psychiatric disorders, and antidepressant drugs that block the NET have shown efficacy in stress-associated mood (Cipriani et al., 2009) and anxiety disorders (Stahl et al., 2005). In animal studies it was shown that most PFC NE axons have an unrecognized, latent capacity to enhance the synthesis and recovery of transmitter which could be an important mechanism in the capacity of adapting to stress which could have been vanished in individuals with PTSD. Chronic exposure to stress leads to increase of plasmalemmal NET expression in the PFC suggesting that this mechanism is an attempt to maintain the normal availability and consequently normal function of dopamine and NE in the PFC (Miner et al., 2006). In the LC, however, chronic stress leads to a reduction of NET availability (Rusnak et al., 2001), which may result in exaggerated synaptic availability of NE in projection areas. Despite these convincing animal models, it is unclear to date whether these models can be applied to humans. The availability of novel radiotracers for the NET (Ding et al., 2005) using positron emission tomography provide an opportunity to study these mechanisms in vivo. Manifestations of NET abnormalities could be important markers for identifying and subtyping patients with PTSD which could become relevant to the treatment of PTSD because NET's are high-affinity targets of antidepressant agents and NET inhibitors, e.g. desmethylimipramine, reboxetine or atomoxetine which are highly selective NET inhibitors have been used as antidepressants for many years (Cipriani et al., 2009) but their role in the treatment of PTSD is unclear yet.
Alpha 2 Adrenoreceptors
Recent transgenic experiments suggest that the alpha-2 adrenoreceptor may emerge as a target of specific interest to PTSD. Knockout of the gene for the α2a receptor increases immobility in the forced swim test and eliminates the augmentation of forced swim test activity by imipramine (Schramm et al., 2001). In contrast, other recent experiments suggest that mice lacking α2c receptors perform on the forced swim test in the same fashion as mice treated with antidepressants (Sallinen et al., 1999). Thus, the α2a and the α2c receptors may have complementary and opposing roles in the regulation of mood and anxiety (Small et al., 2000). If reducing α2c activity is to be used as an antidepressant/anxiolytic strategy it may require some method of targeting only those receptors in the CNS since it was shown recently that an α2c (Del322-325) polymorphism reduces feedback inhibition of sympathetic NE released (Neumeister et al., 2005). Recent evidence that a mutation of the α2a receptor impairs working memory could also help us understand the cognitive symptoms observed in PTSD (Franowicz et al., 2002), and drugs that specifically enhance function of the α2a receptor may be a novel way to treat symptoms of PTSD, even though unspecific α2 receptor agonists failed to show superiority over placebo in the treatment of chronic PTSD (Davis et al., 2008; Neylan et al., 2006).
Interactive Effects of Norepinephrine with Other Neurobiologic Systems
Crosstalk with Serotonin and Dopamine
It is clear that NE transmission does not fully explain the neurobiology of PTSD and changing the set point of NE transmission cannot fully explain antidepressant action and their effects, but there is a growing body of evidence showing that plastic changes in the limbic target areas of monoamine neuron projections are important in the mechanism of action of antidepressants and therefore of relevance to the neurobiology of PTSD. It appears that the behavioral effects of NE, 5-HT, and dopamine have considerable overlap such that augmenting levels of any one may have antidepressant effects, and increasing synaptic levels of more than a single neurotransmitter may be synergistic (Thase et al., 2001). Crosstalk between NE neurons, dopamine, and 5-HT neurons has been documented such that increased NE stimulates 5-HT and dopamine release, and 5-HT release at NE neurons reduces NE release. As another example, blockade of the NET can reduce the uptake of dopamine in the frontal cortex since the NET has high affinity for dopamine (in fact NET has a higher affinity for dopamine than does the dopamine transporter itself), and dopamine transporters in any event are found at low levels in frontal cortex, suggesting that drugs that inhibit the NET may be capable of specifically effective in PTSD by affecting prefrontal dopamine signaling behavior (for review (Arnsten and Li, 2005)).
Interactive Effects with Neurosteroids
Crosstalk between the catecholamine system and steroids may be another novel mechanism through which NE and epinephrine by increasing the sensitivity of glucocorticoid receptors to ligand activation could alter symptoms of PTSD (Zhu et al., 1999). Augmentation effects of catecholamines on GR signaling may thus be important in cognitive and emotional processing. The PI3-K signaling pathway activation through beta receptors appears to be responsible for this putative enhancement of glucocorticoid receptor activity and it is tempting to conjecture that antidepressants which are known to down regulate beta receptors and influence PI3-K signaling could act by glucocorticoid receptor sensitization.
Interaction of Norepinephrine with Neuropeptide Y
Neuropeptide Y (NPY), a 36–amino acid peptide, is one of the most abundant and highly evolutionarily conserved polypeptides found in the brain. Its highest concentrations are in the LC, hypothalamus, septum, and periaqueductal grey, with moderate levels in the hippocampus, amygdala, and brainstem (Silva et al., 2005), areas, which are implicated in arousal and in the assignment of emotional valences to stimuli and memories. Of the 5 NPY receptor subtypes found in mammals (Y1 – Y5), the NPY-Y1 receptor is most closely studies in stress and anxiety models (for review (Thorsell, 2008)). NPY has been shown to be involved in fear consolidation, with studies showing that administration of NPY impairs retention of traumatic memories, reduces anxiety during stressful tasks, enhanced extinction of fear-potentiated startle (Gutman et al., 2008), and the haplotype-driven NPY expression predicts brain responses to emotional and stress challenges (Zhou et al., 2008). NPY also mediates the response to chronic stress, by increasing expression of amygdala NPY mRNA (de Lange et al., 2008).
