Abstract
To study in vivo the export of mitochondrially synthesized protein from the matrix to the intermembrane space, we have fused a synthetic mitochondrial gene, ARG8m, to the Saccharomyces cerevisiae COX2 gene in mitochondrial DNA. The Arg8mp moiety was translocated through the inner membrane when fused to the Cox2p C terminus by a mechanism dependent on topogenic information at least partially contained within the exported Cox2p C-terminal tail. The pre-Cox2p leader peptide did not signal translocation. Export of the Cox2p C-terminal tail, but not the N-terminal tail, was dependent on the inner membrane potential. The mitochondrial export system does not closely resemble the bacterial Sec translocase. However, normal translocation of both exported domains of Cox2p was defective in cells lacking the widely conserved inner membrane protein Oxa1p.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altamura N., Capitanio N., Bonnefoy N., Papa S., Dujardin G. The Saccharomyces cerevisiae OXA1 gene is required for the correct assembly of cytochrome c oxidase and oligomycin-sensitive ATP synthase. FEBS Lett. 1996 Mar 11;382(1-2):111–115. doi: 10.1016/0014-5793(96)00165-2. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Andersson H., von Heijne G. Membrane protein topology: effects of delta mu H+ on the translocation of charged residues explain the 'positive inside' rule. EMBO J. 1994 May 15;13(10):2267–2272. doi: 10.1002/j.1460-2075.1994.tb06508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M., Behrens M., Esser K., Michaelis G., Pratje E. PET1402, a nuclear gene required for proteolytic processing of cytochrome oxidase subunit 2 in yeast. Mol Gen Genet. 1994 Nov 1;245(3):272–278. doi: 10.1007/BF00290106. [DOI] [PubMed] [Google Scholar]
- Behrens M., Michaelis G., Pratje E. Mitochondrial inner membrane protease 1 of Saccharomyces cerevisiae shows sequence similarity to the Escherichia coli leader peptidase. Mol Gen Genet. 1991 Aug;228(1-2):167–176. doi: 10.1007/BF00282462. [DOI] [PubMed] [Google Scholar]
- Bonnefoy N., Chalvet F., Hamel P., Slonimski P. P., Dujardin G. OXA1, a Saccharomyces cerevisiae nuclear gene whose sequence is conserved from prokaryotes to eukaryotes controls cytochrome oxidase biogenesis. J Mol Biol. 1994 Jun 3;239(2):201–212. doi: 10.1006/jmbi.1994.1363. [DOI] [PubMed] [Google Scholar]
