Abstract
Background
The effect of inhalational anesthetics on sensory-evoked unit activity in the cerebral cortex has been controversial. Desflurane has desirable properties for in vivo neurophysiologic studies but its effect on cortical neuronal activity and neuronal responsiveness is not known. We studied the effect of desflurane on resting and visual evoked unit activity in rat visual cortex in vivo.
Methods
Desflurane was administered to adult albino rats at steady-state concentrations at 2%, 4%, 6% and 8%. Flashes from a light emitting diode were delivered to the left eye at 5-second intervals. Extracellular unit activity within the right visual cortex was recorded using a 49-electrode array. Individual units were identified using principal components analysis.
Results
At 2% desflurane 578 active units were found. Of these, 75% increased their firing rate in response to flash. Most responses contained early (0–100ms) and late (150–1000ms) components. With increasing desflurane concentration, the number of units active at baseline decreased (−13%), the number of early responding units increased (+31%), and number of late responding units decreased (−15%). Simultaneously, baseline firing rate decreased (−77%), the early response was unchanged, and the late response decreased (−60%).
Conclusions
The results indicate that visual cortex neurons remain responsive to flash stimulation under desflurane anesthesia but the long-latency component of their response is attenuated in a concentration-dependent manner. Suppression of the long-latency response may be related to a loss of cortico-cortical feedback and loss of consciousness.
Introduction
How general anesthetics influence neuronal reactivity in the brain is important to know for an understanding of how anesthetics work and for an appreciation of the limitations of neurophysiologic and neuroimaging studies conducted in anesthetized subjects. Although numerous investigations have been devoted to the subject, the effect of inhalational agents on the responsiveness of central nervous system neurons to sensory stimuli has been controversial 1–14. Volatile anesthetics differ in their potency and regional effects on central nervous system neurons 15 and it is difficult to extrapolate the effect of one agent to that of another. Desflurane’s effect on the electroencephalogram is similar to that of isoflurane 15–17 but the two agents may differ in their effect on somatosensory evoked potentials. This issue is currently controversial as isoflurane appears more suppressive at equi-minimum alveolar concentration levels with propofol as background anesthetic 18, whereas desflurane appears more suppressive at equi-bispectral index levels with no background anesthetic 19. Still others found no difference between the two agents’ effects 20,21. In rats, somatosensory evoked potentials seem to be preserved up to 1 minimum alveolar concentration but abolished at 2 minimum alveolar concentration of desflurane 22. The effect of desflurane on cortical unit responses to sensory stimuli has not been studied.
Desflurane has desirable properties for rapid induction and emergence and ease of control with small cardiovascular side effects 23 and minimal toxicity 24. It may thus be the anesthetic of choice for in vivo neurophysiologic studies, particularly for repeated use in chronically instrumented animals and for functional brain imaging experiments that require immobility with an adequate depth of anesthesia. It would be important to know the extent cortical neurons remain responsive to sensory stimulation under desflurane anesthesia. Of particular interest is the nature of neuronal changes at an anesthetic depth associated with loss of consciousness. With desflurane alone, unconsciousness supposedly ensues above 4.5% inhaled concentration 25. Is there a characteristic change in neuronal activity or neuronal excitability at this critical anesthetic level?
In this work we examined the concentration-dependent effect of desflurane on the visual cortex neurons in the rat in vivo before and after visual stimulation with light flashes. This follows our previous interest in the effect of general anesthesia on cortical function using the rodent visual system as a model 26–29. We targeted four steady-state anesthetic depths ranging from sedation to unconscious immobility. This range included the critical anesthetic depth that was previously associated with the loss of righting reflex – an accepted behavioral index of unconsciousness in this species 26,30–33. We tested the effect of desflurane on the magnitude of early (middle-latency) and late (long-latency) poststimulus unit responses and found that desflurane exerted a differential effect on these response components. The results are interpreted in the context of current hypotheses about the mechanism of anesthetic action influencing cortical sensory information processing.
Materials and Methods
The experimental procedures and protocols used in this investigation were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin (Milwaukee, Wisconsin). All procedures conformed to the Guiding Principles in the Care and Use of Animals of the American Physiologic Society and were in accordance with the Guide for the Care and Use of Laboratory Animals, National Academy Press, Washington, D.C., 1996.
Surgical preparation
Adult male Sprague-Dawley rats were housed on 12-h light/dark cycle at a constant temperature of 23±1°C with free access to food and water for two weeks prior to each experiment. Animals were anesthetized for surgery with desflurane, tracheotomized, paralyzed with gallamine triethiodide (80mg, IV), and artificially ventilated using a rodent ventilator (SAR 830/P, CWE, Ardmore, PA) with the mixture of 30% oxygen in nitrogen plus desflurane. Inspired and expired desflurane, oxygen and carbon dioxide concentrations were monitored using a gas analyzer (POET II; Criticare Systems, Inc., Waukesha, WI). Femoral arterial and venous lines were placed for monitoring blood pressure and periodic check of blood gases. Rectal temperature was maintained at 37±1°C with a thermostat-controlled heating apparatus (TC-1000, CWE).
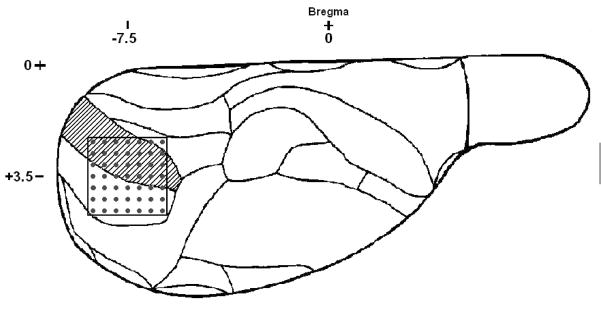
The head was secured in a stereotaxic frame (model 900, Kopf, Tujunga, CA). A craniotomy of approximately 5×5 mm over the right occipital cortex was prepared; the dura was left intact. A 7×7 rectangular array of electrodes (“Utah” array, length: 1.5 mm, spacing: 0.4 mm) was inserted into the cerebral cortex using a pneumatic inserter (Cyberkinetics Neurotechnology Systems, Salt Lake City, UT). The electrodes extended over a 2.4×2.4 mm area containing the primary visual cortex with about half of the electrodes in the monocular and the other half in the binocular visual cortex according to the map of Zilles 34 (fig. 1). The electrode array was initially inserted to a depth of 0.6 mm. The array was then advanced in 0.1 mm increments using a micromanipulator to a depth of 1.0–1.5 mm to facilitate recording from infragranular neurons. For local analgesia, all surgical sites were infiltrated with bupivacaine. Following surgery the room was darkened and desflurane concentration was kept at 8% for a 1-hour equilibration period before neuronal recording was initiated.
Figure 1.
Outline of electrode placement as viewed from the dorsal surface of the right hemisphere of the rat brain. Each dot represents an electrode penetrating at approximately a right angle to the brain surface. Coordinates indicate the location of the center of the electrode array relative to Bregma. Shaded area is the monocular region of the primary visual cortex. The region immediately lateral is the binocular region of the primary visual cortex.
