Abstract
Background
House dust mite (HDM) induces allergic asthma in sensitized individuals, although the mechanisms by which HDM is sensed and recognized by the airway mucosa, leading to dendritic cell (DC) recruitment, activation, and subsequent TH2-mediated responses, are unknown.
Objective
We sought to define the pathways by which HDM activates respiratory epithelium to induce allergic airway responses.
Methods
Using a human airway epithelial cell line (16HBE14o-), we studied secretion of the DC chemokine CCL20 after exposure to HDM or other allergens, investigated components of the HDM responsible for the induction of chemokine release, and examined activation of signaling pathways. Central findings were also confirmed in primary human bronchial cells.
Results
We demonstrate that exposure of airway epithelium to HDM results in specific and rapid secretion of CCL20, a chemokine attractant for immature DCs. The induction of CCL20 secretion is dose and time dependent and quite specific to HDM because other allergens, such as ragweed pollen and cockroach antigen, fail to significantly induce CCL20 secretion. Induction of CCL20 secretion is not protease or Toll-like receptor 2/4 dependent but, interestingly, relies on β-glucan moieties within the HDM extract, as evidenced by the ability of other β-glucans to competitively inhibit its secretion and by the fact that disruption of these structures by treatment of HDM with β-glucanase significantly reduces subsequent chemokine secretion.
Conclusion
Taken together, our results describe a novel mechanism for specific pattern recognition of HDM-derived β-glucan moieties, which initiates allergic airway inflammation and, through recruitment of DCs, might link innate pattern recognition at the airway surface with adaptive immune responses.
Keywords: Asthma, allergy, house dust mite, epithelium, dendritic cell, chemokine, pattern recognition, innate immunity
Allergic asthma is a chronic debilitating disease marked by intense infiltration of the bronchial mucosa by lymphocytes and eosinophils, goblet cell hyperplasia, and increased serum IgE levels.1 Exposure to ubiquitous environmental allergens, such as house dust mite (HDM), is a major trigger for asthma exacerbations, and HDM sensitization has been associated with the development of chronic asthma.2 We have previously demonstrated that asthma and allergy are associated with an expansion of TH2-polarized CD4+ cells,3 and it has been shown that the development of the allergic phenotype in mice is absolutely dependent on CD4+ T cells producing a TH2 profile of cytokines.4–6 Although TH2-polarized CD4+ T cells have been shown to be a hall-mark of asthma, the pathways that lead to TH2-driven immune responses are not clear.
Dendritic cells (DCs), the primary antigen-presenting cell in the lung, play a unique role in initiating and regulating primary immune responses.7 They are known to influence T-cell differentiation through cytokine production, as well as through signals provided through costimulatory molecules expressed on their surfaces. Rapid recruitment of DCs into the bronchial mucosa has been well documented in animal studies and in human subjects in response to allergen challenge.8,9 Central to early DC recruitment, CCL20 (also known as macrophage inflammatory protein 3α) is the only known chemokine ligand for CCR6, a receptor preferentially expressed on immature DCs10 and CCL20 has been shown to induce CD1a+ DC migration in vitro.11 The importance of CCR6 in allergic pulmonary inflammation has recently been demonstrated by using a cockroach antigen model with CCR6−/− mice, showing that lack of CCR6 attenuates the allergic airway response to cockroach antigen.12 Although recruitment of DCs and presentation of antigenic peptides is a critical step in the initiation of immune responses, other stimuli might be required for DC activation. These innate danger signals involve inflammatory cytokines or signals transmitted through pattern-recognition receptors (PRRs),13 and the epithelium is a rich source of these mediators. At present, the mechanism by which HDM is sensed by the airway epithelium and induces the secretion of DC-attractant chemokines is unknown.
The major allergenic peptides of HDM (Der p 1 and Der p 2) are digestive enzymes of the mites, which are secreted in their feces.14 These antigenic peptides are responsible for adaptive immune responses, with the majority of mite-sensitive patients demonstrating both T-cell recognition and IgE serologic reactivity to Der p 1 and Der p 2.15–18 Other effects of HDM exposure have been attributed to its protease activity, including direct injury to the epithelium with clear demonstration of epithelial damage and desquamation, loss of tight junctions, and subsequent entrance of antigen into the submucosal space.19 The cysteine protease of dust mite, Der p 1, has also been shown to activate the innate immune response through cleavage of complement components into their active forms at the mucosal surface.20 Others have reported that the ability of allergenic peptides to activate intracellular signaling pathways in airway epithelium is dependent on their protease activity.21,22
HDMs are complex organisms, and in addition to their well-characterized proteases, they produce thousands of proteins and macromolecules that might serve as ligands for PRRs. Toll-like receptors (TLRs) are the best-described PRRs and are present on mucosal surfaces, but epithelial cells are relatively unresponsive to many TLR ligands. A more recently described class of PRRs is the non-Toll PRRs, such as the C-type lectin dectin-1, which recognizes fungal pathogens. However, the process by which HDM is recognized at the epithelial surface is not well understood at this time.
In this study we sought to define the mechanism by which airway epithelium recognizes the potent and common aeroallergen HDM and to delineate its role in the initiation of immune responses in the lung through recruitment of DCs. We demonstrate that HDM induces CCL20 secretion through TLR-independent, protease-independent, and β-glucan–dependent processes. These data indicate that recognition of β-glucan moieties in HDM is involved in early allergic airway responses.
