Abstract
Many accounts of memory suggest that an initial learning experience initiates a cascade of cellular and molecular events that are required for the consolidation of memory from a labile into a more permanent state. Studies of memory in many species have routinely found that altered gene activity and new protein synthesis are the critical components of this memory consolidation process. During extinction, when organisms learn that previously established relations between stimuli have been severed, new memories are formed and consolidated. However, the nature of the learning that underlies extinction remains unclear and there are many processes that may contribute to the weakening of behavior that occurs during extinction. In this review, we suggest that the molecular mechanisms that underlie extinction may differ depending on the learning process that is engaged by extinction. We review evidence that extinction, like initial learning, requires transcription and translation, as well as evidence that extinction occurs when protein synthesis is inhibited. We suggest that extinction occurs through the interaction of multiple behavioral and molecular mechanisms.
Keywords: Extinction, memory, Lymnaea, molecular mechanisms, behavioral mechanisms
Molecular approaches to learning have delineated many of the signal transduction pathways that are critical for the formation of long-term memory. A common finding from many studies using a variety of species is that gene transcription and protein synthesis are critical for making memories long-lasting (reviewed in McGaugh 2000; Rodriguez et al 2004). Indeed, the requirement for protein synthesis during memory consolidation is one of the most theoretically important, experimentally pervasive, and evolutionarily conserved findings in the neurobiological analysis of memory. Historically, most studies of the molecular mechanisms of memory have focused on those mechanisms that are required for memory encoding and consolidation after an initial learning experience, ranging from associating a context with a shock in rodents to associating an odor with an aversive event in snails. The last several years have seen a dramatic increase in the investigation of the molecular mechanisms underlying extinction, an experience-dependent change in behavior that occurs as organisms learn that the relation between previously associated stimuli (such as a context and a shock) is severed. The study of the extinction has led to many insights into general learning processes and numerous applied studies have demonstrated that extinction has great power as a behavioral intervention for many psychiatric disorders, including anxiety disorders, substance abuse, and developmental disorders (Barad 2005; Davis et al 2006).
Extinction has been an exciting process to study at the cellular and molecular levels because, although it shares much in common with initial learning, unique associative and behavioral processes also are engaged during extinction. Perhaps because of the overlapping, yet distinct, behavioral properties between initial learning and extinction, molecular studies of extinction have shown that common and unique mechanisms may operate during initial memory formation and during formation of the extinction memory. In this review, we describe studies from vertebrates and invertebrates demonstrating a common critical requirement for protein synthesis during extinction. We then describe studies demonstrating that different molecular processes may operate during acquisition and extinction, and suggest some possible protein synthesis-independent mechanisms that may mediate extinction.
Theoretical Approaches to Initial Learning and Extinction Suggest Overlapping, but Distinct, Processes
In their seminal reviews of conditioning and extinction, Pavlov (1927) and Konorski (1948) argued persuasively that the associative and neurobiological mechanisms that underlie extinction may overlap with those mechanisms responsible for initial learning. For example, Konorski (1948) hypothesized that both initial learning and extinction caused the formation and multiplication of synaptic connections. But, just as important, these early theorists recognized that extinction also was a different learning process that engaged neurobiological mechanisms that were thought to be distinct from those initially engaged by conditioning. The most widely recognized difference between initial learning and extinction that was articulated by Pavlov and Konorski was that initial learning likely involved excitatory synaptic connections, whereas extinction likely involved inhibitory synaptic connections (Konorski 1948, pp. 134). This general idea of inhibition is present in many behavioral theories of extinction, but a consistent finding from studies of extinction is that many processes contribute to the development of extinction. Indeed, theories continue to suggest that extinction may cause inhibitory associations between multiple stimuli (such as between a conditioned and unconditioned stimulus) or between stimuli and responses (such as between a conditioned stimulus and a conditioned response), but other theories appeal to new excitatory associations that develop during extinction (Konorski 1967). Common to these theories is the idea that different associations compete for expression in behavior (for reviews of behavioral theories of extinction, see Delamater 2004; Rescorla 2004; Weidemann and Kehoe 2004). There also is evidence that nonassociative factors may influence extinction, including changes in the ways in which the animal processes the conditioned stimulus (Kamprath and Wotjak 2004; Pavlov 1927; Robbins 1990) or the unconditioned stimulus (Rescorla and Heath 1975; Rescorla and Cunningham 1977; Rescorla and Cunningham 1978).
Thus there are many processes that may contribute to the loss of responding that occurs during extinction. Rescorla (2004) suggested that the conditions that favor one extinction process over another remain poorly understood and that it is likely that extinction processes may differ depending on the procedures and preparations used to generate extinction. Some of these processes may overlap with those that support the initial generation of associative learning, whereas other processes may be quite different. Indeed, given that the conditions that favor the contribution of one extinction process over another remain poorly understood after 80 years of research on extinction at the behavioral level, it is perhaps not surprising that molecular studies of extinction have also found evidence for sometimes overlapping, yet sometimes, distinct mechanisms.