Unit Recording and Flash Stimulation
Multiunit activity from 49 electrodes was simultaneously recorded using a 50-channel neural signal acquisition system (Cyberkinetics Neurotechnology Systems). Extracellular spikes were auto-thresholded using a root mean square multiplier of 2.4 at the end of the equilibration period at 8% desflurane; the settings were then left unchanged for the remainder of the experiment. Desflurane concentration was adjusted sequentially to 4, 2, 6 and 8 %, allowing a 30-minute equilibration time. The neuronal response to flash was recorded at each level of the anesthetic. Flashes were generated using a blue (468 nm) light emitting diode positioned 10 mm from the eye. The light cone emanating from the diode produced diffuse illumination of the entire eye. The diode “on” time was 2 ms at +5V Transistor-Transistor-Logic pulse. Sixty flashes were delivered at 5-second intervals, yielding a total stimulation time of 5 minutes in each condition. The left eye was stimulated in all experiments and at all anesthetic levels while the right eye was covered. In addition, in six of the animals at 2 % desflurane, the left eye and the right eye were stimulated in alternation at 5 s intervals. In addition to multichannel unit activity, a time marker corresponding to each flash was recorded through an analog channel.
Data Analysis and Statistics
The activities of individual units from the chosen channels were distinguished using the public domain offline spike sorter PowerNAP (OSTG, Inc., Fremont, CA). The software applies Principal Component Analysis followed by cluster analysis to sort the spikes. Principal Component Analysis defines the linearly dependent factors in the spike data and transforms them to an ordered set of orthogonal basis vectors that capture the directions of largest variation. As the first step, T-distribution was applied to remove synchronous noise artifacts from data. Synchronous artifacts were related to external noise, such as an electrical pulse related to flash generation. This was based on the detection of spike events that occurred on at least half of the units within an interval of 0.5 ms. A scatter plot was then created using the first two principal components, and K-means clustering was applied to define the cluster boundaries of individual units. Occasional remaining outliers were removed manually. On average, 11% of the traces were rejected as noise. Once the spikes were sorted and artifact-free, peristimulus raster plots and peristimulus time-frequency histograms were generated using the software NeuroExplorer (Version 3.02, Nex Technologies, Littleton, MA). Further analysis was limited to units with an average spike rate of at least 0.3 spikes per second representing approximately 90% of all active units.
To determine the number of units responding to flash, we tested the statistical significance of the change in spike rate after flash across the trials in each unit at each anesthetic condition. The flash response was divided into an early component (0–100 ms) and a late component (150–1000 ms). We tested for the significance of both response components as compared to the mean firing rate of each unit during a 1-second prestimulus baseline. To this end, individual t-tests with a generalized Bonferroni correction were applied. Since on the average we found approximately 50 units per experiment, a uniform alpha value of 0.001 was used. Statistical testing was done using Matlab Statistical Toolbox (Mathworks, Inc., Natick, MA). Once the responding units were identified, baseline firing rates and magnitudes of early and late responses (i.e., changes in firing rate from baseline) were averaged across all units within each animal.
The effects of desflurane on baseline and flash-induced firing rates were estimated with linear regression with the anesthetic concentration as continuous and the subject as categorical independent variables. A significant deviation of the slopes from zero was tested with ANOVA. The same procedure was used to test whether the choice of stimulation side (left vs. right eye) had a significant effect on firing rates. To test for statistical significance of desflurane effect on the number of active and flash responding units, logistic regression was used; again with the anesthetic concentration or stimulation side as continuous and subject as categorical independent variables. The odds ratio of having a late response with versus without early response was compared using chi square test with subject as a blocking variable. Reported means and standard deviations were calculated by averaging within subject means. Statistical calculations were performed with the software SATA/IC 10.0 for Windows (StataCorp LP, College Station, TX).
The distribution of prestimulus spike rate data was left skewed and could be normalized by logarithmic transformation. Therefore, as appropriate, geometric means and 95% confidence intervals of the original distributions of the prestimulus spike rate were presented.
Results
In eight animals, a total of 578 units from 398 sites were recorded. The average yield was 1.5 ± 0.6 units per electrode when the overall baseline activity was the highest. A few electrodes did not reveal distinguishable spikes. On those electrodes that carried spikes, usually two, occasionally three units could be separated by spike sorting. The average baseline (prestimulus) firing rate at the lightest plane of anesthesia (2% desflurane) was 1.69 (1.60–1.78) spikes/s.
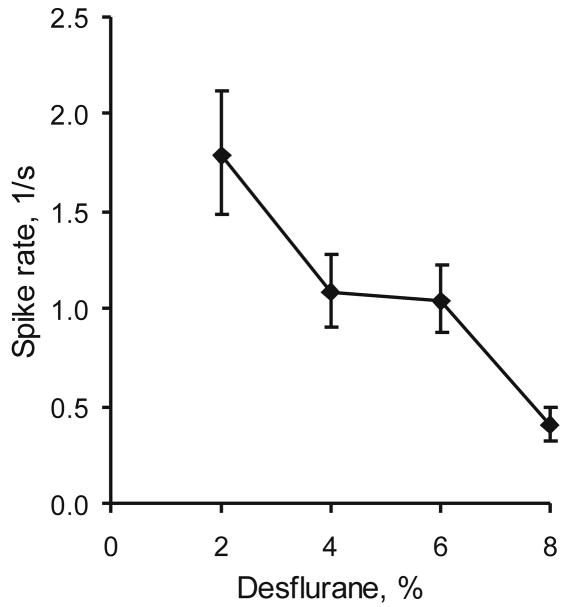
When the concentration of desflurane was increased, there was a small but significant reduction in the number of units active at baseline (table 1, p<0.001, linear trend). In addition, the baseline firing rate decreased in a gradual manner (fig. 2, p<0.001).
Table 1.
Number of units active at baseline and the number of units responding to flash as a function of desflurane concentration in eight rats
| Desflurane concentration | ||||
|---|---|---|---|---|
| 2% | 4% | 6% | 8% | |
| Baseline* | 53±25 | 47±16 | 47±18 | 46±24 |
| Early response* | 70±22% | 80±18% | 79±18% | 92±5% |
| Late response# | 71±25% | 67±29% | 54±34% | 60±33% |
Responding unit counts are in percent of baseline.
p<0.001,
p<0.01 linear trends
Figure 2.
Concentration-dependent effect of desflurane on the average baseline (prestimulus) unit activity in eight rats. Data represent geometric means ± 95% confidence intervals.