Human studies of NPY in people exposed to extreme stress support the idea that NPY not only confers anxiolytic activity but may also be involved in stress resilience. It was shown (Morgan et al., 2000), and subsequently replicated (Morgan et al., 2002), that Special Forces soldiers who underwent an extremely stressful training program had higher and sustained NPY levels than non-Special Forces soldiers during extreme stress, which was associated with better performance and lower stress-induced dissociation (Figure 2). In PTSD, patients relative to non-stressed healthy controls showed lower baseline plasma NPY levels and a blunted yohimbine-induced NPY increase suggesting impaired reagibility of the system to a pharmacologic stressor (Rasmusson et al., 2000). These results were independently confirmed by another group reporting that combat exposed veterans without PTSD had higher NPY levels than non-combat-exposed veterans, but comparable to combat-exposed veterans with PTSD (Yehuda et al., 2006a). They also reported that those veterans with past PTSD had higher plasma NPY than those without past PTSD suggesting that plasma NPY levels may represent a biologic correlate of resilience to or recovery from the adverse effects of stress exposure. These data suggest that NPY may not only play an unspecific role in the psychobiology of stress responses, but is also involved in mechanisms of resilience and PTSD (Eaton et al., 2007), and available data are consistent with the function of NPY as an anxiolytic peptide. Altogether, it can be hypothesized (Figure 3.) that whereas NE mediates the fight and flight response to stress, NPY may have a role in dampen the impact of NE and may therefore be a system of interest for the development of novel treatment approaches in PTSD.
Figure 2.
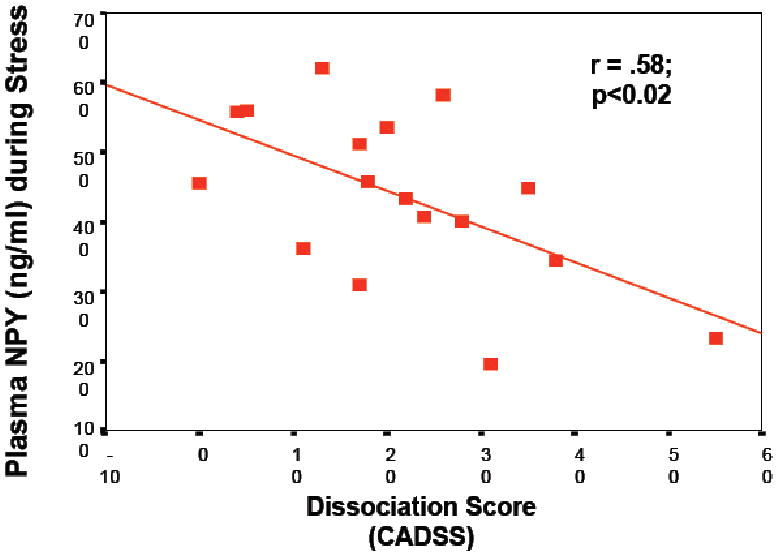
Correlation between psychologic symptoms of dissociation at baseline predict significantly less NPY release during stress in a group of N=25 active duty U.S. Navy personnel participating in survival school training.
Figure 3.
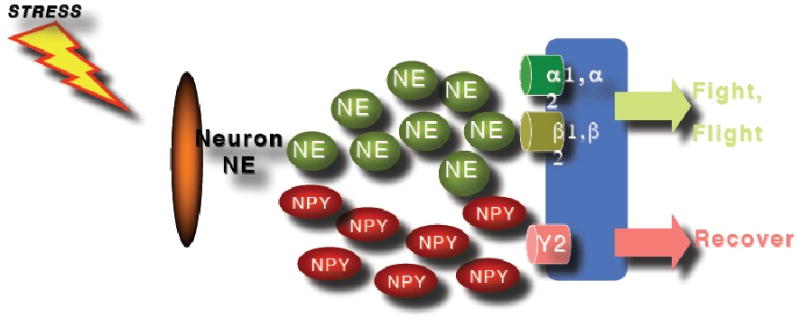
The effects of the sympathetic nervous system are mediated via release of neurotransmitters and neuropeptides from sympathetic neurons. NPY and tyrosinhydroxylase are likely to modulate NPY and/or norepinephrine (NE) release whereby NE seems to moderate the flight and fight response during stress whereas NPY contributes to dampen down the effects of NE during stress response.
The Role of Serotonin
The brain 5-HT system is involved in the regulation of stress and anxiety (Chaouloff, 1993; Griebel, 1995; Harvey et al., 2004) and several preclinical studies have reported an increase in 5-HT release, enhanced neuronal activity in the dorsal raphe nuclei, and increased 5-HT synthesis and turnover in response to stress (Chaouloff et al., 1999; Dunn, 1988). These stress-induced alterations in 5-HT activity occur in multiple brain regions, which have been implicated in the pathophysiology of PTSD, including the amygdala (Mitsushima et al., 2006), ventral striatum (Amato et al., 2006), and the PFC (Bruening et al., 2006; Gobert et al., 1998; Smith et al., 2006).
Brain 5-HT systems have been linked to the neurobiology of PTSD because the administration of m-chlorophenylpiperazine (mCPP), a 5-HT agonist, could transiently evoke symptoms of PTSD but these effects were not observed when mCPP was administered to patients with other psychiatric disorders (Charney et al., 1988; Krystal et al., 1996; Price et al., 1997). Brain 5-HT systems are also implicated in PTSD treatment. Currently, two exemplars of a single class of medications, drugs that block re-uptake of 5-HT, are the only FDA-approved treatment for PTSD. However, in the absence of knowledge about the regulation of specific 5-HT receptors in PTSD, it is difficult to directly link the efficacy of these medications to the neurobiology of the disorder. Neurons, glia, and endothelial cells possess at least 14 distinct receptors, and 5-HT is involved “in more behaviors, physiological mechanisms, and disease processes than any other brain neurotransmitter” (reviewed in (Pineyro and Blier, 1999)). Agents that enhance serotonergic activity such as the 5-HT reuptake inhibitors (SSRI's), which block the 5-HT transporter, are partially effective in PTSD (Stein et al., 2006). Serotonin, in development and adulthood has an important role in CNS neuroplasticity. Clinical and preclinical studies have thus far mostly implicated stimulation and interaction of 5-HT1A, 5-HT1B, and 5-HT2A or 5-HT2C receptors in antidepressant/anxiolytic action, but this emphasis may be in part an artifact related to the availability of selective ligands for these receptor subtypes.