Protein Synthesis-Dependent Mechanisms in Extinction
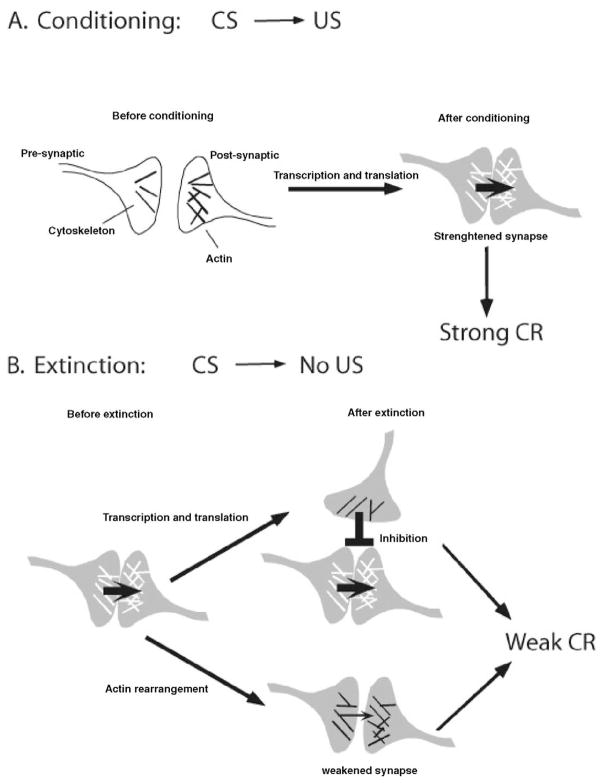
Many studies have demonstrated that gene transcription and protein synthesis are required for initial memory consolidation after a learning experience. Although there is evidence that the nature of that learning experience may alter the protein synthesis requirement of initial consolidation (Bourtchouladze et al 1998; Flood et al 1973; Wittstock et al 1993; reviewed in Routtenberg and Rekart 2005), experiments with many species have demonstrated that if protein synthesis is inhibited, memory consolidation is disrupted. The requirement for transcription and translation in memory has been demonstrated in numerous mammalian studies and forms the cornerstone for modern thinking about molecular mechanisms of memory consolidation (see Figure 1A). Much of this work demonstrating the importance of gene transcription and protein synthesis for memory consolidation comes from studies of initial learning. However, many recent studies have now demonstrated that protein synthesis also is critical for consolidating memories formed during extinction.
Figure 1.
Protein synthesis dependent and independent synaptic changes that may underlie memories for conditioning and extinction. (A) As a consequence of Pavlovian conditioning, synapses are strengthened through a variety of protein synthesis-dependent mechanisms. (B) During extinction, transcription and translation may be required for changes in synapses not previously strengthened through conditioning. These changes may be excitatory or inhibitory, but the net effect of these newly formed connections is inhibition of those synapses encoding the original memory, resulting in a weakening of the conditioned response (CR). Thus, responding may attenuate even though the original synaptic connections themselves are unchanged. Alternatively, rearrangement of actin within the existing circuit may result in a reduction of the synaptic strength (Fischer and Radulovic, unpublished data). Protein synthesis may be required for the generation of inhibition between multiple stimuli or between stimuli and responses, whereas extinction may occur through protein synthesis independent mechanisms when excitation is lost within the previously established circuits representing some part of the original memory, such as the conditioned stimulus (CS), unconditioned stimulus (US), or CS-US association. In addition to these possibilities, extinction is likely to involve several circuits and brain areas. Thus, a decreased activity in one brain region may enhance extinction processes mediated by another area.
Vertebrate Studies
Early work by Flood and colleagues on the role of protein synthesis in memory found that inhibition of protein synthesis impaired extinction of avoidance learning in rodents (Flood et al 1977). More recent rodent studies have found similar effects of protein synthesis inhibition during extinction, consistent with a large body of work demonstrating a requirement for protein synthesis in initial memory consolidation. This requirement for protein synthesis during extinction has been demonstrated in studies of extinction in many preparations, including spatial learning in the water maze (Lattal et al 2004) and extinction after contextual fear conditioning (Suzuki et al 2004). These experiments demonstrating a requirement for protein synthesis during extinction using systemic injections of a protein synthesis inhibitor have found that the effects of the drug on extinction depend greatly on the duration of protein synthesis inhibition and the behavioral extinction protocol. Other recent studies have used microinjection techniques to demonstrate that those brain regions required for initial learning also play a critical role in extinction. For example, intra-hippocampal injection of the protein synthesis inhibitor anisomycin immediately after acquisition of fear conditioning appears to impair the consolidation of the context-shock memory (Debiec et al 2002; Fischer et al 2004; Quevedo et al 2004, 2005). Similarly, injections of anisomycin after extinction trials in the hippocampus have been shown to impair the consolidation of the extinction memory (Power et al 2006; Vianna et al 2001, 2003). Other studies have demonstrated a requirement for protein synthesis in other brain regions and other extinction preparations (Berman and Dudai 2001; Santini et al 2004; Yang and Lu 2005). These findings, coupled with others demonstrating a requirement for certain receptors and protein kinases during extinction suggest that some of the critical cellular and molecular processes needed for establishing initial memories also are needed for establishing extinction memories (Cammarota 2005; Falls et al 1992; Santini et al 2001).
Invertebrate Studies
Much of what is known about extinction and learning in general comes from the study of behavioral processes in vertebrates, particularly in mammals. There are many advantages to studying mammals, but those advantages (such as extensive response repertoires and interactions between multiple neural and behavioral systems) also can be disadvantages when one wants to isolate the molecular cascades involved in learning. Indeed, the extreme complexity of the mammalian nervous system coupled with the difficulty of ascertaining whether a drug (e.g., a transcription or translation inhibitor) actually gets to the necessary neurons at the proper time to affect its target (e.g., transcription or translation) makes it difficult to isolate molecular circuits in mammals. Several studies of extinction in invertebrates have now demonstrated a requirement for some of the same molecular events involved in extinction in vertebrates (McComb et al 2002; Pedreira and Maldonado 2003; Sangha et al 2003b, 2003c). These studies circumvent many of the challenges in isolating molecular events in vertebrates, while also extending the generality of the findings from vertebrate preparations.