METHODS
Reagents and antibodies
HDM, Der p 1, and ragweed were purchased from Greer (Lenoir, NC); protease inhibitor cocktail (1 complete Mini tablet dissolved in 25 mL of media) was purchased from Roche (Indianapolis, Ind); laminarin, ovalbumin (OVA), chitin, zymosan, curdlan, β-glucanase, and piceatannol were purchased from Sigma (St Louis Mo); cycloheximide was purchased from Calbiochem (San Diego, Calif); and LPS was purchased from Invitrogen (Carlsbad, Calif). Cockroach antigen was a gift from Dr Kristen Page (Cincinnati, Ohio). Ambient urban Baltimore particulate matter was collected as previously described.23 β-Glucanase–treated HDM (200 μg of β-glucanase/100 μg of HDM) and HDM treated with vehicle (maleic acid, pH 5.2) were incubated at 50°C for 1 hour to maximize enzymatic activity.
Cell culture
An SV40-transformed human bronchial epithelial cell line (16HBE14o-; California Pacific Medical Center, San Francisco, Calif) was grown in minimum essential medium supplemented with 10% FBS, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were grown on collagen/fibronectin-coated tissue-culture plates and passaged by means of trypsinization at 80% to 85% confluence. Cells were serum deprived for 18 to 24 hours before treatment with HDM extract (100 μg/mL).
Human bronchial epithelial cell isolation
Eight volunteers, aged between 20 and 35 years, were recruited from the Asthma Clinic at Hôpital Laval (Quebec, Canada). The Ethics Committee of Hôpital Laval approved the study, and all subjects provided written informed consent. Epithelial cells were isolated from bronchial biopsy specimens obtained by means of bronchoscopy. Epithelial cells were characterized by means of immunofluorescence and flow cytometry by using anti-cytokeratin antibody from Calbiochem. This identification confirmed the purity of the bronchial cell culture, as has been previously described.24 Bronchial epithelial cells were cultured in a combination of Dulbecco modified Eagle medium with Ham F12 in a 3:1 proportion (Invitrogen, Burlington, Ontario, Canada), supplemented with 10 ng/mL human epidermal growth factor (Austral Biologicals, San Ramon, Calif); 24.3 μg/mL adenine; 5 μg/mL crystallized bovine insulin; 5 μg/mL human transferrin, 2 × 109 mol/L 3, 3′, 5′, triiodo-L-thyronine (Sigma); 0.4 μg/mL hydrocortisone (Calbiochem); 10−10 mol/L cholera toxin (Sigma Chemicals); 10% FBS (Invitrogen); 100 U/mL penicillin G and streptomycin; and 25 μg/mL gentamicin (Sigma). The culture medium was changed 3 times per week.
ELISA
Chemokine protein levels in epithelial culture supernatants were measured by means of ELISA with matched antibody pairs from R & D Systems (Minneapolis, Minn). The sensitivity of the assay per the manufacturer’s instructions is 20 pg/mL.
RT-PCR analysis
RNA was isolated by using a standard TRIzol method of phenol extraction per the manufacturer’s instructions (Invitrogen), and first-strand cDNA was synthesized with the Superscript First-Strand Synthesis kit (Invitrogen). Quantitative RT-PCR assays were performed as described previously.25 PCR primer pairs were as follows: CCL20 sense, CTGCTTTGATGT CAGTGCTGC; CCL20 antisense, TCACCCAAGTCTGTTTTGG.
Luciferase assay for nuclear factor κB
16HBE14o- cells were transiently transfected with the nuclear factor (NF) κB TATA Luc reporter plasmid (Stratagene, La Jolla, Calif), serum starved, and treated with HDM extract (100 μg/mL) or TNF-α (3 ng/mL). Sixteen hours later, cells were harvested and analyzed for luciferase activity by using a Promega Luciferase Assay (Promega, Madison, Wis).
Statistical analysis
Results are presented as the mean of 3 determinations ± SEM. One-way ANOVA was used to determine differences between multiple groups, with post-hoc comparisons using the Bonferroni method. For comparison between 2 groups, the Student t test was performed (GraphPad Prism; GraphPad Software, Inc, La Jolla, Calif)). Significance was assumed at a P value of less than .05 (n = 3 per experiment). Data are representative of at least 3 separate experiments.