The 5-HT1A Receptor
The 5-HT1A receptor is a seven transmembrane G-protein coupled receptor found both at presynaptic locations in the raphe nucleus and at postsynaptic locations, and is critically involved in regulating mood and anxiety levels. Postsynaptic stimulation in the hippocampus augments synaptogenesis in adult animals via a trophic factor referred to as S-100β (Whitaker-Azmitia and Azmitia, 1989; Whitaker-Azmitia et al., 1990). 5-HT1A receptors signals via a Gαi coupled inhibition of adenylyl cyclase and by hyperpolarization via the opening of a K+ channel. The density and mRNA expression of 5-HT1A receptors appear insensitive to reductions in 5-HT transmission associated with lesioning the raphe or administering the 5-HT depleting agent, PCPA (Frazer and Hensler, 1990; Hensler, 2002; Verge et al., 1986). Similarly, elevations of 5-HT transmission resulting from chronic administration of SSRI or monoamine oxidase inhibitors (MAOI) does not consistently alter 5-HT1A receptor density or mRNA in the cortex, hippocampus, amygdala, or hypothalamus (Carli et al., 1996; Spurlock et al., 1994; Welner et al., 1989).
The 5-HT1A receptor may counteract the effects of activation of the 5-HT2A receptor. Activation of the 5-HT1A receptor exerts a hyperpolarizing effect on cortical neurons whereas activation of the 5-HT2A receptor is depolarizing. Activation of 5-HT2A receptors results in glutamate release from thalamocortical afferents and increased levels of glutamate reduces neural, vascular, and glial trophic factors which, in combination with direct glucocorticoid effects, contribute to disruption of neurogenesis, and even neural death, in limbic and cortical brain regions (Hoebel et al., 2007). Thus, loss of neural connectivity may impede behavioral resilience to stress, giving rise to features of PTSD (“learned helplessness”) and impaired learning/memory in animal models. Therefore, it is tempting to speculate that a drug designed to combine 5-HT1A agonism with postsynaptic 5-HT2A antagonism would have robust anxiolytic action.
Recent knockout experiments of the 5-HT1A receptor indicate that the receptor is important early in development with respect to affect-regulated behaviors. 5HT1A null mice have increased anxiety, but ‘rescue’ at a later age in conditional knockouts does not reduce anxiety if the receptor was absent at a developmentally crucial early period (Mayorga et al., 2001). Knockout of the 5HT1A receptor, possibly by eliminating feedback inhibitor, has the effect of reducing immobility in the tail suspension test, simulating antidepressant action. However, rather than being the result of simply increasing synaptic serotonin, challenge studies employing AMPT have implicated augmentation of catecholamine function in the antidepressant-like behavioral effects of 5-HT1A receptor deletions.
It was unclear, however, whether these animal models of anxiety (Bruening et al., 2006; Groenink et al., 2003a; Groenink et al., 2003b) have relevance to disease models of PTSD, and the role of the 5-HT1A receptor in adult PTSD was not directly studied. Data from a relatively small brain imaging study using a selective 5-HT1A receptor antagonist radioligand and PET did not support a direct role of this receptor subtype in PTSD (Bonne et al., 2005) (Figure 4.), even though these studies do not exclude the possibility that 5-HT1A receptors play an important role in the treatment of PTSD.
Figure 4.
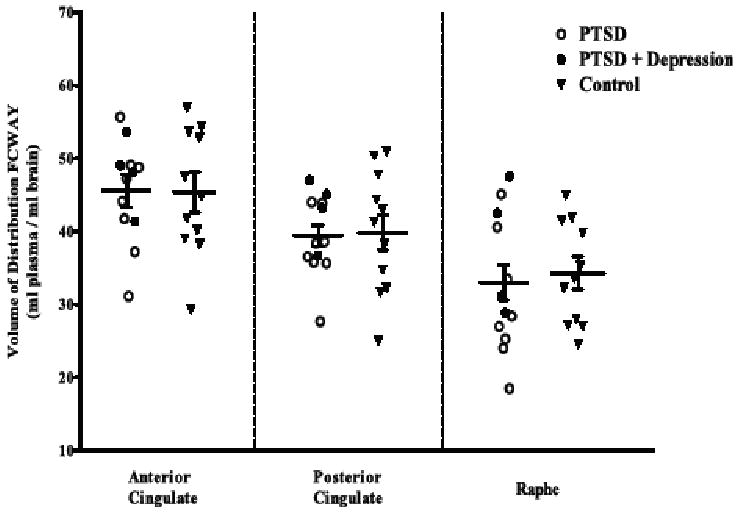
Positron emission tomography study of 5-HT1A receptors with the radioligand [18F]Trans-4-fluoro-N-2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide (FCWAY), a selective 5-HT1A receptor antagonist ligand. No difference in receptor expression was found in PTSD or PTSD and depression vs. healthy control subjects.