- Bonnefoy N., Kermorgant M., Groudinsky O., Minet M., Slonimski P. P., Dujardin G. Cloning of a human gene involved in cytochrome oxidase assembly by functional complementation of an oxa1- mutation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11978–11982. doi: 10.1073/pnas.91.25.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G., Kuhn A., Dalbey R. E. The translocation of negatively charged residues across the membrane is driven by the electrochemical potential: evidence for an electrophoresis-like membrane transfer mechanism. EMBO J. 1995 Mar 1;14(5):866–875. doi: 10.1002/j.1460-2075.1995.tb07068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
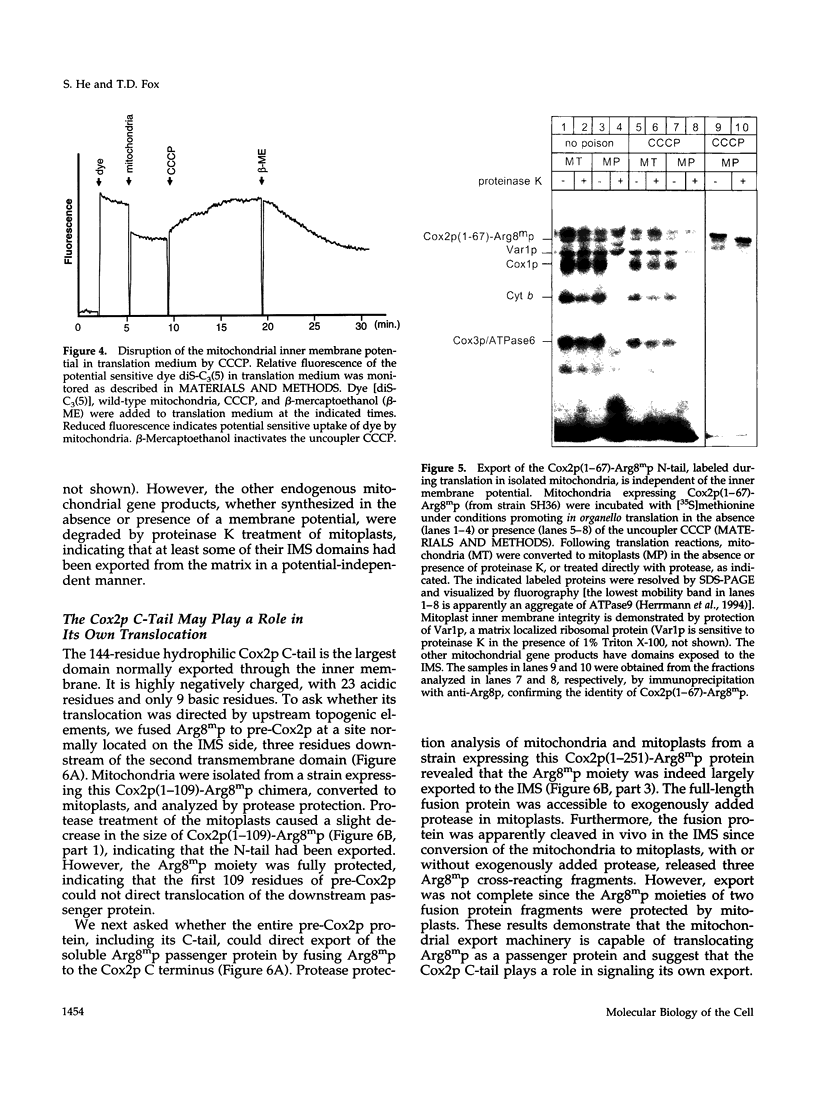
- Clarkson G. H., Poyton R. O. A role for membrane potential in the biogenesis of cytochrome c oxidase subunit II, a mitochondrial gene product. J Biol Chem. 1989 Jun 15;264(17):10114–10118. [PubMed] [Google Scholar]
- Corsi A. K., Schekman R. Mechanism of polypeptide translocation into the endoplasmic reticulum. J Biol Chem. 1996 Nov 29;271(48):30299–30302. doi: 10.1074/jbc.271.48.30299. [DOI] [PubMed] [Google Scholar]
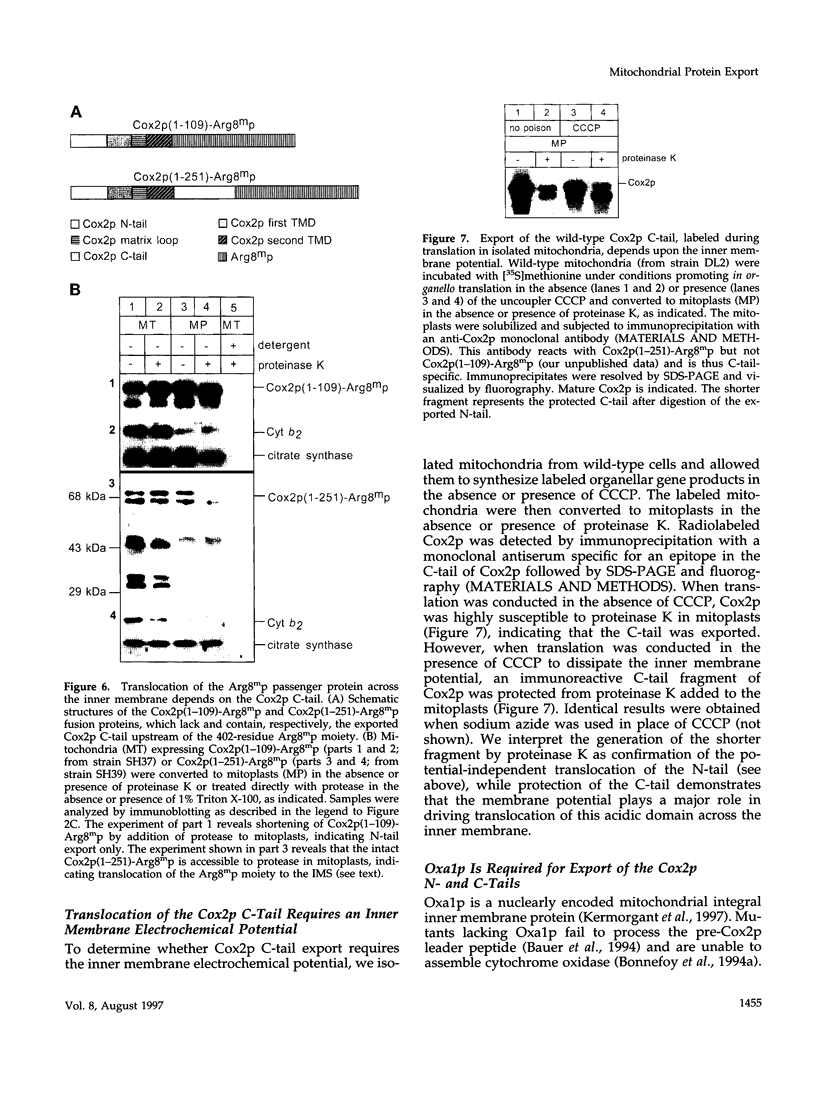
- Costanzo M. C., Fox T. D. Suppression of a defect in the 5' untranslated leader of mitochondrial COX3 mRNA by a mutation affecting an mRNA-specific translational activator protein. Mol Cell Biol. 1993 Aug;13(8):4806–4813. doi: 10.1128/mcb.13.8.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr D. M., Douglas M. G. Early events in the transport of proteins into mitochondria. Import competition by a mitochondrial presequence. J Biol Chem. 1991 Nov 15;266(32):21700–21708. [PubMed] [Google Scholar]
- Dalbey R. E., Kuhn A., von Heijne G. Directionality in protein translocation across membranes: the N-tail phenomenon. Trends Cell Biol. 1995 Oct;5(10):380–383. doi: 10.1016/s0962-8924(00)89079-0. [DOI] [PubMed] [Google Scholar]
- Errington J., Appleby L., Daniel R. A., Goodfellow H., Partridge S. R., Yudkin M. D. Structure and function of the spoIIIJ gene of Bacillus subtilis: a vegetatively expressed gene that is essential for sigma G activity at an intermediate stage of sporulation. J Gen Microbiol. 1992 Dec;138(12):2609–2618. doi: 10.1099/00221287-138-12-2609. [DOI] [PubMed] [Google Scholar]
- Folley L. S., Fox T. D. Site-directed mutagenesis of a Saccharomyces cerevisiae mitochondrial translation initiation codon. Genetics. 1991 Nov;129(3):659–668. doi: 10.1093/genetics/129.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Folley L. S., Mulero J. J., McMullin T. W., Thorsness P. E., Hedin L. O., Costanzo M. C. Analysis and manipulation of yeast mitochondrial genes. Methods Enzymol. 