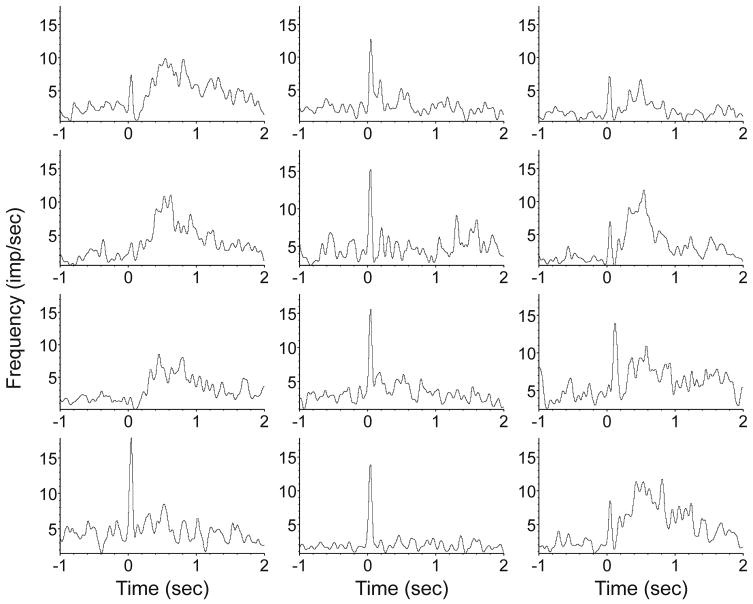
When the left eye was stimulated with flash, approximately 75% of the units responded with a significant (p<0.001) increase in firing rate. Although the response pattern varied from unit to unit; a few typical variants could be identified (fig. 3). The majority of responding units at a concentration of 2% showed a distinct increase in firing rate within the first 100 ms, usually peaking at 40 ms. In this paper we call this the “early” response. In approximately 60% of the units the early response was followed by a secondary increase in firing rate that we call “late” or “long-latency” response. The late response usually started around 150 ms after flash; it was smaller but more prolonged than the early response, extending up to 1 s poststimulus. It often consisted of multiple bouts. A short period of suppressed firing between the early and late responses was often seen.
Figure 3.
Typical patterns of the visual cortex unit response to flash at 2% desflurane level. Time 0 marks the start of the flash stimulus. The early response (large peak at 40 ms) occurs in most units and most frequently is followed by a late response that can extend beyond 1 second. Some units display an early response component or a late component only. Each histogram represents an average of responses from 60 trials. Bin size is 10 ms, displayed values are after 5-point Gaussian smoothing. Ordinate units are in spikes/s.
A small number of units produced a late response in the absence of an early response. The odds of observing a late response was twice as high with, than without, a preceding early response of the same unit (chi-square, p<0.001). This proportion was independent of the depth of anesthesia (logistic regression, p=0.179). The dependence of the late response on anesthetic concentration and stimulation side was similar with or without an early response. Therefore, all units that produced a late response were pooled in the subsequent analysis.
A small fraction (approximately 4%) of the units showed a negative late response that is, a decrease in firing rate after flash. They did not seem to be affected by desflurane concentration and, in light of their scarcity, were not analyzed any further.
When desflurane concentration was increased, there was also an increase in the number of units with an early response (table 1, p<0.001). In contrast, the number of late responding units was reduced (table 1, p<0.01).
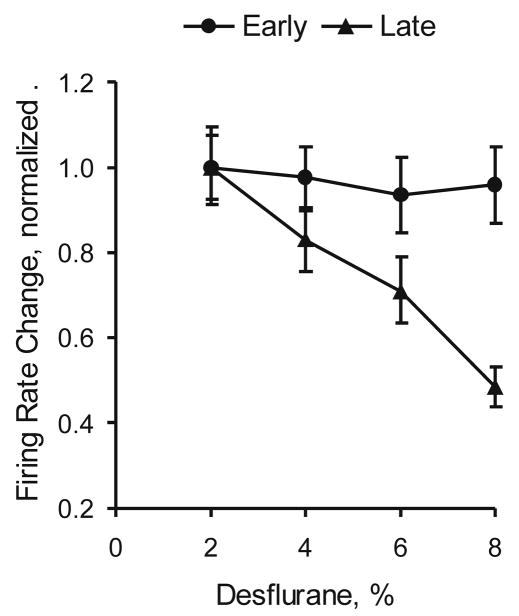
The effect of desflurane on the magnitude of unit response to flash was calculated separately for the early response (0–100 ms vs. baseline) and late responses (150–1000 ms vs. baseline). Only those units that showed a statistically significant increase in firing rate according to the preceding t-test were included. As figure 4 shows, the early response in firing rate did not change with anesthetic concentration (p=0.158). In contrast, the late response was significantly reduced as the anesthetic concentration increased (p<0.001). The overall reduction in firing rate was approximately 60% from the lowest to the highest desflurane concentration.
Figure 4.
Effect of desflurane on visual cortex unit response to flash. The early response (0–100ms) is unchanged, whereas the late response (150–1000ms) is significantly attenuated (p<0.001, linear trend).
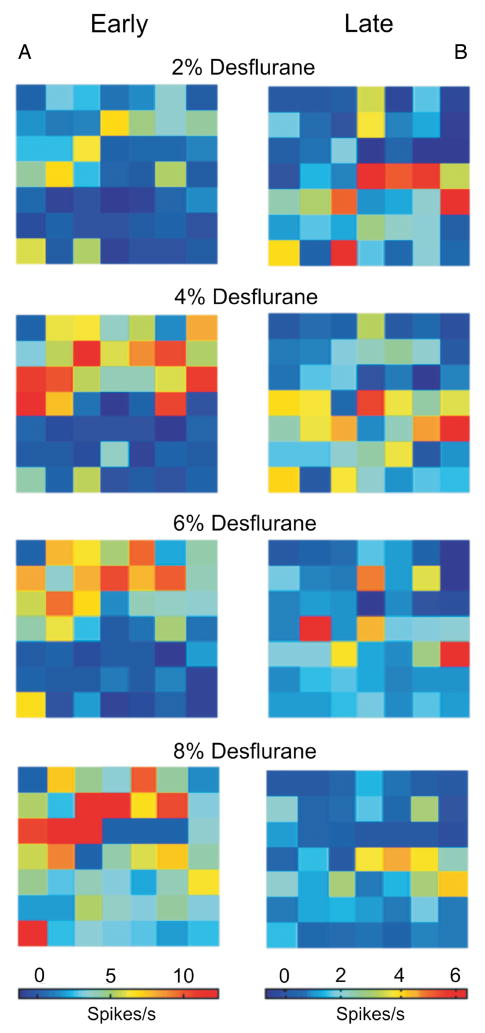
The spatial distribution of unit response to flash is illustrated in figure 5. As seen there, the majority of early responding units to flash are distributed toward the upper left quadrant of the electrode array (same orientation as in fig. 1), corresponding to the monocular visual cortical area. Increasing desflurane concentration augments the early spike response and also recruits units in a wider area. The late responding units are distributed more widely at shallow anesthesia, weighted toward the binocular cortical area. The late response is attenuated and the number of responding units is reduced with increasing desflurane.
Figure 5.