The 5-HT1B Receptor in a Model of Adaptive and Maladaptive Responses to Stress
Given that epistasis among pre-synaptic components of the 5-HT transmitter system appears to be important in the 5-HT system's regulation of synaptic 5-HT levels (Stoltenberg, 2005), the 5-HT1B receptor is a particularly attractive candidate for further study (Clark and Neumaier, 2001). 5-HT1B receptor knock-out (KO) studies (Groenink et al., 2003b) and the viral-mediated gene transfer approach (Clark et al., 2002) leading to 5-HT1B receptor overexpression support the concept that increased dorsal raphe 5-HT1B autoreceptor tone would predispose animals to increased anxiety (Clark et al., 2002) and altered stress reactivity (Neumaier et al., 2002) by reducing 5-HT availability in forebrain terminal fields. Decreased 5-HT1B receptor responsiveness is believed to occur in response to agonist stimulation (Janoshazi et al., 2007) and results in increased synaptic 5-HT availability (Figure 5.). We and others (Kilpatrick et al., 2007; Ursano et al., 2008) propose that increased synaptic availability of 5-HT in the amygdala (Mitsushima et al., 2006), cortical regions (Bruening et al., 2006; Smith et al., 2006), and 5-HT mediated alterations in dopamine release in the ventral striatum (Amato et al., 2006) in response to trauma is critical to avoid symptom development after trauma resulting in the PTSD phenotype and the 5-HT1B receptor may play a critical role in this process. It can be speculated that reductions in 5-HT1B receptors in cortical-striatal-limbic circuits either predict adaptive responses to stress or are a persisting feature of resilient stress responses. This hypothesis is supported by directly linking disturbances in 5-HT1B receptor function to the development stress-induced disorders (Sari, 2004) and also to characteristic symptoms of PTSD, i.e. anxiety, irritability and impulsivity (Clark and Neumaier, 2001). Therefore, we believe that proper 5-HT1B receptor function is a critical mechanism that may prevent symptom development after trauma exposure whereas compromised 5-HT1B receptor function may increase the risk to develop PTSD after trauma exposure. Because 5-HT1B autoreceptors positively regulate 5-HT uptake by 5-HT transporters, these coinciding proteins may provide an opportunity for synergistic effects to modulate serotonergic function. Therefore, our findings are in line with recent reports suggesting that 5-HT1B receptor antagonists may enhance the efficacy of selective 5-HT reuptake inhibitors (Muraki et al., 2008; Starr et al., 2007) which are typically used as first line treatments for patients with PTSD (Stein et al., 2006).
Figure 5.
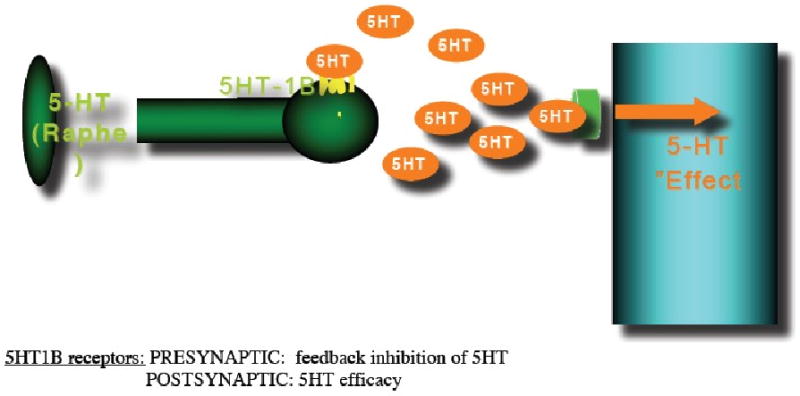
The occurrence of a traumatic event and/or chronic stress leads to increased synaptic 5-HT levels in the amygdala and cortical regions, as well as alterations in dopamine release in the ventral striatum (VST). These effects are at least partially mediated by 5-HT1B receptors. A PTSD-resilience model that implicates a central role for the 5-HT1B receptor would assume that PTSD patients, in contrast to resilient people are unable to downregulate 5-HT1B receptors which will lead to amygdala hyperresponsiveness because of reduced 5-HT activity which may disinhibit excitatory activity by reducing the stimulation of 5HT1A receptors located on pyramidal cells where they inhibit action potential formation, and of 5-HT3 receptors, that are located on GABAergic interneurons where they stimulate GABA release, alterations in dopamine release in the VST and changes in ventromedial prefrontal cortex (vmPFC) function resulting in inadequate top-down governance over the amygdala by the vmPFC which is characteristic for PTSD.
Conclusions
Despite the large increase in knowledge about the neurobiology of stress as well as adaptive and maladaptive responses to stress exposure resulting in the phenotype of PTSD, there is concern that there is a slow-down in the development of truly innovative novel treatments for patients with PTSD. In the area of PTSD, some of this difficulty reflects the high rate of negative and failed trials, related in part to the tremendous genetic and phenotypic heterogeneity in PTSD, and the lack of biological markers to guide drug development. Existing or novel endophenotypes that reduce the syndrome of PTSD to discrete component units and ultimately fundamental units linked to pathophysiology may help move translational research forward. Clinical and genomic approaches are needed to clinically subgroup patients more precisely. Refinement of measurement tools including imaging techniques may lead to the definition of new endpoints, and biomarkers may be developed based on dissected components of current consensus syndromes that measure disease state with accuracy and objectivity.
Acknowledgments
Supported by the National Center for Posttraumatic Stress Disorder, West Haven VA Connecticut Clinical Neurosciences Division, a Merit Award from the Veterans Health Administration of the Department of Veterans Affairs, The Patrick and Catherine Weldon Donaghue Medical Research Foundation (DF 07-101), and an Independent Investigator Award (NARSAD).