1991;194:149–165. doi: 10.1016/0076-6879(91)94013-3. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. S., Brandt A., Cunningham K., Müller S., Hallberg R. L., Schatz G. Cytochromes c1 and b2 are sorted to the intermembrane space of yeast mitochondria by a stop-transfer mechanism. Cell. 1992 May 29;69(5):809–822. doi: 10.1016/0092-8674(92)90292-k. [DOI] [PubMed] [Google Scholar]
- Glick B. S. Pathways and energetics of mitochondrial protein import in Saccharomyces cerevisiae. Methods Enzymol. 1995;260:224–231. doi: 10.1016/0076-6879(95)60140-6. [DOI] [PubMed] [Google Scholar]
- Glick B. S., Pon L. A. Isolation of highly purified mitochondria from Saccharomyces cerevisiae. Methods Enzymol. 1995;260:213–223. doi: 10.1016/0076-6879(95)60139-2. [DOI] [PubMed] [Google Scholar]
- Glick B. S., Von Heijne G. Saccharomyces cerevisiae mitochondria lack a bacterial-type sec machinery. Protein Sci. 1996 Dec;5(12):2651–2652. doi: 10.1002/pro.5560051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. M., Koll H., Cook R. A., Neupert W., Stuart R. A. Topogenesis of cytochrome oxidase subunit II. Mechanisms of protein export from the mitochondrial matrix. J Biol Chem. 1995 Nov 10;270(45):27079–27086. doi: 10.1074/jbc.270.45.27079. [DOI] [PubMed] [Google Scholar]
- Herrmann J. M., Stuart R. A., Craig E. A., Neupert W. Mitochondrial heat shock protein 70, a molecular chaperone for proteins encoded by mitochondrial DNA. J Cell Biol. 1994 Nov;127(4):893–902. doi: 10.1083/jcb.127.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermorgant M., Bonnefoy N., Dujardin G. Oxa1p, which is required for cytochrome c oxidase and ATP synthase complex formation, is embedded in the mitochondrial inner membrane. Curr Genet. 1997 Apr;31(4):302–307. doi: 10.1007/s002940050209. [DOI] [PubMed] [Google Scholar]
- Lee J. I., Kuhn A., Dalbey R. E. Distinct domains of an oligotopic membrane protein are Sec-dependent and Sec-independent for membrane insertion. J Biol Chem. 1992 Jan 15;267(2):938–943. [PubMed] [Google Scholar]
- Lill R., Nargang F. E., Neupert W. Biogenesis of mitochondrial proteins. Curr Opin Cell Biol. 1996 Aug;8(4):505–512. doi: 10.1016/s0955-0674(96)80028-7. [DOI] [PubMed] [Google Scholar]