Spatial distribution of spike rate responses to flash in one of the experiments. Panel A shows the early change in spike rate (0–100 ms poststimulus minus baseline). Panel B shows the late change (150–1000 ms poststimulus minus baseline). Early responding units congregate in the monocular visual area (upper left area of the map); the distribution of late responding units is more widespread. Increasing desflurane exerts a differential effect on the early and late responses: the former is augmented whereas the latter is suppressed by the anesthetic in a concentration-dependent manner. The magnitude and spatial extent the responses appear to change in parallel.
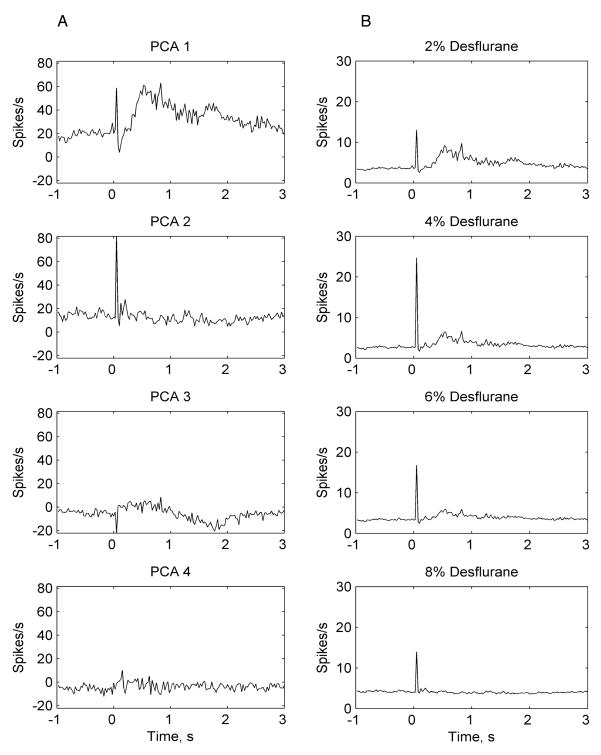
For an additional, model-based analysis of the early and late flash response, we examined the effect of desflurane on the unit population response. Peristimulus spike rate histograms were calculated from all trials at a resolution of 25 ms time bins at the four examined anesthetic concentrations. The spike rate histogram obtained at the lightest anesthetic level (2% desflurane) was taken as a reference and was subjected to Principal Component Analysis. Predicted spike rate histograms (component scores) were calculated by matrix-multiplying the principal components with the original spike rate histograms obtained at 2% desflurane. Predicted spike rate histograms obtained from the first two or three statistically significant principal components, were used as canonical patterns to model the spike rate response of individual units at all four anesthetic concentrations. The latter was accomplished by fitting the canonical histograms to the original peristimulus spike rate histograms using a general linear model 35. For further data reduction, a population response was calculated by averaging the modeled peristimulus spike rate histograms from all units. As illustrated in figure 6, the results confirmed the concentration-dependent suppression of the late population response along with an (transient) augmentation of the early population response.
Figure 6.
Illustration of modeling the population response to flash using Principal Components Analysis and the General Linear Model in one experiment. Panel A shows the first four predicted peristimulus spike rate histograms (component scores) from the corresponding four principal components. Panel B shows spike rate responses at various anesthetic concentrations as modeled by the first two canonicals on Panel A. Although three principal components were significant, the first two components captured the responses of interest. Note the early + late response represented in the first principal component (PCA 1) and the early only response represented in the second principal component (PCA 2). A concentration-dependent suppression of the late population response by desflurane is evident from Panel B. The early response is not attenuated in the examined anesthetic range.
Finally, in six of the animals we compared the responses to left and right eye stimulation at 2% desflurane. The results are summarized in table 2. Looking at the early response, a smaller number of units responded to right eye stimulation than to left eye stimulation (p<0.001). The early response in firing rate was also smaller with right eye than left eye stimulation (p<0.001). In case of the late response, the side of stimulation made no significant difference in the number of responding units (p=0.529) or in the change in firing rate (p=0.251).
Table 2.
Number of responding units and their change in firing rate in the right hemisphere with left and right eye stimulation
| Response type | Number of responding units | Change in firing rate (1/s) | ||
|---|---|---|---|---|
| Left eye | Right eye | Left eye | Right eye | |
| Early | 76±17% | 59±30%* | 4.74±1.19 | 3.22±1.26* |
| Late | 64±17% | 63±30% | 1.78±0.57 | 1.89±1.04 |
Data are percent of all active units ± SD and mean with 95% confidence intervals.
p<0.001 vs. left eye
Discussion
Methodological considerations
This study employed flash activation of the rat visual system as a model to test the concentration-dependent effect of desflurane on the reactivity of cortical sensory neurons. The rat is often considered a ‘nonvisual’ animal, however, ample evidence supports that it can perform cognitive-behavioral tasks demanding complex visual discriminations 36. A surprisingly large area of the rat cerebral cortex is devoted to processing visual stimuli – a region that approximates the size of the barrel cortex used for vibrissal exploration 37. Furthermore, visual cortex neurons show learning and reward-dependent plasticity 38. Our prior experience with this model shows that the cortical response to flash stimulation in albino rats is highly reproducible and robust 26–28,39. Recently, brain imaging studies have adopted flash stimulation in the rat as a useful model to study neurofunctional networks 29.
There are several sources of variance in the data of the present study, some of which have not been fully explored. These include unit-to-unit, trial-to-trial, animal-to-animal, and anesthetic-dependent variations. In our analysis, unit-to-unit variance was reduced by prescreening the units that responded significantly (t-tests) and subsequently categorizing them into subsets for further analysis. Animal-to-animal variance and unit-to-unit variance were explicitly represented in the statistical models used to test the anesthetic effect. Trial-to-trial variation was not considered explicitly; it was averaged over by reducing the data to peristimulus histograms. There are novel ways to address the effect of trial-to-trial systematic variations of spike trains 40 this may be considered for further analysis in the future.
Prestimulus unit activity
First we found, perhaps not unexpectedly, that spontaneous unit activity in visual cortex was reduced by desflurane in a concentration-dependent manner. It has been known that older general anesthetics reduce baseline neuron firing in visual 1 and other sensory systems 41,42. Ogawa et al 43,44 found that volatile anesthetics halothane, isoflurane, enflurane and sevoflurane suppressed multiunit activity in the reticular formation, but they did not study cortical units and only investigated the effect of anesthetic concentrations higher than those that produce loss of consciousness. Villeneuve and Casanova45 found that volatile anesthetics suppressed spontaneous cell firing in cat primary visual cortex; isoflurane was more potent than halothane. Again, mainly deeper levels of anesthesia were studied.
Early response to flash
Our main question was how desflurane influenced the cortical unit response to visual stimulation. When we stimulated the contralateral eye with light flashes at the lightest anesthetic level, approximately 3/4 of the units responded with an increase in firing rate. Burne and colleagues 4 found that in awake animals, 90% of rat visual cortical neurons responded to visual stimuli. They used stationary and moving stimuli, not flash, therefore these figures are not directly comparable. We cannot exclude that the number of responding neurons at 2% desflurane may have been reduced compared to that in waking. Nevertheless, in the range of 2% to 8%, desflurane did not attenuate the early response and the number of responding units was even increased.