Studies of the pond snail Lymnaea stagnalis have been particularly informative in isolating the molecular cascades involved in extinction because of the ability to study a 3-interneuron network that drives an important homeostatic function, aerial respiratory behavior. By using a combination of cell culture and in vivo transplantation techniques the Lukowiak lab has demonstrated both the sufficiency and necessity of this 3-neuron central pattern generator (CPG) to drive aerial respiratory behavior (Syed et al 1990, 1992). If the behavior mediated by this circuit could be modified by experience (i.e., learning) and then be consolidated into a long-lasting non-declarative memory, this would demonstrate this to be a useful preparation to directly study the causal mechanisms of learning, memory formation and extinction (Lukowiak et al 1996). By surgically removing the soma of RPeD1, and thus the genes without interfering with on-going CPG activity, the Lukowiak lab showed that the necessary molecular changes for memory formation occurred in a single CPG neuron, RPeD1 (Lukowiak et al 2003; Scheibenstock et al 2002).
The behavioral attributes of extinction in Lymnaea are similar to those found in mammalian systems. For example, extinction in Lymnaea is context specific (McComb et al 2002), it is more difficult to induce in partially reinforced animals (partial reinforcement extinction effect; Sangha et al 2002) and spaced extinction training is more effective in producing extinction than massed extinction training. Moreover, extinction in Lymnaea exhibits the phenomenon of spontaneous recovery (Sangha et al 2003a, 2003c). Thus, extinction in Lymnaea, as in other model systems, is not the unlearning of the ‘old’ memory. These behavioral data are consistent with the view that during extinction training new learning occurs and this new extinction memory suppresses, but does not abolish, the conditioning memory. Thus a distinction has to be made between the learning event (i.e., an extinction trial without reinforcement) and the memory of that new learning (i.e., the memory of that extinction trial).
The most direct compelling data showing the dependence of extinction on altered gene activity comes from the experiments demonstrating that following RPeD1 soma ablation, extinction could not occur (Sangha et al 2003c). By ablating the soma of RPeD1 (i.e., removal of the nucleus) RNA synthesis is inhibited specifically in RPeD1. Thus, if following the soma-ablation procedure extinction were no longer possible it would indicate that RNA synthesis in the soma of this neuron was a necessary prerequisite. The experimental procedure is relatively straightforward. Snails receive two 45 min operant conditioning sessions separated by 1 hr. This procedure results in LTM. One hour later, animals undergo surgery in which either the soma of LPeD1 (control) or RPeD1 is ablated. Two days later (to allow for full surgical recovery so that all snails could still perform aerial respiratory behavior), extinction training is administered (two 45 min extinction training sessions separated by 1 hr). All animals are tested for savings 4 hr after extinction training. Extinction is observed in the LPeD1 soma ablated snails but not in the RPeD1 ablated snails. Combined with data showing that extinction could be blocked using either the protein synthesis blocker anisomycin or cooling, these data showed for the first time that molecular processes (RNA synthesis) in the soma of a single neuron, RPeD1, is required for extinction (presumably, a newer extinction memory occluding the older conditioning memory).
The Lukowiak lab also demonstrated that it was possible to extend the persistence of extinction (i.e., extend the duration of the newer, weaker memory). This was done by: 1) cooling snails after extinction training and its consolidation into extinction-memory had occurred; and 2) preventing the occurrence of aerial breathing behavior after extinction had been committed to memory (McComb et al 2002). Thus, preventing the forgetting process of extinction by retroactive interference extends extinction, just as it does the original conditioning memory (Sangha et al 2005). Taken together, the data from Lymnaea studies strongly point to extinction being dependent on altered gene activity and new protein synthesis.
As noted, a major breakthrough in the study of extinction in Lymnaea is the ability to investigate this learning process in isolated neurons. Studies of extinction and learning in general in mammalian systems are inherently more complicated because of the interaction between neural systems and behavioral responses. Nonetheless, studies of invertebrate and vertebrate systems point to many molecular similarities between initial learning and extinction. Molecular models of memory consolidation have incorporated these findings into a general framework that suggests that long-term memory requires activation of protein kinases, such as protein kinase A (PKA) and MAP kinase, which activate transcription factors, such as CREB and Elk-1, ultimately leading to de novo protein synthesis, which is thought to be required for the synaptic changes that underlie long-term memory. These processes may be triggered by an initial learning experience as well as by an extinction learning experience.