RESULTS
CCL20 and HDM
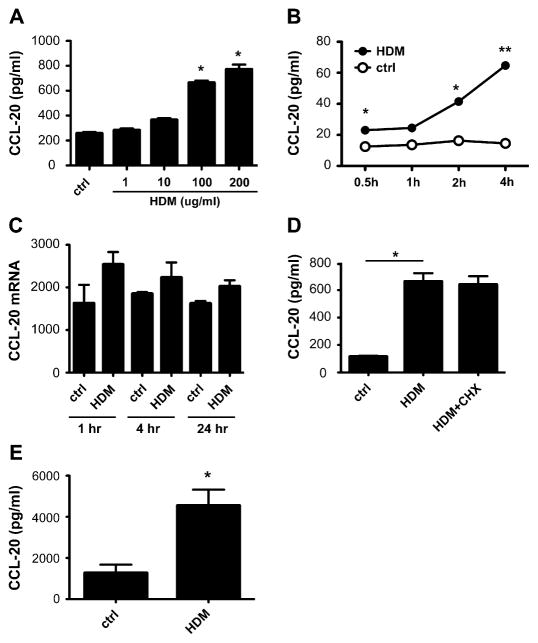
CCL20 is crucial for the initial recruitment of immature DCs to the lung during allergic airway responses and has been found to be upregulated by a variety of inflammatory cytokines.26–29 To determine whether HDM initiates allergic airway responses at the mucosal surface, we examined the ability of HDM to induce CCL20 secretion from human respiratory epithelial cells. Specifically, we treated nearly confluent 16HBE14o- cells that were serum starved overnight with increasing concentrations of HDM (1, 10, 100, and 200 μg/mL) and examined CCL20 protein levels in the cell supernatants after 24 hours. We show that HDM increases CCL20 levels in a dose-dependent manner, with an increase in CCL20 level initially seen at 10 μg/mL HDM and a plateau reached at 100 μg/mL (P <.001; Fig 1, A). No detectable levels of CCL20 were found in the HDM extract or the growth medium itself (data not shown). We demonstrate that HDM induction of CCL20 is time dependent, with an increase in CCL20 levels seen as early as 30 minutes after exposure (P < .05 and P <.001; Fig 1, B).
FIG 1.
CCL20 levels in HDM-treated epithelium. A, CCL20 protein levels determined by means of ELISA in supernatant from 16HBE14o- cells treated with varying doses of HDM. *P < .001. B, Time course of CCL20 secretion from 16HBE14o- cells treated with HDM (100 μg/mL). *P < .05 and **P < .001. C, CCL20 message from 16HBE14o- cells treated with HDM. P = not significant. D, CCL20 protein levels in HDM-exposed 16HBE14o- cells pretreated with cycloheximide (CHX) at 100 μg/mL. *P < .001. E, CCL20 protein levels determined by means of ELISA in supernatant from HDM-treated primary human bronchial epithelial cells. P < .001. ctrl, Control.
Because rapid release of CCL20 was noted after HDM exposure, we examined whether HDM induces transcriptional changes in the message for CCL20. RT-PCR was used to assay the level of CCL20 gene expression in cDNA from HDM-treated 16HBE14o- cells. Constitutive expression of CCL20 was detected at baseline, but no significant increase in message was seen at 1, 4, or 24 hours after HDM treatment (P = not significant; Fig 1, C). This suggests that transcriptional regulation plays only a small part in the increased secretion of CCL20 observed in HDM-exposed 16HBE14o- cells. To further explore the role of transcriptional regulation in HDM-induced CCL20 secretion, 16HBE14o- cells were pretreated with cycloheximide (100 μg/mL) before HDM application to inhibit de novo protein biosynthesis. Cycloheximide treatment failed to inhibit the HDM-induced CCL20 secretion (P <.001; Fig 1, D).
To confirm the applicability of our findings, cultured primary human bronchial epithelial cells from adult volunteers were treated with 100 μg/mL HDM extract for 24 hours, and CCL20 levels were measured in the cell supernatants. We found robust secretion of CCL20 from HDM-exposed cells, which is consistent across the panel of donors (P < .001; Fig 1, E). Protein levels of CCL20 were within a range that has been demonstrated to induce DC migration in vitro (1–10 ng/mL).30 We conclude that HDM induces rapid release of CCL20 from preformed stores and that this rapid response is an important initial event in DC recruitment.
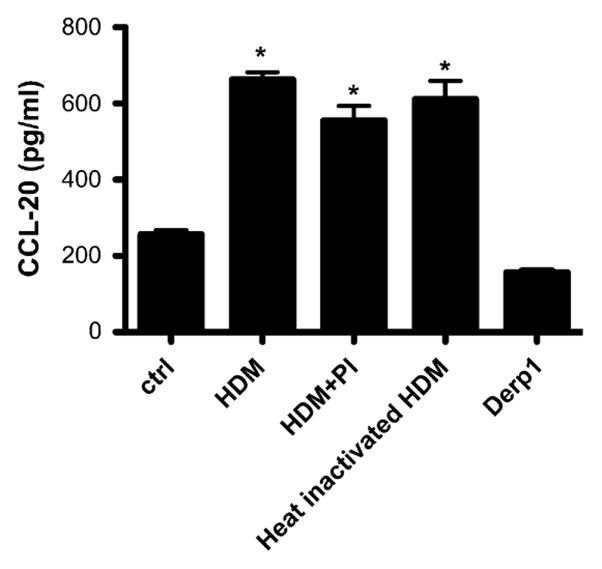
HDM-induced CCL20 production is not protease dependent
Because it has previously been shown that HDM possesses potent proteolytic activity and HDM’s effects on epithelial cells are often ascribed to this activity, we investigated the role of proteases in HDM-induced CCL20 production using several different approaches. First, HDM was heat inactivated at 60°C or treated with a chemical protease inhibitor cocktail (which neutralizes both serine and cysteine proteases) before exposure of 16HBE14o- cells, as described above. Interestingly, the increased CCL20 levels seen after HDM exposure were unchanged when the HDM was heat inactivated or when it was pretreated with the protease inhibitor cocktail (P <.001, Fig 2). Further evidence that protease activity in our model does not contribute to CCL20 secretion is the fact that treatment of cells with the allergenic peptide Der p 1 (5 μg/mL), which is known to be a cysteine protease, did not have any effect on CCL20 secretion. Collectively, these studies strongly suggest that the mechanism of HDM-induced CCL20 production is independent of its protease activity.