Previous studies examined the concentration-dependent effects of various volatile anesthetics, but not of desflurane, on visual cortical neuronal responses. The early investigations by Adams and Forrester revealed that chloroform in small doses enhanced the responsiveness to flash stimulation 1. Ikeda and Wright 10 found that halothane increased from 0.2 to 2.2 % reduced the sensitivity of cat striate cortex neurons to stimulus orientation, spatial frequency and contrast. Halothane suppressed the later components of firing more than the initial component -both within the time frame of our early response. In contrast, Tigwell and Sauter 14 found reliable neuron responses in monkey striate cortex at isoflurane concentrations 0.5 to 0.9 %. Villeneuve and Casanova 45 compared the concentration-dependent effects of halothane/nitrous oxide and isoflurane/nitrous oxide on cat striate cortex neuron responses and found that isoflurane produced a greater suppression than did halothane. Neither anesthetic influenced the orientation and direction sensitivity and receptive field organization of the neurons.
Our results on the early response appear to be at variance with the general observation that the visual evoked potential (VEP) in humans is sensitive to general anesthesia 46. Clearly, we have to be cautious of comparing intracortical unit recordings in rats with scalp-recorded field potentials in humans. However, the most important factor may be the stimulation frequency. We previously showed 26,39 that the VEP in the rat is preserved if light flashes are presented at long (equal or greater than 5 s) interstimulus intervals. These data are relevant because the early unit response peaking at 40ms in layer 4–5 coincides with the N40 evoked potential with a dominant current sink in the thalamocortical input layer 4C 47,48. Likewise, Rabe et al 49 found that VEP in the rat was not depressed by halothane in the concentration range of 0.25 – 1.0% 49. Ogawa et al 43 found that VEP derived from the first 100 ms after flash was not suppressed by isoflurane at a concentration (0.8%) that presumably produces loss of consciousness. Moreover, it was shown that in human patients halothane did not attenuate the VEP when tested with low-frequency (0.5 or 1 Hz) stimuli 50. We surmise that the human VEP, commonly recorded with flickering light, is attenuated by general anesthetics because thalamic and cortical neurons increasingly fail to follow high-frequency driving under anesthesia. However, the results from rats and humans appear to be consistent with each other when tested with low-frequency visual stimulation.
The reason for the preserved visual cortical responsiveness in spite of reduced background activity is unclear but it appears to be true to various sensory modalities 51. It may be due to a transient increase in neuronal excitability as has been seen in the hippocampus in vitro 9. Also, it may reflect a preferential anesthetic depression of feed forward inhibition 52, leading to a larger response and/or more units being able to respond. Erchova et al 7 suggested that with increasing anesthetic depth, sensory specificity may increase due to a reduction of associative inputs and so the early response may be augmented by increasing spike synchronization. Others showed that cortical neuronal receptive fields may expand in light anesthesia and suppressed at deeper levels42.
Volatile anesthetics may also augment the sensory response by suppressing feedforward inhibition through γ-aminobutyric acid-mediated effects. Orth and Antognini 53 studied the effect of mesencephalic reticular formation stimulation on electroencephalogram activation during halothane and isoflurane anesthesia. They concluded that cortical neurons remain responsive at anesthetic concentrations associated with unconsciousness.
Late response to flash
In contrast to the preserved reactivity of cortical units within 100 ms, the late component of the response was gradually reduced by deepening anesthesia. Very few studies have examined the effect of volatile anesthetics on cortical unit reactivity at latencies longer than 100 ms and, as far as we know, none of them have studied desflurane. Early on, Robson 13 noted that the unit response to flash in cat visual cortex had several late components between 200 and 500 ms which were more sensitive to anesthesia than was the primary response within the 100 ms. Their results obtained with trichloroethylene are difficult to extrapolate to modern anesthetics such as desflurane. Ikeda and Wright 11 studied the effect of halothane/nitrous oxide on the reactivity of neurons in cat visual cortex and found that in stage III anesthesia characterized by slow-wave electroencephalogram, the sustained response component (up to 5 seconds) of certain neurons was suppressed, while the primary response was preserved. Likewise, Chapin et al 5 found that 0.75% halothane depressed the long-latency (300 ms) excitatory firing of rat somatosensory cortical neurons following cutaneous stimulation of the forepaw while short-latency responses within 50 ms were little affected. At variance with these findings, Tigwell and Sauter 14 reported that the sustained response up to 300 ms of monkey striate cortex neurons was preserved under anesthesia with 0.5 – 0.9% isoflurane + 73% nitrous oxide. While the exact reason for this discrepancy is unclear, it may have been due to the presentation of a prolonged visual stimulus as opposed to flash. Recently, Villeneuve and Casanova 45 reported that halothane and isoflurane reduced in a concentration-dependent manner single cell responses in primary visual cortex of cats. Isoflurane was more potent, producing a 50% response reduction at 0.8 minimum alveolar concentration - roughly eqivalent to our 4% desflurane level. A notable difference from our study was the use of nitrous oxide which likely potentiated the anesthetic affects. Since the visual stimuli were complex and temporally extended, the effect of anesthesia on short and long-latency components of the unit response could not be investigated.
The reason for the differential reduction of the late versus early response to flash is not entirely clear. General anesthetics are thought to produce a general decrease neuronal excitability 3,54. Several investigators suggested that various anesthetics suppressed the reactivity of thalamocortical neurons 2,6,12. Detsch 6 in particular showed that isoflurane at >0.8% concentration suppressed the responsiveness of thalamocortical cells when driven by high-frequency (30–100 Hz) somatosensory stimuli. The initial response to the onset of the stimulus train was present but the responses to subsequent stimuli were quickly attenuated. We presented the flash stimuli at substantially longer intervals (5 seconds) to facilitate the recovery of neuronal excitability after each stimulus. None of these findings can be easily reconciled with the anesthetics’ differential effect on the early and late response.
A possible explanation may lie in the difference in the underlying mechanisms of the early and late response. Chapin et al 5 suggested that the cortical long-latency response was brought about by “nonspecific” spino-reticulo-thalamic pathways. In awake animals, this response was exhibited by cells with large receptive fields, it attenuated rapidly to high stimulus frequencies (>2 Hz), and could be elicited by nonspecific, arousing stimuli. The early and late components were separated by an inhibitory period similar to that observed in this study. Long-latency responses could be selectively abolished by cryogenic blockade of centromedian thalamic nuclei55. It is also possible that the selective depression of the late response is due to its dependence on cortical polysynaptic pathways 46. Anesthetic depression of synaptic transmission may result in a cumulative loss of signaling such that the more synapses involved the greater overall suppression is produced. In turn, the most complex information processing would be affected the most.