Protein Synthesis-Independent Mechanisms in Extinction
Behavioral Studies
Many studies have now demonstrated that protein synthesis inhibition may impair the development of both initial learning and extinction. However, the effects of protein synthesis inhibition on the development of extinction are complicated because the effects are less consistent compared to the effects on initial learning. The different effects of protein synthesis inhibition during extinction are most obviously illustrated in studies of extinction after contextual fear conditioning. For example, after extinction, hippocampal protein synthesis inhibition sometimes impairs extinction (i.e., causes conditioned responding to remain high; Vianna et al 2001) and sometimes facilitates extinction (i.e., causes conditioned responding to rapidly decrease; Debiec et al 2002). Such a behavioral facilitation of extinction has been used to suggest that the reconsolidation of the original memory may be disrupted (Debiec et al 2002; Tronson et al 2006), whereas others have suggested that the development of extinction may be enhanced (Fischer et al 2004; Isiegas et al, unpublished data). Some studies have demonstrated that the enhanced extinction effect observed in behavior is long-lasting (Debiec et al 2002; Duvarci and Nader 2004), whereas others have demonstrated that it reverses with time (i.e., spontaneous recovery occurs) or through reminder treatments (Eisenberg and Dudai 2004; Fischer et al 2004; Judge and Quartermain 1982; Isiegas et al, unpublished data; Lattal and Abel 2004; Power et al 2006; Prado-Alcala et al 2006; Vianna et al 2001). The challenge in interpreting these results is to determine the mechanism that sometimes causes these effects to be long-lasting, but sometimes does not. It is quite possible that the original memory may be disrupted under some circumstances and that extinction may be enhanced under others. The pattern that occurs may depend critically on the amount of protein synthesis inhibitor that is injected, whether that injection causes lasting cellular damage (Rudy et al 2006), whether repeated testing is used (Lattal and Abel 2004), or how performance is assessed (Denniston et al 2001).
The major theoretical challenge in interpreting results that show a behavioral enhancement of extinction is mapping that behavioral change onto a change in memory. At the level of behavior, recent evidence suggests that protein synthesis inhibition may enhance extinction, but the mechanism that underlies this behavioral effect remains unknown. Indeed, the elimination of behavior after nonreinforcement is the defining feature of behavioral extinction, but determining how the absence of behavior maps into learning and memory processes continues to be a fundamental challenge facing learning theorists (Bouton 2004; Denniston et al 2001; Rescorla 2003).
Although there have been theoretical accounts proposed for the differences in the effects of protein synthesis during extinction (Dudai and Eisenberg 2004; Duvarci and Nader 2004; Fischer et al 2004; Power et al 2006), the mechanisms that cause rapid behavioral extinction when protein synthesis is inhibited remain unknown. Adding to the complexity of this analysis are behavioral studies showing that extinction can occur normally when protein synthesis is inhibited (Lattal and Abel 2001; Lattal et al 2004), as can latent inhibition (Lewis and Gould, 2004). Latent inhibition is similar to extinction in that both involve stimulus exposure in the absence of reinforcement and both may share a dependence on L-type voltage-gated calcium channels (Barad et al 2004) and NMDA receptors (Lewis and Gould 2004). Findings such as these suggest that, at the very least, the protein synthesis requirements of initial learning and extinction may be different, although by themselves should not be used to suggest that extinction is independent of protein synthesis (see Lattal and Abel 2001). However, it is clear that although the effect of systemic protein synthesis inhibition on acquisition is robust, the effect during extinction may be restricted to very specific extinction parameters (Flood et al 1977; Lattal and Abel 2001; Lattal et al 2004; Suzuki et al 2004). These findings suggest that additional molecular mechanisms may operate during extinction.
Cellular Basis
At a cellular level, there is evidence that some forms of long-term synaptic plasticity may occur when protein synthesis is inhibited. Studies in hippocampal cultures or slices demonstrate that, depending on the neuronal history, some forms of long-term depression (LTD) (Kauderer and Kandel 2000) and long-term potentiation (LTP) (Fonseca et al 2004) of hippocampal synapses can be established when protein synthesis is blocked. The crucial role of the strength and frequency of prior synaptic input has been underlined as a major determinant of the protein synthesis dependence or independence of these forms of synaptic plasticity (Kauderer and Kandel 2000; Fonseca et al 2004). In some respect, these in vitro studies parallel the observations that mechanisms of extinction may significantly vary depending on the initial learning paradigm. Although these findings do not support the view that de novo protein synthesis is universally required for all forms of learning, they are in agreement with several concepts indicating that reorganization of pre-existing protein complexes can produce stable information and readout (Lynch and Baudry 1984; Lisman and Zhabotinsky 2001; Routtenberg and Rekart 2005).
Molecular Basis
These behavioral and cellular demonstrations that learning can occur when protein synthesis is inhibited suggest that protein synthesis-independent mechanisms may operate in some learning situations. There are several possible molecular mechanisms that may allow extinction (and learning in general) to occur in the absence of protein synthesis. One possible mechanism involves cytoskeletal rearrangement that may occur during extinction. Fischer and colleagues (Fischer et al 2004) found that cytoskeletal rearrangement in the hippocampus is involved in extinction of context-evoked fear. The reorganization of actin fibers of the dynamic actin (F-actin) pool profoundly affects neuronal plasticity by regulating the clustering of presynaptic proteins (Hatada et al 2000; Antonova et al 2001; Wang et al 2005), anchoring of membrane receptors (Zhou et al 2001), distribution of signalling molecules (Meng et al 2003) and morphology of dendritic spines (Matus 1999). Some of these mechanisms have been implicated in long-term facilitation of neuronal activity (Hatada et al 2000; Antonova et al 2001), and have been supported by the observations that actin rearrangement is involved in learning (Robertson 1994; Fischer et al 2004). Other mechanisms, such as internalization of AMPA receptors, could decrease synaptic function (Zhou et al 2001). Accordingly, hippocampal actin polymerization regulates the induction of LTP (Chen et al 2004; Fukazawa al 2003) as well as the magnitude of LTD (Chen et al 2004).