FIG 2.
Effect of protease inhibition on CCL20 secretion. HDM was pretreated with a chemical protease inhibitor cocktail (PI) for 1 hour or heat treated for 20 minutes at 60°C. CCL20 protein levels were measured in supernatants from 16HBE14o- cells exposed to HDM, protease inhibitor–treated HDM, heat-inactivated HDM, or purified Der p 1. *P < .001. ctrl, Control.
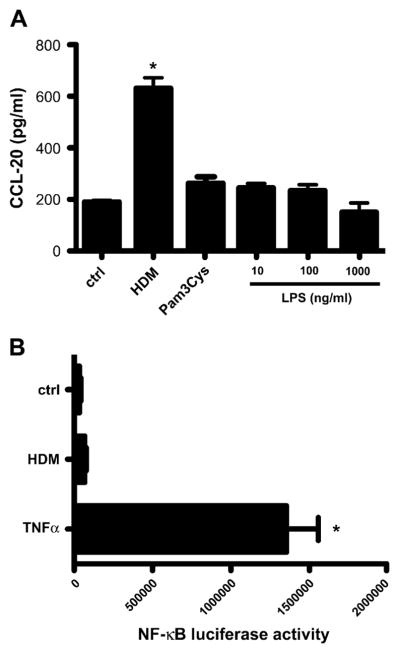
HDM induction of CCL20 is not mediated through TLR stimulation
The mechanism by which the innate arm of the immune system recognizes and senses HDM is unknown at this point. HDM is a complex mixture of antigenic peptides and biologic materials and is likely to include endotoxin and other bacterial products. To investigate the role of TLRs in HDM induction of CCL20, we treated 16HBE14o- cells with LPS, an ubiquitous component of almost all biologic samples, which signals through TLR4 and TLR2. We found that a range of doses of LPS were unable to replicate the effects of HDM in stimulating CCL20 release from the airway epithelial cells. The TLR2 agonist Pam3Cys (10 μg/mL) also failed to elicit CCL20 secretion from 16HBE14o- cells (P < .001; Fig 3, A). Cell lysates were analyzed for NF-κB activation by using an NF-κB luciferase assay. NF-κB levels were only increased by HDM 2-fold over control values, in contrast to the positive control of TNF-α, which increases NF-κB 25-fold, indicating minimal activation of this pathway after HDM exposure (P <.001; Fig 3, B). We conclude that the classic pathway of TLR2 and TLR4 ligand binding and signaling through NF-κB is not responsible for the release of CCL20 from epithelium exposed to HDM.
FIG 3.
Role of TLRs in mediating HDM effects on epithelium. A, CCL20 levels were measured in 16HBE14o- cells exposed to HDM, Pam3Cys, or LPS. *P < .001. B, 16HBE14o- cells transfected with a NF-κB TATA Luc reporter plasmid were stimulated with HDM or TNF-α, and NF-κB activation was assessed by using a luciferase activity. *P < .001. ctrl, Control.
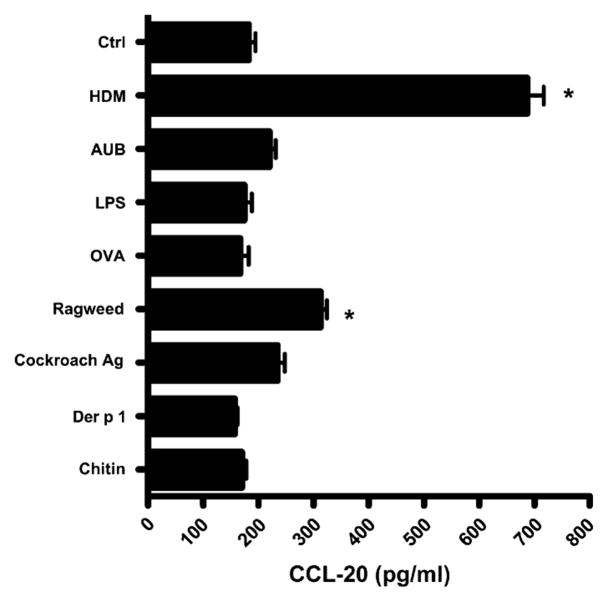
HDM is a specific stimulus for CCL20 production
16HBE14o- cells were treated with a variety of antigenic compounds (HDM, 100 μg/mL; ragweed, 100 μg/mL; cockroach, 10 μg/mL; Der p 1, 5 μg/mL; ambient urban Baltimore particulate matter, 50 μg/mL; OVA, 800 μg/mL; chitin, 100 μg/mL; and LPS, 0.1 μg/mL) to determine the specificity of the effect of HDM on CCL20 secretion. HDM was found to be a very specific and consistent stimulus for CCL20 secretion from these cells, increasing CCL20 levels almost 4-fold over control values (P < .001, Fig 4). In contrast, ragweed induced a less than 2-fold increase in CCL20 secretion, and levels of CCL20 from cockroach extract–, purified Der p 1–, OVA-, chitin-, and LPS-treated cells were indistinguishable from those from media-treated cells. We conclude that HDM is a unique allergen in its ability to induce early and robust secretion of the DC chemokine CCL20.