Ocular dependence of the flash response
Flash stimulation of the ipsilateral eye also produced a substantial neuronal response; although the early component was attenuated, the number of late responding neurons and the magnitude of their firing rate increase was just as large as that obtained with the contralateral eye stimulation. As approximately half of the recording electrodes were targeted to the monocular and the other half to the binocular sensitive area of the primary visual cortex, assuming a nearly even illumination of the retina, we anticipated that approximately twice that many units will respond to contralateral than to ipsilateral stimulation. As we saw no such difference, we surmise that the observed response, especially the late component, may reflect nonspecific or integrative processing that can be elicited from either eye.
Mechanism anesthesia
The present data suggest that under anesthesia, the visual cortex does not lose its initial reactivity to visual stimuli but the sustained component of its reactivity is attenuated. What implications may this have for information processing in the anesthetized brain? We know that general anesthetics at increasing doses produce sedation, amnesia and behavioral unresponsiveness, first to verbal and then also to painful stimuli. Unconsciousness ensues at an intermediate dose or concentration, well before nociceptive responses are lost. The concentration of desflurane for unconsciousness falls in the middle of those examined in this work (between 4% and 6%) 27. At this anesthetic depth, the cortex remains reactive but presumably is no longer capable to integrate information at the conscious level 56.
Chapin et al 5 suggested that the selective reduction of long-latency response may contribute to the loss of consciousness associated with anesthesia. Whether they are related to so-called “nonspecific” activation or more complex sensory specific processes is not clear at this time. Although the flash is a simple stimulus, the evoked response likely reflects the brain’s ability to process information because the pattern of cortical population activity evoked by various stimuli is stereotypical, presumably reflecting the wiring of cortical local circuits, and similar to that associated with the performance of complex cognitive functions 57.
It has also been suggested that the long-latency response in primary visual cortex may be mediated by recurrent activity between sensory-specific and higher cortical processing regions and is necessary for conscious perception 58–60. Conscious awareness of visual stimuli has been linked to cortical activation seen at 130–280 ms 61 or 270–300 ms 62,63. We previously found that volatile anesthetics reduced feedback information transfer from frontal and parietal cortex to visual cortex at the time unconsciousness ensued 27. Fronto-occipital electroencephalogram coherence at rest or after flash stimulation was also reduced 28,64. Thus, the attenuation of the late response may also indicate the suppression of recurrent integrative information processing responsible for the anesthetic-induced unconsciousness. Although the mechanism of this effect has not been elucidated, it may, as already mentioned, be due to the accumulation of anesthetic effects at each synapse in a chain so that the complex integrative responses are more depressed because more synapses are involved.
Future extensions of this work could include a more detailed analysis of the temporal details of the flash response and its dependence on various anesthetics. Adams and Forrester 1 saw more than one component of the primary response and we noted that the sustained response may consist of several components as well. Also, histological identification and categorization of the recorded neurons would give further insight as Evarts 8 found that different populations of neurons may respond to anesthesia different ways.
In summary, our results indicate that the majority of visual cortex neurons do not become “undrivable” by flash stimuli under a range of anesthetic conditions with desflurane that include the transition from consciousness to unconsciousness. At the same time, the long-latency component of the neuronal response to flash is attenuated by desflurane in a concentration-dependent manner. Taken together with previous findings, the attenuation of long-latency neuron response may be related to a suppression of cortical communication and integration associated with the commencement of unconsciousness during general anesthesia.
Acknowledgments
Financial support: This work was supported by the grant R01 GM-56398 from the Institute of General Medical Sciences, National Institutes of Health, Bethesda, Maryland.
The authors express their sincere gratitude to James D. Wood, R.L.A.T., in memoriam, for his relentless help in performing the experiments. They also thank Kristina M. Ropella, Ph.D., Professor and Chair, Department of Biomedical Engineering, Marquette University, Milwaukee, Wisconsin for her advice with respect to signal analysis and Richard Rys, B.S., Senior Research Engineer, Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin, for his contribution in constructing components of the electrophysiological recording system.
Footnotes
Meeting presentation: Part of this work was presented at the Annual Meeting of the American Society of Anesthesiologists in Orlando, Florida on October 20, 2008.
References
- 1.Adams AD, Forrester JM. The projection of the rat’s visual field on the cerebral cortex. Q J Exp Physiol Cogn Med Sci. 1968;53:327–36. doi: 10.1113/expphysiol.1968.sp001974. [DOI] [PubMed] [Google Scholar]
- 2.Angel A. The G. L. Brown lecture. Adventures in anaesthesia. Exp Physiol. 1991;76:1–38. doi: 10.1113/expphysiol.1991.sp003471. [DOI] [PubMed] [Google Scholar]
- 3.Berg-Johnsen J, Langmoen IA. Mechanisms concerned in the direct effect of isoflurane on rat hippocampal and human neocortical neurons. Brain Res. 1990;507:28–34. doi: 10.1016/0006-8993(90)90517-f. [DOI] [PubMed] [Google Scholar]
- 4.Burne RA, Parnavelas JG, Lin CS. Response properties of neurons in the visual cortex of the rat. Exp Brain Res. 1984;53:374–83. doi: 10.1007/BF00238168. [DOI] [PubMed] [Google Scholar]
- 5.Chapin JK, Waterhouse BD, Woodward DJ. Differences in cutaneous sensory response properties of single somatosensory cortical neurons in awake and halothane anesthetized rats. Brain Res Bull. 1981;6:63–70. doi: 10.1016/s0361-9230(81)80069-x. [DOI] [PubMed] [Google Scholar]
- 6.Detsch O, Vahle-Hinz C, Kochs E, Siemers M, Bromm B. Isoflurane induces dose-dependent changes of thalamic somatosensory information transfer. Brain Res. 1999;829:77–89. doi: 10.1016/s0006-8993(99)01341-4. [DOI] [PubMed] [Google Scholar]