Relatedly, several studies suggest that extinction may be mediated by protein phosphatases (Isiegas et al, unpublished data; Lin et al 2003a, 2003b). Isiegas et al have found that transgenic inhibition of PKA in forebrain neurons enhances extinction of context-evoked fear without affecting spontaneous recovery (Koh and Bernstein 2003). They suggested that inhibition of PKA may remove an inhibitory constraint on calcineurin and protein phosphatase 1, which allows extinction to develop more quickly. Thus, although inhibition of PKA impairs initial memory consolidation, it may facilitate extinction learning and consolidation of the extinction memory. Similarly, indirect activation of PKA through rolipram, a type IV-specific phosphodiesterase inhibitor that can be administered systemically, may enhance memory consolidation in fear conditioning (Barad et al 1998), but may retard the development of extinction (Monti et al 2006).
It is not yet clear which of these processes underlies extinction, but all of them could be regulated through cytoplasmic mechanisms that may operate in the absence of new protein synthesis (Routtenberg and Rekart 2005). Thus, it is likely that multiple molecular mechanisms contribute to the development of extinction. New protein synthesis may be required for new associations, either excitatory or inhibitory, that emerge during extinction, whereas actin rearrangement may allow changes in some part of those synapses that represent the CS or the US (Figure 1B). Either mechanism will ultimately result in a weakening of the conditioned response. The identification of the individual molecular substrates affected by cytoskeletal rearrangement and their roles in synaptic excitability is crucial for the further delineation of the distinctive mechanisms regulating specific extinction processes.
Regional Interactions: Disruptions in Fear-Generating Structures May Enhance Extinction
At a systems level, some hippocampal immediate early genes, such as cFos, arc, and egr-1, show reduced levels after nonreinforced (extinction) trials when compared to the initial reinforced learning trial (Milanovic et al 1998; Malkani and Rosen 2000), supporting the possibility that hippocampal activity during extinction may be down-regulated. Similarly, extinction activates calcineurin and decreases the active cAMP-dependent-response element binding protein in the amygdala (Lin et al 2003b). Again, regional differences are likely to occur: enhancement of synaptic plasticity in the prefrontal cortex has been implicated in extinction of tone-dependent fear conditioning (Herry and Garcia 2002; Milad and Quirk 2002), whereas a decrease of neuronal activity in the cerebellum paralleled extinction of eyelid conditioning (Mauk and Ohyama 2004). Similarly, McNally et al (2005) showed that reductions in cAMP in the ventrolateral area of the midbrain periacqueductal gray is required for fear extinction, whereas other studies have shown that increased cAMP in the hippocampus and amygdala are required for fear extinction (Szapiro et al 2003). This down regulation of activity in certain systems during extinction could help to explain why protein synthesis inhibition appears to facilitate extinction in some circumstances (Fischer et al 2004; Lattal and Abel 2004). By depressing the representation of the original memory, which appears to be mediated by hippocampal mechanisms in contextual fear conditioning, the feedback between the neural systems responsible for maintaining the original memory and those responsible for developing the extinction memory may be disrupted, allowing extinction to proceed unchecked by the original memory. The idea that learning, or the expression of learning, may be enhanced by the prevention of inhibitory feedback between different neuronal systems also has been proposed as an account of the enhancement of operant learning that occurs when protein synthesis is inhibited in the dorsolateral striatum (Hernandez et al 2002).
The Molecular Mechanisms of Extinction May Depend on the Behavioral Process Engaged during Extinction
At a theoretical level, there are a number of different accounts for the associative changes that underlie extinction (Bouton 1993; Konorski 1967; Pavlov 1927; Rescorla and Cunningham 1978; Robbins 1990; reviewed in Delamater 2004; Rescorla 2004). These theories likely all capture different aspects of what is learned during extinction, ranging from temporary depressions in the representations of the conditioned stimulus (Pavlov 1927; Robbins 1990) or unconditioned stimulus (Ledgerwood et al 2005; Rescorla and Cunningham 1978), to the development of new associations of an excitatory (Konorski 1967) or inhibitory (Rescorla 1993) form. Certainly, there is evidence that all of these processes contribute to extinction (Delamater 2004; Rescorla 2004). The relative contribution of one extinction process over another likely depends on how extinction is arranged experimentally and what features are most salient during extinction (Weidemann and Kehoe 2004). At a molecular level, protein synthesis-independent processes may be responsible for the depressions in the CS or US representations because this type of change may occur within the neural circuit that represents the CS or US without requiring lasting synaptic modification. However, new associations that are established between the CS and other stimuli, such as the context, may require de novo protein synthesis because these associative connections are likely to involve the strengthening of new synapses, perhaps between different brain structures. Thus, the synthesis of new proteins may be required for some, but not all aspects of extinction.
Conclusions and Clinical Implications
Extinction is a rich learning process that involves changes in behavior that do not always reflect changes in the state of the original memory. Although it is widely accepted that extinction is new learning, the nature of this learning is unclear. What is abundantly clear is that there is not a single extinction memory mechanism that operates at the behavioral or molecular level. Extinction-induced changes in memory and behavior may not have the same dependence on transcription and translation because extinction to a large extent is a modification of a pre-existing memory. The nature of this modification could be a change in that original memory or the development of a new memory. The systems and signalling molecules required for extinction may therefore be identical to those required for initial learning under some circumstances, but may be different under others. Indeed, because extinction is such an important and evolutionarily conserved behavioral strategy, it is likely that multiple mechanisms have evolved to allow extinction to occur. The challenge is determining the conditions that favor the development of one extinction process over another (Weidemann and Kehoe 2004).