Dr. Krystal reports receiving consulting fees from Astra-Zeneca, Bristol-Meyers Squibb, Cypress Bioscience, Inc., Eli Lilly and Co., Forest Laboratories, Glaxo-SmithKline, Houston Pharma, Janssen Research Foundation, Lohocla Research Corporation, Merz Pharmaceuticals, Organon Pharmaceuticals, Pfizer Pharmaceuticals, Takeda Industries, Tetragenex Pharmaceuticals (compensation in exercisable warrant options until March 21, 2012; value less than $10K), Transcept Pharmaceuticals, and is a co-sponsor for three pending patents, “glutamatergic agents for psychiatric disorders (depression, OCD)”, “antidepressant effects of oral ketamine”, “ oral ketamine for depression”; Dr. Neumeister reports grant support from Pfizer Inc., Eli Lilly, UCB Pharma Inc., and Ortho-McNeil Janssen Scientific Affairs, LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alim TN, Feder A, Graves RE, Wang Y, Weaver J, Westphal M, Alonso A, Aigbogun NU, Smith BW, Doucette JT, Mellman TA, Lawson WB, Charney DS. Trauma, resilience, and recovery in a high-risk African-American population. Am J Psychiatry. 2008;165:1566–75. doi: 10.1176/appi.ajp.2008.07121939. [DOI] [PubMed] [Google Scholar]
- Amato JL, Bankson MG, Yamamoto BK. Prior Exposure to Chronic Stress and MDMA Potentiates Mesoaccumbens Dopamine Release Mediated by the 5-HT(1B) Receptor. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301174. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. The biology of being frazzled. Science. 1998;280:1711–2. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Steere JC, Jentsch DJ, Li BM. Noradrenergic influences on prefrontal cortical cognitive function: opposing actions at postjunctional alpha 1 versus alpha 2-adrenergic receptors. Adv Pharmacol. 1998;42:764–7. doi: 10.1016/s1054-3589(08)60859-5. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–84. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, DC: 1994. Vol. [Google Scholar]
- Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AF. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23:240–9. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Galea S, Bucciarelli A, Vlahov D. What predicts psychological resilience after disaster? The role of demographics, resources, and life stress. J Consult Clin Psychol. 2007;75:671–82. doi: 10.1037/0022-006X.75.5.671. [DOI] [PubMed] [Google Scholar]
- Bonne O, Bain E, Neumeister A, Nugent AC, Vythilingam M, Carson RE, Luckenbaugh DA, Eckelman W, Herscovitch P, Drevets WC, Charney DS. No change in serotonin type 1A receptor binding in patients with posttraumatic stress disorder. Am J Psychiatry. 2005;162:383–5. doi: 10.1176/appi.ajp.162.2.383. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Posttraumatic stress disorder and mortality among U.S. Army veterans 30 years after military service. Ann Epidemiol. 2006;16:248–56. doi: 10.1016/j.annepidem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Ng CK, Staib LH, Salomon RM, Bronen RA, Duncan J, Southwick SM, Krystal JH, Rich D, Zubal G, Dey H, Soufer R, Charney DS. Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:246–54. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55:626–32. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RC, Peterson EL, Lucia VC. Vulnerability to assaultive violence: further specification of the sex difference in post-traumatic stress disorder. Psychol Med. 1999;29:813–21. doi: 10.1017/s0033291799008612. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL, Schultz LR. A second look at prior trauma and the posttraumatic stress disorder effects of subsequent trauma: a prospective epidemiological study. Arch Gen Psychiatry. 2008;65:431–7. doi: 10.1001/archpsyc.65.4.431. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–66. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Bruening S, Oh E, Hetzenauer A, Escobar-Alvarez S, Westphalen RI, Hemmings HC, Jr, Singewald N, Shippenberg T, Toth M. The anxiety-like phenotype of 5-HT receptor null mice is associated with genetic background-specific perturbations in the prefrontal cortex GABA-glutamate system. J Neurochem. 2006;99:892–9. doi: 10.1111/j.1471-4159.2006.04129.x. [DOI] [PubMed] [Google Scholar]
- Carli M, Afkhami-Dastjerdian S, Reader TA. [3H]8-OH-DPAT binding and serotonin content in rat cerebral cortex after acute fluoxetine, desipramine, or pargyline. J Psychiatry Neurosci. 1996;21:114–22. [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F. Physiopharmacological interactions between stress hormones and central serotonergic systems. Brain Res Brain Res Rev. 1993;18:1–32. doi: 10.1016/0165-0173(93)90005-k. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacology. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Charney DS, Goodman WK, Price LH, Woods SW, Rasmussen SA, Heninger GR. Serotonin function in obsessive-compulsive disorder. A comparison of the effects of tryptophan and m-chlorophenylpiperazine in patients and healthy subjects. Arch Gen Psychiatry. 1988;45:177–85. doi: 10.1001/archpsyc.1988.01800260095012. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009 doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Clark CR, McFarlane AC, Morris P, Weber DL, Sonkkilla C, Shaw M, Marcina J, Tochon-Danguy HJ, Egan GF. Cerebral function in posttraumatic stress disorder during verbal working memory updating: a positron emission tomography study. Biol Psychiatry. 2003;53:474–81. doi: 10.1016/s0006-3223(02)01505-6. [DOI] [PubMed] [Google Scholar]
- Clark MS, Neumaier JF. The 5-HT1B receptor: behavioral implications. Psychopharmacol Bull. 2001;35:170–85. [PubMed] [Google Scholar]
- Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22:4550–62. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JR. Remission in post-traumatic stress disorder (PTSD): effects of sertraline as assessed by the Davidson Trauma Scale, Clinical Global Impressions and the Clinician-Administered PTSD scale. Int Clin Psychopharmacol. 2004;19:85–7. doi: 10.1097/00004850-200403000-00005. [DOI] [PubMed] [Google Scholar]
- Davis LL, Ward C, Rasmusson A, Newell JM, Frazier E, Southwick SM. A placebo-controlled trial of guanfacine for the treatment of posttraumatic stress disorder in veterans. Psychopharmacol Bull. 2008;41:8–18. [PubMed] [Google Scholar]
- de Lange RP, Wiegant VM, Stam R. Altered neuropeptide Y and neurokinin messenger RNA expression and receptor binding in stress-sensitised rats. Brain Res. 2008;1212:35–47. doi: 10.1016/j.brainres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Dieperink M, Erbes C, Leskela J, Kaloupek D, Farrer MK, Fisher L, Wolf E. Comparison of treatment for post-traumatic stress disorder among three Department of Veterans Affairs medical centers. Mil Med. 2005;170:305–8. doi: 10.7205/milmed.170.4.305. [DOI] [PubMed] [Google Scholar]
- Ding YS, Lin KS, Logan J, Benveniste H, Carter P. Comparative evaluation of positron emission tomography radiotracers for imaging the norepinephrine transporter: (S,S) and (R,R) enantiomers of reboxetine analogs ([11C]methylreboxetine, 3-Cl-[11C]methylreboxetine and [18F]fluororeboxetine), (R)-[11C]nisoxetine, [11C]oxaprotiline and [11C]lortalamine. J Neurochem. 2005;94:337–51. doi: 10.1111/j.1471-4159.2005.03202.x. [DOI] [PubMed] [Google Scholar]
- DuMont KA, Widom CS, Czaja SJ. Predictors of resilience in abused and neglected children grown-up: the role of individual and neighborhood characteristics. Child Abuse Negl. 2007;31:255–74. doi: 10.1016/j.chiabu.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Changes in plasma and brain tryptophan and brain serotonin and 5-hydroxyindoleacetic acid after footshock stress. Life Sci. 1988;42:1847–53. doi: 10.1016/0024-3205(88)90023-9. [DOI] [PubMed] [Google Scholar]
- Eaton K, Sallee FR, Sah R. Relevance of neuropeptide Y (NPY) in psychiatry. Curr Top Med Chem. 2007;7:1645–59. doi: 10.2174/156802607782341037. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J Neurosci. 2002;22:8771–7. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A, Hensler JG. 5-HT1A receptors and 5-HT1A-mediated responses: effect on treatments that modify serotonergic neurotransmission. In: Whitaker-Azmitia PM, Peroutka SJ, editors. The Neuropharmacology of Serotonin. The New York Academy of Sciences; New York: 1990. pp. 460–475. [DOI] [PubMed] [Google Scholar]
- Galletly C, Clark CR, McFarlane AC, Weber DL. Working memory in posttraumatic stress disorder--an event-related potential study. J Trauma Stress. 2001;14:295–309. doi: 10.1023/A:1011112917797. [DOI] [PubMed] [Google Scholar]
- Gobert A, Rivet JM, Audinot V, Newman-Tancredi A, Cistarelli L, Millan MJ. Simultaneous quantification of serotonin, dopamine and noradrenaline levels in single frontal cortex dialysates of freely-moving rats reveals a complex pattern of reciprocal auto- and heteroreceptor-mediated control of release. Neuroscience. 1998;84:413–29. doi: 10.1016/s0306-4522(97)00565-4. [DOI] [PubMed] [Google Scholar]
- Griebel G. 5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research. Pharmacol Ther. 1995;65:319–95. doi: 10.1016/0163-7258(95)98597-j. [DOI] [PubMed] [Google Scholar]
- Groenink L, Pattij T, De Jongh R, Van der Gugten J, Oosting RS, Dirks A, Olivier B. 5-HT1A receptor knockout mice and mice overexpressing corticotropin-releasing hormone in models of anxiety. Eur J Pharmacol. 2003a;463:185–97. doi: 10.1016/s0014-2999(03)01281-0. [DOI] [PubMed] [Google Scholar]
- Groenink L, van Bogaert MJ, van der Gugten J, Oosting RS, Olivier B. 5-HT1A receptor and 5-HT1B receptor knockout mice in stress and anxiety paradigms. Behav Pharmacol. 2003b;14:369–83. doi: 10.1097/01.fbp.0000087737.21047.75. [DOI] [PubMed] [Google Scholar]
- Gutman AR, Yang Y, Ressler KJ, Davis M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J Neurosci. 2008;28:12682–90. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habecker BA, Willison BD, Shi X, Woodward WR. Chronic depolarization stimulates norepinephrine transporter expression via catecholamines. J Neurochem. 2006;97:1044–51. doi: 10.1111/j.1471-4159.2006.03792.x. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Naciti C, Brand L, Stein DJ. Serotonin and stress: protective or malevolent actions in the biobehavioral response to repeated trauma? Ann N Y Acad Sci. 2004;1032:267–72. doi: 10.1196/annals.1314.035. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Differential regulation of 5-HT1A receptor-G protein interactions in brain following chronic antidepressant administration. Neuropsychopharmacology. 2002;26:565–73. doi: 10.1016/S0893-133X(01)00395-5. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 2007;7:617–27. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, Terhakopian A, Castro CA, Messer SC, Engel CC. Association of posttraumatic stress disorder with somatic symptoms, health care visits, and absenteeism among iraq war veterans. Am J Psychiatry. 2007;164:150–3. doi: 10.1176/ajp.2007.164.1.150. [DOI] [PubMed] [Google Scholar]
- Janoshazi A, Deraet M, Callebert J, Setola V, Guenther S, Saubamea B, Manivet P, Launay JM, Maroteaux L. Modified receptor internalization upon coexpression of 5-HT1B receptor and 5-HT2B receptors. Mol Pharmacol. 2007;71:1463–74. doi: 10.1124/mol.106.032656. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164:1693–9. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Cooney NL, Kranzler HR, Southwick SW, Heninger GR, Charney DS. Serotonergic and noradrenergic dysregulation in alcoholism: m-chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry. 1996;153:83–92. doi: 10.1176/ajp.153.1.83. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Spiro A, 3rd, Vokonas PS, Sparrow D. Prospective Study of Posttraumatic Stress Disorder Symptoms and Coronary Heart Disease in the Normative Aging Study. Arch Gen Psychiatry. 2007;64:109–116. doi: 10.1001/archpsyc.64.1.109. [DOI] [PubMed] [Google Scholar]