- 7.Erchova IA, Lebedev MA, Diamond ME. Somatosensory cortical neuronal population activity across states of anaesthesia. Eur J Neurosci. 2002;15:744–52. doi: 10.1046/j.0953-816x.2002.01898.x. [DOI] [PubMed] [Google Scholar]
- 8.Evarts EV. Relation of cell size to effects of sleep in pyramidal tract neurons. Prog Brain Res. 1965;18:81–91. doi: 10.1016/s0079-6123(08)63585-2. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara N, Higashi H, Nishi S, Shimoji K, Sugita S, Yoshimura M. Changes in spontaneous firing patterns of rat hippocampal neurones induced by volatile anaesthetics. J Physiol. 1988;402:155–75. doi: 10.1113/jphysiol.1988.sp017198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda H, Wright MJ. Effect of halothane-nitrous oxide anaesthesia on the behaviour of ‘sustained’ and ‘transient’ visual cortical neurones. J Physiol. 1974;237:20P–1P. [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda H, Wright MJ. Sensitivity of neurones in visual cortex (area 17) under different levels of anaesthesia. Exp Brain Res. 1974;20:471–84. doi: 10.1007/BF00238014. [DOI] [PubMed] [Google Scholar]
- 12.Ries CR, Puil E. Mechanism of anesthesia revealed by shunting actions of isoflurane on thalamocortical neurons. J Neurophysiol. 1999;81:1795–801. doi: 10.1152/jn.1999.81.4.1795. [DOI] [PubMed] [Google Scholar]
- 13.Robson JG. The effects of anesthetic drugs on cortical units. Anesthesiology. 1967;28:144–54. doi: 10.1097/00000542-196701000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Tigwell DA, Sauter J. On the use of isofluorane as an anaesthetic for visual neurophysiology. Exp Brain Res. 1992;88:224–8. doi: 10.1007/BF02259146. [DOI] [PubMed] [Google Scholar]
- 15.Murrell JC, Waters D, Johnson CB. Comparative effects of halothane, isoflurane, sevoflurane and desflurane on the electroencephalogram of the rat. Lab Anim. 2008;42:161–70. doi: 10.1258/la.2007.06019e. [DOI] [PubMed] [Google Scholar]
- 16.Rampil IJ, Lockhart SH, Eger EI, 2nd, Yasuda N, Weiskopf RB, Cahalan MK. The electroencephalographic effects of desflurane in humans. Anesthesiology. 1991;74:434–9. doi: 10.1097/00000542-199103000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Rehberg B, Bouillon T, Zinserling J, Hoeft A. Comparative pharmacodynamic modeling of the electroencephalography-slowing effect of isoflurane, sevoflurane, and desflurane. Anesthesiology. 1999;91:397–405. doi: 10.1097/00000542-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Rehberg B, Ruschner R, Fischer M, Ebeling BJ, Hoeft A. Concentration-dependent changes in the latency and amplitude of somatosensory-evoked potentials by desflurane, isoflurane and sevoflurane. Anasthesiol Intensivmed Notfallmed Schmerzther. 1998;33:425–9. doi: 10.1055/s-2007-994279. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher JE, Hinn AR, Heard CM, Georges LS, Freid EB, Keifer A, Brooks SD, Bailey AG, Valley RD. The effects of isoflurane and desflurane titrated to a bispectral index of 60 on the cortical somatosensory evoked potential during pediatric scoliosis surgery. Anesth Analg. 2005;100:1797–803. doi: 10.1213/01.ANE.0000152193.90756.4E. [DOI] [PubMed] [Google Scholar]
- 20.Bernard JM, Pereon Y, Fayet G, Guiheneuc P. Effects of isoflurane and desflurane on neurogenic motor- and somatosensory-evoked potential monitoring for scoliosis surgery. Anesthesiology. 1996;85:1013–9. doi: 10.1097/00000542-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Freye E, Bruckner J, Latasch L. No difference in electroencephalographic power spectra or sensory-evoked potentials in patients anaesthetized with desflurane or sevoflurane. Eur J Anaesthesiol. 2004;21:373–8. doi: 10.1017/s0265021504005046. [DOI] [PubMed] [Google Scholar]
- 22.Haghighi SS, Sirintrapun SJ, Johnson JC, Keller BP, Oro JJ. Suppression of spinal and cortical somatosensory evoked potentials by desflurane anesthesia. J Neurosurg Anesthesiol. 1996;8:148–53. doi: 10.1097/00008506-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Eger EI, 2nd, Johnson BH. Rates of awakening from anesthesia with I-653, halothane, isoflurane, and sevoflurane: A test of the effect of anesthetic concentration and duration in rats. Anesth Analg. 1987;66:977–82. [PubMed] [Google Scholar]
- 24.Koblin DD, Eger EI, 2nd, Johnson BH, Konopka K, Waskell L. I-653 resists degradation in rats. Anesth Analg. 1988;67:534–8. [PubMed] [Google Scholar]
- 25.Schwender D, Klasing S, Conzen P, Finsterer U, Poppel E, Peter K. Midlatency auditory evoked potentials during anaesthesia with increasing end expiratory concentrations of desflurane. Acta Anaesthesiol Scand. 1996;40:171–6. doi: 10.1111/j.1399-6576.1996.tb04416.x. [DOI] [PubMed] [Google Scholar]
- 26.Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG. Volatile anesthetics enhance flash-induced gamma oscillations in rat visual cortex. Anesthesiology. 2005;102:937–47. doi: 10.1097/00000542-200505000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG. Volatile anesthetics disrupt frontal-posterior recurrent information transfer at gamma frequencies in rat. Neurosci Lett. 2005;387:145–50. doi: 10.1016/j.neulet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Imas OA, Ropella KM, Wood JD, Hudetz AG. Isoflurane disrupts anterio-posterior phase synchronization of flash-induced field potentials in the rat. Neurosci Lett. 2006;402:216–21. doi: 10.1016/j.neulet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Pawela CP, Hudetz AG, Ward BD, Schulte ML, Li R, Kao DS, Mauck MC, Cho YR, Neitz J, Hyde JS. Modeling of region-specific fMRI BOLD neurovascular response functions in rat brain reveals residual differences that correlate with the differences in regional evoked potentials. Neuroimage. 2008;41:525–34. doi: 10.1016/j.neuroimage.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutton RC, Rampil IJ, Eger EI., 2nd Inhaled nonimmobilizers do not alter the middle latency auditory-evoked response of rats. Anesth Analg. 2000;90:213–7. doi: 10.1097/00000539-200001000-00042. [DOI] [PubMed] [Google Scholar]
- 31.Ma J, Shen B, Stewart LS, Herrick IA, Leung LS. The septohippocampal system participates in general anesthesia. J Neurosci. 2002;22:RC200. doi: 10.1523/JNEUROSCI.22-02-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kissin I, Morgan PL, Smith LR. Anesthetic potencies of isoflurane, halothane, and diethyl ether for various end points of anesthesia. Anesthesiology. 1983;58:88–92. doi: 10.1097/00000542-198301000-00012. [DOI] [PubMed] [Google Scholar]
- 33.MacIver MB, Mandema JW, Stanski DR, Bland BH. Thiopental uncouples hippocampal and cortical synchronized electroencephalographic activity. Anesthesiology. 1996;84:1411–24. doi: 10.1097/00000542-199606000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Zilles K, Wree A. In: Cortex: Areal and Laminar Structure, The Rat Nervous System. 2. Paxinos G, editor. San Diego: Academic Press; 1995. pp. 649–85. [Google Scholar]