FIG 4.
Induction of CCL20 production by various environmental antigens. 16HBE14o- cells were exposed to HDM, ambient urban Baltimore particulate matter (AUB), LPS, OVA, ragweed, cockroach antigen (Ag), Der p 1, or chitin, and subsequent CCL20 secretion was measured by means of ELISA. *P < .001. ctrl, Control.
β-Glucan structures in HDM mediate its induction of CCL20
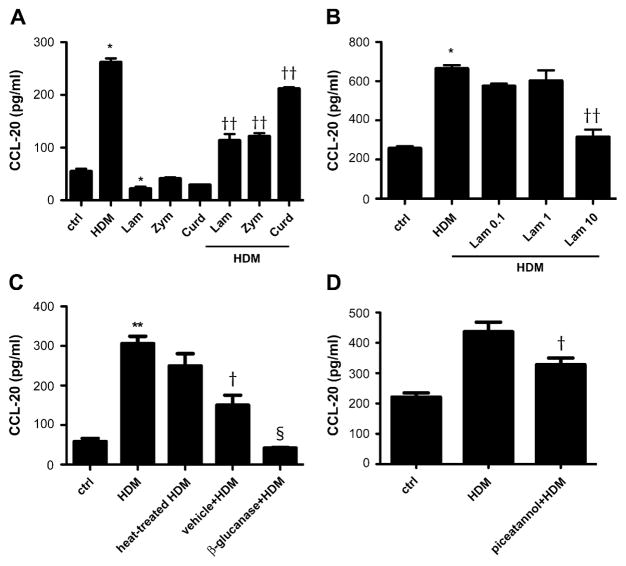
Because HDM does not activate epithelial cells through pure TLR signaling, we explored the possibility that HDM is specifically recognized by a non-TLR PRR. A β-glucan receptor, such as dectin-1, was an excellent candidate because fungal species have been demonstrated in the gastrointestinal tract of HDMs.31 We competitively inhibited the dectin-1 receptor with other β-glucan moieties (laminarin, 10 mg/mL; zymosan, 100 μg/mL; and curdlan, 100 μg/mL). We demonstrated that pretreatment of 16HBE14o- cells with these dectin-1 ligands competitively inhibits HDM-induced CCL20 production (P < .001 vs control and P < .001 vs HDM; Fig 5, A). None of the β-glucan analogs themselves were able to induce CCL20 secretion from the respiratory epithelium. This suggests that HDM provides a ligand for a unique β-glucan receptor or induces a novel signaling pathway in contrast to the classic β-glucan moieties. In studying a range of laminarin concentrations, we found competitive inhibition was most effective at 10 mg/mL (P < .001 vs control and P < .001 vs HDM; Fig 5, B). To further explore the role of HDM-derived β-glucans, we used β-glucanase to digest these structures in the HDM extract. No effect on the viability of the cells was noted by means of Trypan blue exclusion (data not shown). Our data demonstrate that β-glucanase treatment (200 μg/mL) dramatically reduces epithelial CCL20 secretion significantly more than vehicle alone (P < .001 vs control, P < .001 vs HDM, and P <.01 vs maleic acid plus HDM; Fig 5, C). We conclude that specific recognition by a non-Toll β-glucan–dependent process mediates the effects of HDM on the epithelium.
FIG 5.
Role of β-glucan structures in HDM-induced CCL20 production. A, CCL20 secretion from 16HBE14o-cells treated with HDM, zymosan (Zym), laminarin (Lam), curdlan (Curd), and a combination of these β-glucans. *P < .001 vs control and ††P < .001 vs HDM. B, Dose-dependent inhibition of HDM-induced CCL20 secretion by laminarin. *P < .001 vs control and ††P < .001 vs HDM. C, Decreased CCL20 production by 16HBE14o- cells exposed to HDM pretreated with a β-glucanase. **P < .001 vs control, †P < .001 vs HDM, and §P < .01 vs maleic acid plus HDM. D, CCL20 secretion from HDM-exposed 16HBE14o- cells pretreated with the Syk inhibitor piceatannol (4 μmol/L final concentration). †P < .001 vs HDM. ctrl, Control.
HDM’s effects on CCL20 are mediated through the spleen tyrosine kinase pathway
We hypothesized that a non-Toll PRR recognizes HDM-derived β-glucan moieties, and thus we looked for evidence of involvement of transcription factors known to be downstream of the β-glucan family of receptors, including dectin-1. The cytoplasmic tail of dectin-1 has been demonstrated to interact with spleen tyrosine kinase (Syk), and we used a Syk inhibitor (piceatannol) to examine the contributions of this pathway. 16HBE14o- cells were pretreated with various doses of piceatannol for 1 hour and then exposed to HDM, as previously described. Syk inhibition at 4 μmol/L decreased HDM-induced CCL20 secretion, whereas higher concentrations showed some cellular toxicity (P <.001 vs HDM; Fig 5, D). The dependence on the Syk pathway is supportive of the model in which HDM is recognized by and signals through a β-glucan receptor similar to dectin-1.