Identifying the molecular substrates of extinction has the potential for the development of pharmacological treatments for a number of psychiatric disorders that involve failures to suppress invasive memories or inhibit undesirable behaviors. However, the many demonstrations that multiple behavioral, systems, and molecular processes contribute to the development and persistence of extinction suggest that pharmacological interventions designed to facilitate extinction should be explored carefully. It is quite possible that a given pharmacological agent (such as a PKA inhibitor) may enhance extinction under one set of circumstances, but may impair extinction under other sets of circumstances. Thus, the effect of a systemically delivered agent on extinction in clinical populations may be closely tied to how extinction is arranged behaviorally, what type of associative mechanism is driving extinction, and what brain systems are most affected by that agent. Determining the interplay between different molecular mechanisms and neural systems representing memories from an original learning experience and memories from extinction will be critical for future extrapolations of basic research to clinical settings.
Acknowledgments
Preparation of the manuscript was supported by National Institute of Health Grants MH073669 (to JR) and MH074547 (to KML), a Grant from the Portland Alcohol Research Center (AA010760 to KML), and a Grant from CIHR (to KL).
Footnotes
Aspects of this work were presented at the conference, “Extinction: The Neural Mechanisms of Behavior Change,” held February 2–6, 2005, in Ponce, Puerto Rico. The conference was sponsored by the National Institute of Mental Health, National Institute of Drug Abuse, Ponce School of Medicine, University of Puerto Rico COBRE Program, Pfizer Global Pharmaceutical, and the Municipality of Ponce.
Contributor Information
K. Matthew Lattal, Department of Behavioral Neuroscience and Portland Alcohol Research Center, Oregon Health & Science University, Portland, Oregon.
Jelena Radulovic, Department of Psychiatry and Behavioral Sciences, Northwestern University, Chicago, Illinois.
Ken Lukowiak, Hotchkiss Brain Institute, University of Calgary, Calgary, Alberta Canada.
References
- Antonova I, Arancio O, Trillat AC, Wang HG, Zablow L, Udo H, Kandel ER, Hawkins RD. Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science. 2001;294:1547–1550. doi: 10.1126/science.1066273. [DOI] [PubMed] [Google Scholar]
- Barad M. Fear extinction in rodents: basic insight to clinical promise. Curr Opin Neurobiol. 2005;15(6):710–5. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Barad M, Blouin AM, Cain CK. Like extinction, latent inhibition of conditioned fear in mice is blocked by systemic inhibition of L-type voltage-gated calcium channels. Learn Mem. 2004;11:536–539. doi: 10.1101/lm.78304. [DOI] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci USA. 1998;95(25):15020–5. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5(4–5):365–374. [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114(1):80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11(5):485–94. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Barros DM, Vianna MR, Izquierdo LA, Medina JH, Izquierdo I. Retrieval and the extinction of memory. Cell Mol Neurobiol. 2005;25:465–474. doi: 10.1007/s10571-005-4009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bourne J, Pieribone VA, Fitzsimonds RM. The role of actin in the regulation of dendritic spine morphology and bidirectional synaptic plasticity. Neuroreport. 2004;15:829–832. doi: 10.1097/00001756-200404090-00018. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM, Chhatwal J, Ressler KJ. Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx. 2006;3(1):82–96. doi: 10.1016/j.nurx.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36(3):527–38. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Delamater AR. Experimental extinction in Pavlovian conditioning: behavioural and neuroscience perspectives. Q J Exp Psychol B. 2004;57(2):97–132. doi: 10.1080/02724990344000097. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Savastano HI, Miller RR. The extended comparator hypothesis: Learning by contiguity, responding by relative strength. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Mahwah, NJ: Erlbaum; 2001. pp. 65–117. [Google Scholar]
- Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24(42):9269–75. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M, Dudai Y. Reconsolidation of fresh, remote, and extinguished fear memory in Medaka: old fears don’t die. Eur J Neurosci. 2004;20:3397–3403. doi: 10.1111/j.1460-9568.2004.03818.x. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J Neurosci. 2004;24(8):1962–6. doi: 10.1523/JNEUROSCI.5112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Jarvik ME, Bennett EL, Orme AE, Rosenzweig MR. Protein synthesis inhibition and memory for pole jump active avoidance and extinction. Pharmacol Biochem Behav. 1977;7:71–77. doi: 10.1016/0091-3057(77)90013-2. [DOI] [PubMed] [Google Scholar]