- 35.Truccolo W, Eden UT, Fellows MR, Donoghue JP, Brown EN. A point process framework for relating neural spiking activity to spiking history, neural ensemble, and extrinsic covariate effects. J Neurophysiol. 2005;93:1074–89. doi: 10.1152/jn.00697.2004. [DOI] [PubMed] [Google Scholar]
- 36.Dean P. Sensory Cortex: Visual Perceptual Functions. In: Kolb B, Tees RC, editors. The Cerebral Cortex of the Rat. Cambridge: MIT Press; 1990. pp. 275–307. [Google Scholar]
- 37.Olavarria J, Montero VM. Relation of callosal and striate-extrastriate cortical connections in the rat: Morphological definition of extrastriate visual areas. Exp Brain Res. 1984;54:240–52. doi: 10.1007/BF00236223. [DOI] [PubMed] [Google Scholar]
- 38.Shuler MG, Bear MF. Reward timing in the primary visual cortex. Science. 2006;311:1606–9. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- 39.Imas OA, Ropella KM, Wood JD, Hudetz AG. Halothane augments event-related gamma oscillations in rat visual cortex. Neuroscience. 2004;123:269–78. doi: 10.1016/j.neuroscience.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Czanner G, Eden UT, Wirth S, Yanike M, Suzuki WA, Brown EN. Analysis of between-trial and within-trial neural spiking dynamics. J Neurophysiol. 2008;99:2672–93. doi: 10.1152/jn.00343.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mountcastle VB, Davies PW, Berman AL. Response properties of neurons of cat’s somatic sensory cortex to peripheral stimuli. J Neurophysiol. 1957;20:374–407. doi: 10.1152/jn.1957.20.4.374. [DOI] [PubMed] [Google Scholar]
- 42.Armstrong-James M, George MJ. Influence of anesthesia on spontaneous activity and receptive field size of single units in rat Sm1 neocortex. Exp Neurol. 1988;99:369–87. doi: 10.1016/0014-4886(88)90155-0. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa T, Shingu K, Shibata M, Osawa M, Mori K. The divergent actions of volatile anaesthetics on background neuronal activity and reactive capability in the central nervous system in cats. Can J Anaesth. 1992;39:862–72. doi: 10.1007/BF03008298. [DOI] [PubMed] [Google Scholar]
- 44.Osawa M, Shingu K, Murakawa M, Adachi T, Kurata J, Seo N, Murayama T, Nakao S, Mori K. Effects of sevoflurane on central nervous system electrical activity in cats. Anesth Analg. 1994;79:52–7. doi: 10.1213/00000539-199407000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Villeneuve MY, Casanova C. On the use of isoflurane versus halothane in the study of visual response properties of single cells in the primary visual cortex. J Neurosci Methods. 2003;129:19–31. doi: 10.1016/s0165-0270(03)00198-5. [DOI] [PubMed] [Google Scholar]
- 46.Banoub M, Tetzlaff JE, Schubert A. Pharmacologic and physiologic influences affecting sensory evoked potentials: Implications for perioperative monitoring. Anesthesiology. 2003;99:716–37. doi: 10.1097/00000542-200309000-00029. [DOI] [PubMed] [Google Scholar]
- 47.Kraut MA, Arezzo JC, Vaughan HG., Jr Intracortical generators of the flash VEP in monkeys. Electroencephalogr Clin Neurophysiol. 1985;62:300–12. doi: 10.1016/0168-5597(85)90007-3. [DOI] [PubMed] [Google Scholar]
- 48.Givre SJ, Schroeder CE, Arezzo JC. Contribution of extrastriate area V4 to the surface-recorded flash VEP in the awake macaque. Vision Res. 1994;34:415–28. doi: 10.1016/0042-6989(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 49.Rabe LS, Moreno L, Rigor BM, Dafny N. Effects of halothane on evoked field potentials recorded from cortical and subcortical nuclei. Neuropharmacology. 1980;19:813–25. doi: 10.1016/0028-3908(80)90077-5. [DOI] [PubMed] [Google Scholar]
- 50.Osa M, Ando M, Adachi-Usami E. Human flash visually evoked cortical potentials under different levels of halothane anesthesia. Nippon Ganka Gakkai Zasshi. 1989;93:265–70. [PubMed] [Google Scholar]
- 51.Vahle-Hinz C, Detsch O. What can in vivo electrophysiology in animal models tell us about mechanisms of anaesthesia? Br J Anaesth. 2002;89:123–42. doi: 10.1093/bja/aef166. [DOI] [PubMed] [Google Scholar]
- 52.Nishikawa K, MacIver MB. Excitatory synaptic transmission mediated by NMDA receptors is more sensitive to isoflurane than are non-NMDA receptor-mediated responses. Anesthesiology. 2000;92:228–36. doi: 10.1097/00000542-200001000-00035. [DOI] [PubMed] [Google Scholar]
- 53.Orth M, Bravo E, Barter L, Carstens E, Antognini JF. The differential effects of halothane and isoflurane on electroencephalographic responses to electrical microstimulation of the reticular formation. Anesth Analg. 2006;102:1709–14. doi: 10.1213/01.ane.0000205752.00303.94. [DOI] [PubMed] [Google Scholar]
- 54.Hentschke H, Schwarz C, Antkowiak B. Neocortex is the major target of sedative concentrations of volatile anaesthetics: Strong depression of firing rates and increase of GABAA receptor-mediated inhibition. Eur J Neurosci. 2005;21:93–102. doi: 10.1111/j.1460-9568.2004.03843.x. [DOI] [PubMed] [Google Scholar]
- 55.O’Brien JH, Rosenblum SM. Contribution of nonspecific thalamus to sensory evoked activity in cat postcruciate cortex. J Neurophysiol. 1974;37:430–42. doi: 10.1152/jn.1974.37.3.430. [DOI] [PubMed] [Google Scholar]
- 56.Hudetz AG. Are we unconscious during general anesthesia? Int Anesthesiol Clin. 2008;46:25–42. doi: 10.1097/AIA.0b013e3181755db5. [DOI] [PubMed] [Google Scholar]
- 57.Mitzdorf U. Properties of cortical generators of event-related potentials. Pharmacopsychiatry. 1994;27:49–51. doi: 10.1055/s-2007-1014274. [DOI] [PubMed] [Google Scholar]
- 58.Shao Z, Burkhalter A. Different balance of excitation and inhibition in forward and feedback circuits of rat visual cortex. J Neurosci. 1996;16:7353–65. doi: 10.1523/JNEUROSCI.16-22-07353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Super H, Spekreijse H, Lamme VA. Two distinct modes of sensory processing observed in monkey primary visual cortex (V1) Nat Neurosci. 2001;4:304–10. doi: 10.1038/85170. [DOI] [PubMed] [Google Scholar]
- 60.Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–2. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- 61.Halgren E, Boujon C, Clarke J, Wang C, Chauvel P. Rapid distributed fronto-parieto-occipital processing stages during working memory in humans. Cereb Cortex. 2002;12:710–28. doi: 10.1093/cercor/12.7.710. [DOI] [PubMed] [Google Scholar]
- 62.Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci. 2005;8:1391–400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- 63.Babiloni C, Vecchio F, Miriello M, Romani GL, Rossini PM. Visuo-spatial consciousness and parieto-occipital areas: A high-resolution EEG study. Cereb Cortex. 2006;16:37–46. doi: 10.1093/cercor/bhi082. [DOI] [PubMed] [Google Scholar]
- 64.John ER, Prichep LS. The anesthetic cascade: A theory of how anesthesia suppresses consciousness. Anesthesiology. 2005;102:447–71. doi: 10.1097/00000542-200502000-00030. [DOI] [PubMed] [Google Scholar]