DISCUSSION
In this study we describe a novel pattern-recognition pathway wherein the allergen HDM binds a non-Toll PRR to initiate innate immune responses. Our data show that stimulation of respiratory epithelium by HDM induces rapid secretion of CCL20, a chemokine central to recruitment of immature DCs to the lung. The effect is specific to HDM because other aeroallergens, such as ragweed and cockroach, fail to elicit this response, and the response is dependent on β-glucan structures in the HDM extract. HDM is understood to be an important trigger of allergic airway responses, but until now, the mechanisms by which HDM stimulates the innate arm of the immune system through the epithelium were unknown.
Previously, proteases were thought to be central to epithelial activation, although initial events at the airway surface remain largely unknown. As shown by the inability of protease inhibitors or heat inactivation to alter the secretion of CCL20 after HDM exposure, we demonstrate that the protease activity of HDM is not responsible for the induction of CCL20 from epithelial cells. This is in contrast to the body of literature that has focused on the direct injury caused by the protease activity of allergens, including damage to tight junctions, cleavage of mediators (eg, complement), and subsequent activation of signaling pathways.19–22 Although there are clearly potent danger signals induced by nonspecific injury to the epithelium and an important role for proteases in mediating these effects, our data suggest a unique pathway of epithelial stimulation through HDM, whereby an allergen is specifically recognized by the airway epithelium to effect intracellular events, leading to DC recruitment to the lung.
As demonstrated by the lack of a similar response to other common aeroallergens, HDM activation of airway epithelium is quite specific and suggests recognition of a distinct molecular pattern. The immunoreceptor tyrosine-based activation motifs found on PRRs are evolutionarily distinct and interface with diverse adaptor proteins, conferring specificity to the intracellular signals that are transduced. Although TLRs are present on mucosal surfaces, epithelial cells are relatively unresponsive to many TLR ligands, and the failure of classic TLR4 (LPS) and TLR2 (Pam3Cys) ligands to induce CCL20 in our study indicates that the epithelial stimulation seen after exposure to HDM is not mediated through TLR2 or TLR4. Although it has been shown in occupational settings that inhalation of dust-containing bacterial components, especially LPS, can cause airway inflammation, decreases in lung function, and airway hyperresponsiveness,32 a clear link between ambient levels of LPS and the development of allergic airway responses has not been demonstrated; exposure to LPS early in life can even decrease allergic responses.33 In contrast to our findings, others have reported NF-κB activation after HDM exposure,34 with reports focused on NF-κB activation in leukocytes. Wong et al34 used bronchial epithelial cells cocultured with eosinophils, suggesting that signals from other cell types might be required for NF-κB activation. We conclude that HDM-induced CCL20 production from epithelium is not TLR dependent.
We speculate that the unique ligation of HDM with a non-Toll PRR is responsible for triggering allergic airway responses and demonstrate that HDM-derived β-glucans are essential for the activation of respiratory epithelium by showing abrogation of HDM-induced CCL20 production by β-glucanase treatment or by competitive inhibition with other β-glucans. It has been suggested that the water-soluble fraction of Candida albicans, which is mainly composed of mannoprotein β-glucans, can be classified as a pathogen-associated molecular pattern because of its toxicologic effects.35 β-Glucans, which are now recognized as potent immunologic activators, have been demonstrated to induce neutrophil chemotaxis and are even being used clinically for immune stimulation in some parts of the world.36,37 Ligation of the classic β-glucan receptor dectin-1 by fungal antigens has been demonstrated to lead to the activation of Syk,38 and our finding that Syk inhibition abrogates the HDM-induced CCL20 production is suggestive of involvement of a dectin-like receptor. Our data suggest for the first time that a non-Toll β-glucan receptor, such as dectin-1, mediates HDM activation of the respiratory epithelium.
As evidenced by the rapid response to HDM in our isolated epithelial cell culture system and the fact that this response can be abrogated by β-glucanase treatment or competitively inhibited by other β-glucans, we demonstrate that β-glucan moieties in HDM are essential for the induction of epithelially derived CCL20 independent of adaptive immunity. Laminarin is composed of (1→3)(1→6)-β-glucan linkages, whereas zymosan is a mixture of β-glucans, mannan, and chitin; these structures were more effective in competitively inhibiting HDM-induced CCL20 than was curdlan, which contains (1→3)-β-glucan linkages with reactive carboxyl groups, suggesting that the particular linkage structure of the β-glucans is crucial in determining their interaction with the epithelium. The role of antigenic peptides, such as Der p 1, in the adaptive immune response to allergens is well established, but our data show that distinct components in the HDM extract are responsible for early and innate epithelial responses. Literature on the association between environmental β-glucan exposure and subsequent wheezing or asthma are contradictory, with increased respiratory symptoms described in adults exposed to indoor environments with increased β-glucan levels39 but a decreased risk of recurrent wheezing in an infant population exposed to high levels of (1→3)-β-glucans.40 In a parallel example of allergic activation of innate immunity, ragweed pollen extracts have been shown to contain intrinsic reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, which induce neutrophil migration to the airways independent of adaptive immune responses. Challenge with Amb a 1, the major antigen in ragweed pollen, which does not contain NADPH oxidase activity, failed to induce robust responses in a murine model of allergic airway inflammation.41 Thus activation of innate immunity by allergen likely involves mechanisms distinct from the antigenic stimulation of the adaptive arm of the immune system.