- Flood JF, Rosenzweig MR, Bennett EL, Orme AE. The influence of duration of protein synthesis inhibition on memory. Physiol Behav. 1973;10:555–562. doi: 10.1016/0031-9384(73)90221-7. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Nagerl UV, Morris RG, Bonhoeffer T. Competing for memory: hippocampal LTP under regimes of reduced protein synthesis. Neuron. 2004;44:1011–20. doi: 10.1016/j.neuron.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Hatada Y, Wu F, Sun ZY, Schacher S, Goldberg DJ. Presynaptic morphological changes associated with long-term synaptic facilitation are triggered by actin polymerization at preexisting varicositis. J Neurosci. 2000;20:RC82. doi: 10.1523/JNEUROSCI.20-13-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PJ, Sadeghian K, Kelley AE. Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nat Neurosci. 2002;5(12):1327–31. doi: 10.1038/nn973. [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge ME, Quartermain D. Characteristics of retrograde amnesia following reactivation of memory in mice. Physiol Behav. 1982;28:585–590. doi: 10.1016/0031-9384(82)90034-8. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Wotjak CT. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn Mem. 2004;11:770–786. doi: 10.1101/lm.86104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauderer BS, Kandel ER. Capture of a protein synthesis-dependent component of long-term depression. Proc Natl Acad Sci USA. 2000;97:13342–7. doi: 10.1073/pnas.97.24.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Bernstein IL. Inhibition of protein kinase A activity during conditioned taste aversion retrieval: interference with extinction or reconsolidation of a memory? Neuroreport. 2003;14(3):405–7. doi: 10.1097/00001756-200303030-00021. [DOI] [PubMed] [Google Scholar]
- Konorski J. Conditioned Reflexes and Neuron Organization. Cambridge: University Press; 1948. [Google Scholar]
- Konorski J. Integrative Activity of the Brain, an Interdisciplinary Approach. Chicago: University of Chicago Press; 1967. [Google Scholar]
- Lattal KM, Abel T. Different requirements for protein synthesis in acquisition and extinction of spatial preferences and context-evoked fear. J Neurosci. 2001;21:5773–5780. doi: 10.1523/JNEUROSCI.21-15-05773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc Natl Acad Sci USA. 2004;101:4667–4672. doi: 10.1073/pnas.0306546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Honarvar S, Abel T. Effects of post-session injections of anisomycin on the extinction of a spatial preference and on the acquisition of a spatial reversal preference. Behav Brain Res. 2004;153(2):327–339. doi: 10.1016/j.bbr.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Gould TJ. Latent inhibition of cued fear conditioning: an NMDA receptor-dependent process that can be established in the presence of anisomycin. Eur J Neurosci. 2004;20(3):818–26. doi: 10.1111/j.1460-9568.2004.03531.x. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Leu TH, Chang WC, Wang ST, Gean PW. Identification of calcineurin as a key signal in the extinction of fear memory. J Neurosci. 2003;23(5):1574–9. doi: 10.1523/JNEUROSCI.23-05-01574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci. 2003;23(23):8310–7. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Lukowiak K, Ringseis E, Spencer G, Wildering W, Syed N. Operant conditioning of aerial respiratory behaviour in Lymnaea stagnalis. J Exp Biol. 1996;199:683–691. doi: 10.1242/jeb.199.3.683. [DOI] [PubMed] [Google Scholar]
- Lukowiak K, Sangha S, McComb C, Varshay N, Rosenegger D, Sadamoto H, Scheibenstock A. Associative learning, long-term memory, and the assignment of ‘marks’ in the pond snail, Lymnaea. J Exp Biol. 2003;206:2097–2103. doi: 10.1242/jeb.00374. [DOI] [PubMed] [Google Scholar]
- Lynch G, Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984;224:1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- Matus A. Postsynaptic actin and neuronal plasticity. Curr Opin Neurobiol. 1999;9:561–565. doi: 10.1016/S0959-4388(99)00018-5. [DOI] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. Specific induction of early growth response gene 1 in the lateral nucleus of the amygdala following contextual fear conditioning in rats. Neuroscie. 2000;97:693–702. doi: 10.1016/s0306-4522(00)00058-0. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Ohyama T. Extinction as new learning versus unlearning: considerations from a computer simulation of the cerebellum. Learn Mem. 2004;11:566–71. doi: 10.1101/lm.83504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb C, Sangha S, Qadry S, Yue J, Scheibenstock A, Lukowiak K. Context extinction and associative learning in Lymnaea. Neurobiol Learn Mem. 2002;78:23–34. doi: 10.1006/nlme.2001.4041. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McNally GP, Lee BW, Chiem JY, Choi EA. The midbrain periaqueductal gray and fear extinction: opioid receptor subtype and roles of cyclic AMP, protein kinase A, and mitogen-activated protein kinase. Behav Neurosci. 2005;119(4):1023–33. doi: 10.1037/0735-7044.119.4.1023. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Falls DL, Jia Z. Regulation of spine morphology and synaptic function by LIMK and the actin cytoskeleton. Rev Neurosci. 2003;14:233–240. doi: 10.1515/revneuro.2003.14.3.233. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milanovic S, Radulovic J, Laban O, Stiedl O, Henn F, Spiess J. Production of the Fos protein after contextual fear conditioning of C57BL/6N mice. Brain Res. 1998;784:37–47. doi: 10.1016/s0006-8993(97)01266-3. [DOI] [PubMed] [Google Scholar]
- Monti B, Berteotti C, Contestabile A. Subchronic Rolipram Delivery Activates Hippocampal CREB and Arc, Enhances Retention and Slows Down Extinction of Conditioned Fear. Neuropsychopharmacol. 2006;31(2):278–86. doi: 10.1038/sj.npp.1300813. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes, An Investigation of the Physiological Activity of the Cerebral Cortex. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38(6):863–9. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- Power AE, Berlau DJ, McGaugh JL, Steward O. Anisomycin infused into the hippocampus fails to block “reconsolidation” but impairs extinction: the role of re-exposure duration. Learn Mem. 2006;13(1):27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Alcala RA, Diaz Del Guante MA, Garin-Aguilar ME, Diaz-Trujillo A, Quirarte GL, McGaugh JL. Neurobiol Learn Mem. 2006. Amygdala or hippocampus inactivation after retrieval induces temporary memory deficit. [epub, ahead of print] [DOI] [PubMed] [Google Scholar]