- Mulero J. J., Fox T. D. Alteration of the Saccharomyces cerevisiae COX2 mRNA 5'-untranslated leader by mitochondrial gene replacement and functional interaction with the translational activator protein PET111. Mol Biol Cell. 1993 Dec;4(12):1327–1335. doi: 10.1091/mbc.4.12.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagley P. Eukaryote membrane genetics: the Fo sector of mitochondrial ATP synthase. Trends Genet. 1988 Feb;4(2):46–51. doi: 10.1016/0168-9525(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Nunnari J., Fox T. D., Walter P. A mitochondrial protease with two catalytic subunits of nonoverlapping specificities. Science. 1993 Dec 24;262(5142):1997–2004. doi: 10.1126/science.8266095. [DOI] [PubMed] [Google Scholar]
- Pinkham J. L., Dudley A. M., Mason T. L. T7 RNA polymerase-dependent expression of COXII in yeast mitochondria. Mol Cell Biol. 1994 Jul;14(7):4643–4652. doi: 10.1128/mcb.14.7.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton R. O., Bellus G., McKee E. E., Sevarino K. A., Goehring B. In organello mitochondrial protein and RNA synthesis systems from Saccharomyces cerevisiae. Methods Enzymol. 1996;264:36–42. doi: 10.1016/s0076-6879(96)64006-3. [DOI] [PubMed] [Google Scholar]
- Poyton R. O., Duhl D. M., Clarkson G. H. Protein export from the mitochondrial matrix. Trends Cell Biol. 1992 Dec;2(12):369–375. doi: 10.1016/0962-8924(92)90049-s. [DOI] [PubMed] [Google Scholar]
- Pratje E., Guiard B. One nuclear gene controls the removal of transient pre-sequences from two yeast proteins: one encoded by the nuclear the other by the mitochondrial genome. EMBO J. 1986 Jun;5(6):1313–1317. doi: 10.1002/j.1460-2075.1986.tb04361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratje E., Mannhaupt G., Michaelis G., Beyreuther K. A nuclear mutation prevents processing of a mitochondrially encoded membrane protein in Saccharomyces cerevisiae. EMBO J. 1983;2(7):1049–1054. doi: 10.1002/j.1460-2075.1983.tb01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport T. A., Jungnickel B., Kutay U. Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu Rev Biochem. 1996;65:271–303. doi: 10.1146/annurev.bi.65.070196.001415. [DOI] [PubMed] [Google Scholar]
- San Millan J. L., Boyd D., Dalbey R., Wickner W., Beckwith J. Use of phoA fusions to study the topology of the Escherichia coli inner membrane protein leader peptidase. J Bacteriol. 1989 Oct;171(10):5536–5541. doi: 10.1128/jb.171.10.5536-5541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G., Dobberstein B. Common principles of protein translocation across membranes. Science. 1996 Mar 15;271(5255):1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- Schatz G. The protein import system of mitochondria. J Biol Chem. 1996 Dec 13;271(50):31763–31766. doi: 10.1074/jbc.271.50.31763. [DOI] [PubMed] [Google Scholar]