Taken together, our data support the novel finding that HDM is specifically recognized by the airway epithelium, setting in motion a cascade of intracellular events that result in secretion of DC-attracting chemokines. We propose a model in which ligation of HDM-derived β-glucans by non-Toll PRRs results in secretion of CCL20 to attract a specific subset of immature DCs to the lung, thereby coupling innate and adaptive immunity. A better understanding of these pathways will allow us to develop therapeutic interventions for the growing epidemic of allergic asthma.
Acknowledgments
M.W.-K. received grant support from the National Institutes of Health (P01 HL076383 and R01 H667737).
Abbreviations
- DC
Dendritic cell
- HDM
House dust mite
- NF
Nuclear factor
- OVA
Ovalbumin
- PRR
Pattern-recognition receptor
- Syk
Spleen tyrosine kinase
- TLR
Toll-like receptor
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
Clinical implications: HDM sensitization is strongly associated with asthma, and better understanding of the mechanism by which HDM is recognized by airway epithelium will improve targeting of therapies to decrease allergic immune responses.
References
- 1.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 2.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 3.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–81. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 4.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC., Jr Allergic and non-allergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–15. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 5.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994;10:587–93. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 6.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 7.Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 8.Jahnsen FL, Moloney ED, Hogan T, Upham JW, Burke CM, Holt PG. Rapid dendritic cell recruitment to the bronchial mucosa of patients with atopic asthma in response to local allergen challenge. Thorax. 2001;56:823–6. doi: 10.1136/thorax.56.11.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt PG, Stumbles PA. Characterization of dendritic cell populations in the respiratory tract. J Aerosol Med. 2000;13:361–7. doi: 10.1089/jam.2000.13.361. [DOI] [PubMed] [Google Scholar]
- 10.Power CA, Church DJ, Meyer A, Alouani S, Proudfoot AE, Clark-Lewis I, et al. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J Exp Med. 1997;186:825–35. doi: 10.1084/jem.186.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dieu-Nosjean MC, Massacrier C, Homey B, Vanbervliet B, Pin JJ, Vicari A, et al. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med. 2000;192:705–18. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukacs NW, Prosser DM, Wiekowski M, Lira SA, Cook DN. Requirement for the chemokine receptor CCR6 in allergic pulmonary inflammation. J Exp Med. 2001;194:551–5. doi: 10.1084/jem.194.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–9. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 14.Tovey ER, Chapman MD, Platts-Mills TA. Mite faeces are a major source of house dust allergens. Nature. 1981;289:592–3. doi: 10.1038/289592a0. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien RM, Thomas WR. Immune reactivity to Der p I and Der p II in house dust mite sensitive patients attending paediatric and adult allergy clinics. Clin Exp Allergy. 1994;24:737–42. doi: 10.1111/j.1365-2222.1994.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 16.Platts-Mills TA, Chapman MD. Dust mites: immunology, allergic disease, and environmental control. J Allergy Clin Immunol. 1987;80:755–75. doi: 10.1016/s0091-6749(87)80261-0. [DOI] [PubMed] [Google Scholar]
- 17.Rawle FC, Mitchell EB, Platts-Mills TA. T cell responses to the major allergen from the house dust mite Dermatophagoides pteronyssinus, antigen P1: comparison of patients with asthma, atopic dermatitis, and perennial rhinitis. J Immunol. 1984;133:195–201. [PubMed] [Google Scholar]
- 18.O’Brien RM, Thomas WR, Wootton AM. T cell responses to the purified major allergens from the house dust mite Dermatophagoides pteronyssinus. J Allergy Clin Immunol. 1992;89:1021–31. doi: 10.1016/0091-6749(92)90225-q. [DOI] [PubMed] [Google Scholar]
- 19.Herbert CA, King CM, Ring PC, Holgate ST, Stewart GA, Thompson PJ, et al. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am J Respir Cell Mol Biol. 1995;12:369–78. doi: 10.1165/ajrcmb.12.4.7695916. [DOI] [PubMed] [Google Scholar]
- 20.Maruo K, Akaike T, Ono T, Okamoto T, Maeda H. Generation of anaphylatoxins through proteolytic processing of C3 and C5 by house dust mite protease. J Allergy Clin Immunol. 1997;100:253–60. doi: 10.1016/s0091-6749(97)70233-1. [DOI] [PubMed] [Google Scholar]