- Lee CM, Javitch JA, Snyder SH. Recognition sites for norepinephrine uptake: regulation by neurotransmitter. Science. 1983;220:626–9. doi: 10.1126/science.6301013. [DOI] [PubMed] [Google Scholar]
- Li BM, Mao ZM, Wang M, Mei ZT. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology. 1999;21:601–10. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- Li Q, Brownfield MS, Levy AD, Battaglia G, Cabrera TM, Van de Kar LD. Attenuation of hormone responses to the 5-HT1A agonist ipsapirone by long-term treatment with fluoxetine, but not desipramine, in male rats. Biol Psychiatry. 1994;36:300–8. doi: 10.1016/0006-3223(94)90627-0. [DOI] [PubMed] [Google Scholar]
- Mao ZM, Arnsten AF, Li BM. Local infusion of an alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol Psychiatry. 1999;46:1259–65. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J Pharmacol Exp Ther. 2001;298:1101–7. [PubMed] [Google Scholar]
- Miner LH, Jedema HP, Moore FW, Blakely RD, Grace AA, Sesack SR. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci. 2006;26:1571–8. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. Eur J Neurosci. 2006;24:3245–54. doi: 10.1111/j.1460-9568.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Wang S, Southwick SM, Rasmusson A, Hazlett G, Hauger RL, Charney DS. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol Psychiatry. 2000;47:902–9. doi: 10.1016/s0006-3223(99)00239-5. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Rasmusson AM, Wang S, Hoyt G, Hauger RL, Hazlett G. Neuropeptide-Y, cortisol, and subjective distress in humans exposed to acute stress: replication and extension of previous report. Biol Psychiatry. 2002;52:136–42. doi: 10.1016/s0006-3223(02)01319-7. [DOI] [PubMed] [Google Scholar]
- Muraki I, Inoue T, Koyama T. Effect of co-administration of the selective 5- HT1A receptor antagonist WAY 100,635 and selective 5-HT1B/1D receptor antagonist GR 127,935 on anxiolytic effect of citalopram in conditioned fear stress in the rat. Eur J Pharmacol. 2008;586:171–8. doi: 10.1016/j.ejphar.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Edwards E, Plotsky PM. 5-HT(1B) mrna regulation in two animal models of altered stress reactivity. Biol Psychiatry. 2002;51:902–8. doi: 10.1016/s0006-3223(01)01371-3. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Charney DS, Belfer I, Geraci M, Holmes C, Sharabi Y, Alim T, Bonne O, Luckenbaugh DA, Manji H, Goldman D, Goldstein DS. Sympathoneural and adrenomedullary functional effects of alpha2C-adrenoreceptor gene polymorphism in healthy humans. Pharmacogenet Genomics. 2005;15:143–9. doi: 10.1097/01213011-200503000-00002. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Lenoci M, Samuelson KW, Metzler TJ, Henn-Haase C, Hierholzer RW, Lindley SE, Otte C, Schoenfeld FB, Yesavage JA, Marmar CR. No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am J Psychiatry. 2006;163:2186–8. doi: 10.1176/appi.ajp.163.12.2186. [DOI] [PubMed] [Google Scholar]
- Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60:409–18. doi: 10.1037//0022-006x.60.3.409. [DOI] [PubMed] [Google Scholar]
- O'Donnell T, Hegadoren KM, Coupland NC. Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology. 2004;50:273–83. doi: 10.1159/000080952. [DOI] [PubMed] [Google Scholar]
- Pineyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev. 1999;51:533–91. [PubMed] [Google Scholar]
- Price LH, Malison RT, McDougle CJ, McCance-Katz EF, Owen KR, Heninger GR. Neurobiology of tryptophan depletion in depression: effects of m-chlorophenylpiperazine (mCPP) Neuropsychopharmacology. 1997;17:342–50. doi: 10.1016/S0893-133X(97)00084-5. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47:526–39. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- Rusnak M, Kvetnansky R, Jelokova J, Palkovits M. Effect of novel stressors on gene expression of tyrosine hydroxylase and monoamine transporters in brainstem noradrenergic neurons of long-term repeatedly immobilized rats. Brain Res. 2001;899:20–35. doi: 10.1016/s0006-8993(01)02126-6. [DOI] [PubMed] [Google Scholar]
- Rutter M. Resilience in the face of adversity. Protective factors and resistance to psychiatric disorder. Br J Psychiatry. 1985;147:598–611. doi: 10.1192/bjp.147.6.598. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, MacDonald E, Viitamaa T, Lahdesmaki J, Rybnikova E, Pelto-Huikko M, Kobilka BK, Scheinin M. Genetic alteration of the alpha2-adrenoceptor subtype c in mice affects the development of behavioral despair and stress-induced increases in plasma corticosterone levels. Mol Psychiatry. 1999;4:443–52. doi: 10.1038/sj.mp.4000543. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–82. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Hayes AF, Lunney CA, McFall M, Uddo M. Longitudinal analysis of the relationship between symptoms and quality of life in veterans treated for posttraumatic stress disorder. J Consult Clin Psychol. 2006;74:707–13. doi: 10.1037/0022-006X.74.4.707. [DOI] [PubMed] [Google Scholar]
- Schramm NL, McDonald MP, Limbird LE. The alpha(2a)-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J Neurosci. 2001;21:4875–82. doi: 10.1523/JNEUROSCI.21-13-04875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Silva AP, Xapelli S, Grouzmann E, Cavadas C. The putative neuroprotective role of neuropeptide Y in the central nervous system. Curr Drug Targets CNS Neurol Disord. 2005;4:331–47. doi: 10.2174/1568007054546153. [DOI] [PubMed] [Google Scholar]