- Quevedo J, Vianna MR, Martins MR, Barichello T, Medina JH, Roesler R, Izquierdo I. Protein synthesis, PKA, and MAP kinase are differentially involved in short- and long-term memory in rats. Behav Brain Res. 2004;154:339–343. doi: 10.1016/j.bbr.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Vianna MR, Roesler R, Martins MR, de-Paris F, Medina JH, Izquierdo I. Pretraining but not preexposure to the task apparatus prevents the memory impairment induced by blockade of protein synthesis, PKA or MAP kinase in rats. Neurochem Res. 2005;30(1):61–7. doi: 10.1007/s11064-004-9686-3. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Contemporary study of Pavlovian conditioning. Span J Psychol. 2003;6(2):185–95. doi: 10.1017/s1138741600005333. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery. Learn Mem. 2004;11(5):501–9. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Cunningham CL. The erasure of reinstated fear. Animal Learn Behav. 1977;5(4):386–394. [Google Scholar]
- Rescorla RA, Cunningham CL. Recovery of the US representation over time during extinction. Learn Motiv. 1978;9(4):373–391. [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol: Animal Behav Proc. 1975;1(1):88–96. [PubMed] [Google Scholar]
- Robbins SJ. Mechanisms underlying spontaneous recovery in autoshaping. J Exp Psychol: Animal Behav Proc. 1990;16(3):235–249. [Google Scholar]
- Robertson JD. Cytochalasin D blocks touch learning in octopus vulgaris. Proc Biol Sci. 1994;258:61–66. doi: 10.1098/rspb.1994.0142. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Rekart JL. Post-translational protein modification as the substrate for long-lasting memory. Trends Neurosci. 2005;28:12–19. doi: 10.1016/j.tins.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Biedenkapp JC, Moineau J, Bolding K. Anisomycin and the reconsolidation hypothesis. Learn Mem. 2006;13(1):1–3. doi: 10.1101/lm.157806. [DOI] [PubMed] [Google Scholar]
- Sangha S, McComb C, Scheibenstock A, Johannes C, Lukowiak K. The effects of continuous versus partial reinforcement schedules on associative learning, memory and extinction in Lymnaea stagnalis. J Exp Biol. 2002;205:1171–1178. doi: 10.1242/jeb.205.8.1171. [DOI] [PubMed] [Google Scholar]
- Sangha S, Scheibenstock A, Morrow R, Lukowiak K. Extinction requires new RNA and protein synthesis and the soma of the cell right pedal dorsal 1 in Lymnaea stagnalis. J Neurosci. 2003;23:9842–9851. doi: 10.1523/JNEUROSCI.23-30-09842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, McComb C, Lukowiak K. Forgetting and the extension of memory in Lymnaea. J Exp Biol. 2003a;206:71–77. doi: 10.1242/jeb.00061. [DOI] [PubMed] [Google Scholar]
- Sangha S, Morrow R, Smyth K, Cooke R, Lukowiak K. Cooling blocks ITM and LTM formation and preserves memory. Neurobiol Learn Mem. 2003b;80:130–139. doi: 10.1016/s1074-7427(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Sangha S, Scheibenstock A, Martens K, Varshney N, Cooke R, Lukowiak K. Impairing forgetting by preventing new learning and memory. Behav Neurosci. 2005;119:787–796. doi: 10.1037/0735-7044.119.3.787. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24(25):5704–10. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibenstock A, Krygier D, Haque Z, Syed N, Lukowiak K. The soma of RPeD1 must be present for LTM formation of associative learning in Lymnaea. J Neurophysiol. 2002;88:1584–1591. doi: 10.1152/jn.2002.88.4.1584. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24(20):4787–95. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed NI, Bulloch AGM, Lukowiak K. In vitro reconstruction of the respiratory central pattern generator of the mollusk Lymnaea. Science. 1990;250:282–285. doi: 10.1126/science.2218532. [DOI] [PubMed] [Google Scholar]
- Syed NI, Ridgway R, Lukowiak K, Bulloch AGM. Transplantation and functional integration of an identified respiratory interneuron in Lymnaea stagnalis. Neuron. 1992;8:767–774. doi: 10.1016/0896-6273(92)90097-w. [DOI] [PubMed] [Google Scholar]
- Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–58. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9(2):167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Vianna MR, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proc Natl Acad Sci USA. 2001;98:12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna MR, Igaz LM, Coitinho AS, Medina JH, Izquierdo I. Memory extinction requires gene expression in rat hippocampus. Neurobiol Learn Mem. 2003;79:199–203. doi: 10.1016/s1074-7427(03)00003-0. [DOI] [PubMed] [Google Scholar]
- Wang HG, Lu FM, Jin I, Udo H, Kandel ER, de Vente J, Walter U, Lohmann SM, Hawkins RD, Antonova I. Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron. 2005;45:389–403. doi: 10.1016/j.neuron.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Weidemann G, Kehoe EJ. Recovery of the rabbit’s conditioned nictitating membrane response without direct reinforcement after extinction. Learn Behav. 2004;32(4):409–26. doi: 10.3758/bf03196038. [DOI] [PubMed] [Google Scholar]
- Wittstock S, Kaatz H-H, Menzel R. Inhibition of brain protein synthesis by cycloheximide does not affect formation of long-term memory in honeybees after olfactory conditioning. J Neurosci. 1993;13:1379–1386. doi: 10.1523/JNEUROSCI.13-04-01379.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Lu KT. Facilitation of conditioned fear extinction by d-cycloserine is mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neurosci. 2005;134(1):247–60. doi: 10.1016/j.neuroscience.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Xiao M, Nicoll RA. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc Natl Acad Sci USA. 2001;98:1261–1266. doi: 10.1073/pnas.031573798. [DOI] [PMC free article] [PubMed] [Google Scholar]