- 21.Grunstein MM, Veler H, Shan X, Larson J, Grunstein JS, Chuang S. Proasthmatic effects and mechanisms of action of the dust mite allergen, Der p 1, in airway smooth muscle. J Allergy Clin Immunol. 2005;116:94–101. doi: 10.1016/j.jaci.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 22.Kondo S, Helin H, Shichijo M, Bacon KB. Cockroach allergen extract stimulates protease-activated receptor-2 (PAR-2) expressed in mouse lung fibroblast. Inflamm Res. 2004;53:489–96. doi: 10.1007/s00011-004-1287-8. [DOI] [PubMed] [Google Scholar]
- 23.Walters DM, Breysse PN, Wills-Karp M. Ambient urban Baltimore particulate-induced airway hyperresponsiveness and inflammation in mice. Am J Respir Crit Care Med. 2001;164:1438–43. doi: 10.1164/ajrccm.164.8.2007121. [DOI] [PubMed] [Google Scholar]
- 24.Goulet F, Boulet LP, Chakir J, Tremblay N, Dube J, Laviolette M, et al. Morphologic and functional properties of bronchial cells isolated from normal and asthmatic subjects. Am J Respir Cell Mol Biol. 1996;15:312–8. doi: 10.1165/ajrcmb.15.3.8810634. [DOI] [PubMed] [Google Scholar]
- 25.Finkelman FD, Yang M, Perkins C, Schleifer K, Sproles A, Santeliz J, et al. Suppressive effect of IL-4 on IL-13-induced genes in mouse lung. J Immunol. 2005;174:4630–8. doi: 10.4049/jimmunol.174.8.4630. [DOI] [PubMed] [Google Scholar]
- 26.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–26. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 27.Scapini P, Crepaldi L, Pinardi C, Calzetti F, Cassatella MA. CCL20/macrophage inflammatory protein-3alpha production in LPS-stimulated neutrophils is enhanced by the chemoattractant formyl-methionyl-leucyl-phenylalanine and IFN-gamma through independent mechanisms. Eur J Immunol. 2002;32:3515–24. doi: 10.1002/1521-4141(200212)32:12<3515::AID-IMMU3515>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Sugita S, Kohno T, Yamamoto K, Imaizumi Y, Nakajima H, Ishimaru T, et al. Induction of macrophage-inflammatory protein-3alpha gene expression by TNF-dependent NF-kappaB activation. J Immunol. 2002;168:5621–8. doi: 10.4049/jimmunol.168.11.5621. [DOI] [PubMed] [Google Scholar]
- 29.Starner TD, Barker CK, Jia HP, Kang Y, McCray PB., Jr CCL20 is an inducible product of human airway epithelia with innate immune properties. Am J Respir Cell Mol Biol. 2003;29:627–33. doi: 10.1165/rcmb.2002-0272OC. [DOI] [PubMed] [Google Scholar]
- 30.Thorley AJ, Goldstraw P, Young A, Tetley TD. Primary human alveolar type II epithelial cell CCL20 (macrophage inflammatory protein-3alpha)-induced dendritic cell migration. Am J Respir Cell Mol Biol. 2005;32:262–7. doi: 10.1165/rcmb.2004-0196OC. [DOI] [PubMed] [Google Scholar]
- 31.Van Asselt L. Review: interactions between domestic mites and fungi. Indoor Built Environ. 1999;8:216–20. [Google Scholar]
- 32.Hodgson MJ, Bracker A, Yang C, Storey E, Jarvis BJ, Milton D, et al. Hypersensitivity pneumonitis in a metal-working environment. Am J Ind Med. 2001;39:616–28. doi: 10.1002/ajim.1061. [DOI] [PubMed] [Google Scholar]
- 33.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 34.Wong CK, Li MLY, Wang CB, Ip WK, Tian YP, Lam CWK. House dust mite allergen Der p 1 elevates the release of inflammatory cytokines and expression of adhesion molecules in co-culture of human eosinophils and bronchial epithelial cells. Int Immunol. 2006;18:1327–35. doi: 10.1093/intimm/dxl065. [DOI] [PubMed] [Google Scholar]
- 35.Tada R, Nagi-Miura N, Adachi Y, Ohno N. Candida albicans derived fungal pathogen-associated molecular patterns (PAMPS), CAWS, water soluble mannoprotein-beta-glucan complex shows similar immunotoxicological activity with bacterial endotoxin from Escherichia coli O9. Biol Pharm Bull. 2006;29:240–6. doi: 10.1248/bpb.29.240. [DOI] [PubMed] [Google Scholar]
- 36.Sato T, Iwabuchi K, Nagaoka I, Adachi Y, Ohno N, Tamura H, et al. Induction of human neutrophil chemotaxis by Candida albicans-derived beta-1,6-long glycoside side-chain-branched beta-glucan. J Leukoc Biol. 2006;80:204–11. doi: 10.1189/jlb.0106069. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Seviour R. Medicinal importance of fungal beta-(1→3), (1→6)-glucans. Mycol Res. 2007;111:635–52. doi: 10.1016/j.mycres.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Brown GD, Herre J, Williams DL, Willment JA, Marshall ASJ, Gordon S. Dectin-1 mediates the biological effects of β-glucans. J Exp Med. 2003;197:1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan GH, Li CS. Indoor endotoxin and glucan in association with airway inflammation and systemic symptoms. Arch Environ Health. 1999;54:172–9. doi: 10.1080/00039899909602256. [DOI] [PubMed] [Google Scholar]
- 40.Iossifova YY, Reponen T, Bernstein DI, Levin L, Kalra H, Campo P, et al. House dust (1–3)-beta-D-glucan and wheezing in infants. Allergy. 2007;62:504–13. doi: 10.1111/j.1398-9995.2007.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, et al. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–79. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]