- Small KM, Forbes SL, Rahman FF, Bridges KM, Liggett SB. A four amino acid deletion polymorphism in the third intracellular loop of the human alpha 2C-adrenergic receptor confers impaired coupling to multiple effectors. J Biol Chem. 2000;275:23059–64. doi: 10.1074/jbc.M000796200. [DOI] [PubMed] [Google Scholar]
- Smith DG, Davis RJ, Gehlert DR, Nomikos GG. Exposure to predator odor stress increases efflux of frontal cortex acetylcholine and monoamines in mice: comparisons with immobilization stress and reversal by chlordiazepoxide. Brain Res. 2006;1114:24–30. doi: 10.1016/j.brainres.2006.07.058. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, Heninger GR, Charney DS. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:266–74. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, Morgan CA, 3rd, Nicolaou AL, Nagy LM, Johnson DR, Heninger GR, Charney DS. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:749–58. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- Spurlock G, Buckland P, O'Donovan M, McGuffin P. Lack of effect of antidepressant drugs on the levels of mRNAs encoding serotonergic receptors, synthetic enzymes and 5HT transporter. Neuropharmacology. 1994;33:433–40. doi: 10.1016/0028-3908(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Grady MM, Moret C, Briley M. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10:732–47. doi: 10.1017/s1092852900019726. [DOI] [PubMed] [Google Scholar]
- Starr KR, Price GW, Watson JM, Atkinson PJ, Arban R, Melotto S, Dawson LA, Hagan JJ, Upton N, Duxon MS. SB-649915-B, a novel 5- HT1A/B autoreceptor antagonist and serotonin reuptake inhibitor, is anxiolytic and displays fast onset activity in the rat high light social interaction test. Neuropsychopharmacology. 2007;32:2163–72. doi: 10.1038/sj.npp.1301341. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2006:CD002795. doi: 10.1002/14651858.CD002795.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Walker JR, Hazen AL, Forde DR. Full and partial posttraumatic stress disorder: findings from a community survey. Am J Psychiatry. 1997;154:1114–9. doi: 10.1176/ajp.154.8.1114. [DOI] [PubMed] [Google Scholar]
- Stein MB, Walker JR, Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behav Res Ther. 2000;38:619–28. doi: 10.1016/s0005-7967(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Stein MB, Kennedy CM, Twamley EW. Neuropsychological function in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:1079–88. doi: 10.1016/s0006-3223(02)01414-2. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF. Epistasis among presynaptic serotonergic system components. Behav Genet. 2005;35:199–209. doi: 10.1007/s10519-004-1019-4. [DOI] [PubMed] [Google Scholar]
- Swann AC, Duman R, Hewitt L. Desipramine binding: relationship to central and sympathetic noradrenergic activity. J Neurochem. 1985;44:611–5. doi: 10.1111/j.1471-4159.1985.tb05455.x. [DOI] [PubMed] [Google Scholar]
- Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry. 2001;178:234–41. doi: 10.1192/bjp.178.3.234. [DOI] [PubMed] [Google Scholar]
- Thorsell A. Central neuropeptide Y in anxiety- and stress-related behavior and in ethanol intake. Ann N Y Acad Sci. 2008;1148:136–40. doi: 10.1196/annals.1410.083. [DOI] [PubMed] [Google Scholar]
- Tiet QQ, Bird HR, Davies M, Hoven C, Cohen P, Jensen PS, Goodman S. Adverse life events and resilience. J Am Acad Child Adolesc Psychiatry. 1998;37:1191–200. doi: 10.1097/00004583-199811000-00020. [DOI] [PubMed] [Google Scholar]
- Ursano RJ, Li H, Zhang L, Hough CJ, Fullerton CS, Benedek DM, Grieger TA, Holloway HC. Models of PTSD and traumatic stress: the importance of research “from bedside to bench to bedside”. Prog Brain Res. 2008;167:203–15. doi: 10.1016/S0079-6123(07)67014-9. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Marcinkiewicz M, Patey A, el Mestikawy S, Gozlan H, Hamon M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci. 1986;6:3474–82. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, White SS, Javors MA, Palmiter RD, Szot P. Regulation of norepinephrine transporter abundance by catecholamines and desipramine in vivo. Brain Res. 2002;946:239–46. doi: 10.1016/s0006-8993(02)02889-5. [DOI] [PubMed] [Google Scholar]
- Welner SA, De Montigny C, Desroches J, Desjardins P, Suranyi-Cadotte BE. Autoradiographic quantification of serotonin1A receptors in rat brain following antidepressant drug treatment. Synapse. 1989;4:347–52. doi: 10.1002/syn.890040410. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Azmitia EC. Stimulation of astroglial serotonin receptors produces culture media which regulates growth of serotonergic neurons. Brain Res. 1989;497:80–5. doi: 10.1016/0006-8993(89)90972-4. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Murphy R, Azmitia EC. Stimulation of astroglial 5-HT1A receptors releases the serotonergic growth factor, protein S-100, and alters astroglial morphology. Brain Res. 1990;528:155–8. doi: 10.1016/0006-8993(90)90210-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006a;59:660–3. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci. 2006b;1071:379–96. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- Zatzick DF, Marmar CR, Weiss DS, Browner WS, Metzler TJ, Golding JM, Stewart A, Schlenger WE, Wells KB. Posttraumatic stress disorder and functioning and quality of life outcomes in a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1997;154:1690–5. doi: 10.1176/ajp.154.12.1690. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Klimek V, Dilley GE, Haycock JW, Stockmeier C, Overholser JC, Meltzer HY, Ordway GA. Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol Psychiatry. 1999;46:1275–86. doi: 10.1016/s0006-3223(99)00135-3. [DOI] [PubMed] [Google Scholar]