- Schneider A., Behrens M., Scherer P., Pratje E., Michaelis G., Schatz G. Inner membrane protease I, an enzyme mediating intramitochondrial protein sorting in yeast. EMBO J. 1991 Feb;10(2):247–254. doi: 10.1002/j.1460-2075.1991.tb07944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevarino K. A., Poyton R. O. Mitochondrial membrane biogenesis: identification of a precursor to yeast cytochrome c oxidase subunit II, an integral polypeptide. Proc Natl Acad Sci U S A. 1980 Jan;77(1):142–146. doi: 10.1073/pnas.77.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Steele D. F., Butler C. A., Fox T. D. Expression of a recoded nuclear gene inserted into yeast mitochondrial DNA is limited by mRNA-specific translational activation. Proc Natl Acad Sci U S A. 1996 May 28;93(11):5253–5257. doi: 10.1073/pnas.93.11.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens G. J., Buse G. Studies on cytochrome c oxidase, IV[1--3]. Primary structure and function of subunit II. Hoppe Seylers Z Physiol Chem. 1979 Apr;360(4):613–619. [PubMed] [Google Scholar]
- Torello A. T., Overholtzer M. H., Cameron V. L., Bonnefoy N., Fox T. D. Deletion of the leader peptide of the mitochondrially encoded precursor of Saccharomyces cerevisiae cytochrome c oxidase subunit II. Genetics. 1997 Apr;145(4):903–910. doi: 10.1093/genetics/145.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler B., Boyd D., Beckwith J. The topological analysis of integral cytoplasmic membrane proteins. J Membr Biol. 1993 Feb;132(1):1–11. doi: 10.1007/BF00233047. [DOI] [PubMed] [Google Scholar]
- Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996 May 24;272(5265):1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Myers A. M. Genetics of mitochondrial biogenesis. Annu Rev Biochem. 1986;55:249–285. doi: 10.1146/annurev.bi.55.070186.001341. [DOI] [PubMed] [Google Scholar]
- Whitley P., Zander T., Ehrmann M., Haardt M., Bremer E., von Heijne G. Sec-independent translocation of a 100-residue periplasmic N-terminal tail in the E. coli inner membrane protein proW. EMBO J. 1994 Oct 3;13(19):4653–4661. doi: 10.1002/j.1460-2075.1994.tb06788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Leonard M. R. Escherichia coli preprotein translocase. J Biol Chem. 1996 Nov 22;271(47):29514–29516. doi: 10.1074/jbc.271.47.29514. [DOI] [PubMed] [Google Scholar]
- Yaffe M. P. Analysis of mitochondrial function and assembly. Methods Enzymol. 1991;194:627–643. doi: 10.1016/0076-6879(91)94046-f. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]