Abstract
Recently we showed Lupeol, a triterpene, found in fruits and vegetables inhibits the growth of tumors originated from human androgen-sensitive prostate cancer (CaP) cells and decreases the serum-PSA levels in a mouse model. Here, we provide evidence that Lupeol inhibits the growth of androgen-sensitive as well as androgen-insensitive CaP cells by inducing G2/M cell cycle arrest without exhibiting any toxicity to normal human prostate epithelial cells (PrEC) at the doses at which it kills cancer cells. We observed that Lupeol treatment to LNCaP and DU145 cells resulted in a dose dependent (i) decrease in the protein levels of Cyclins-A, -B1, -D1, -D2, -E2, cyclin-dependent kinase (cdk)-2 and (ii) increase in the protein level of CDK-inhibitor p21. Since G2/M cell cycle phase is regulated by microtubule assembly, we investigated effect of Lupeol on microtubule assembly, its regulation and down-stream targets in CaP cells. Lupeol treatment significantly modulated the level of (i) microtubule components α-tubulin and β-tubulin, (ii) microtubule-regulatory protein stathmin, and (iii) microtubule-regulatory downstream target/pro-survival protein survivin. Lupeol treatment also decreased the level of anti-apoptotic protein cFLIP. Finally, Lupeol was observed to significantly decrease the transcriptional activation of Survivin and cFLIP genes in CaP cells. We conclude that the Lupeol-induced growth inhibition of CaP cells is a net outcome of simultaneous effects on Stathmin, cFLIP, and Survivin which results in the disruption of microtubule assembly. We suggest that Lupeol alone or as an adjuvant to other microtubule agents could be developed as a potential agent for the treatment of human CaP.
Keywords: Lupeol, G2/M arrest, cFLIP, microtubule, stathmin, survivin, prostate cancer
Introduction
Prostate cancer (CaP) is the most common invasive malignancy in males in the Western World. In recent years there is an intense activity to identify novel therapeutic modalities and preventive approaches for CaP. Epidemiological and laboratory studies suggest that diet-based naturally occurring agents, due to their ability to target multiple signaling pathways, their cost-effectiveness, and most importantly their human acceptability could be ideal candidates for the treatment and prevention of human CaP [1]. There is a growing interest in natural triterpenoids, also known as phytosterols, due to their wide spectrum of biological activities [2]. Triterpenes are natural components of human diets and are largely derived from cereals, fruits, vegetables and plant-derived oils [2]. One such triterpene which has gained wide attention of medical professionals, pharmaceutical marketers and researchers all around the world is Lupeol [Lup-20[29]-en-3β-ol]. Lupeol is found in vegetables such as white cabbage, pepper, cucumber, tomato, in fruits such as olive, fig, mango, strawberry, red grapes and in medicinal plants such as American ginseng and Shea butter plant [3–4]. Lupeol has been shown to inhibit the growth of human pancreatic tumors, melanoma and head and neck cancer in xenograft mouse models [5–8]. We recently showed that Lupeol activates apoptotic machinery and inhibits the tumorigenicity of human androgen-sensitive CaP cells with a concomitant decrease in serum PSA levels under in vivo conditions [9]. In the current study, we provide evidence that Lupeol inhibits the growth of both androgen-sensitive and insensitive CaP cells while sparing normal prostate epithelial cells. We show that Lupeol induces G2/M cell cycle arrest, modulates microtubule assembly and targets microtubule regulatory molecules stathmin, survivin and cFLIP.
Materials and methods
Cell culture
Normal prostate epithelial cells (PrEC) and PrEC medium were obtained from Cambrex Bioscience (Walkersville, MD). Human CaP cells LNCaP, CWR22Rν1, PC-3 and DU145 were obtained from ATCC (Manassas, VA). Cells were cultured in RPMI-1640 medium supplemented with 10% Fetal bovine serum supplemented with 1% Penicillin-Streptomycin (Cellgro Mediatech Inc., Herndon, VA).
Treatment of Cells
For dose dependent studies, the cells (50% confluent) were treated with Lupeol (5–50 μM) for 48 h in complete cell medium. After 48h of treatment with Lupeol, the cells were harvested and cell lysates were prepared and stored at −80°C for later use.
Cell viability assay
The effect Lupeol on the viability of cells was determined by MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazoliumbromide) assay. The cells were plated at 1 × 104 cells per well in 200 μl of complete culture medium. The next day cells were treated with Lupeol (5–50 μM) for 48 hr. Each concentration was repeated in 10 wells. After incubation for specified time at 37 °C in a humidified incubator, MTT [5 mg/ml in phosphate buffered saline] was added to each well and incubated for 2 h after which the plate was centrifuged at 500 g for 5 min at 4 °C. The MTT solution was removed from the wells by aspiration. After careful removal of the medium, 0.1 ml of buffered DMSO was added to each well and plates were shaken. The absorbance was recorded on a microplate reader at the wavelength of 540 nm. The effect on cell growth inhibition was assessed as percent cell viability where vehicle-treated cells were taken as 100% viable.
DNA cell cycle analysis
The cells [60% confluent] were starved for 12 h to arrest them in G0 phase of the cell cycle, after which they were treated with Lupeol (5–50 μM) in RPMI-1640 complete media for 48 h. The cells were trypsinized thereafter, washed twice with cold PBS, and centrifuged. The pellet was resuspended in 50 μl cold PBS and 450 μl cold methanol for 1 h at 4°C. The cells were centrifuged at 110 g for 5 min, pellet washed twice with cold PBS, suspended in 500 μl PBS, and incubated with 5 μl RNAse (20 μg/ml final concentration) at 37°C for 30 min. The cells were chilled over ice for 10 min and stained with propidium iodide (50 μg/ml final concentration) for 1 h and analyzed by flow cytometry.
Western Blot Analysis
Cell and tissue lysates were prepared in cold lysis buffer ([0.05 mmol/L Tris-HCl, 0.15 mmol/L NaCl, 1 mole/L EGTA, 1 mol/L EDTA, 20 mmol/L NaF, 100 mmol/L Na3VO4, 0.5% NP-40, 1% Triton X-100, 1 mol/L phenyl methylsulfonyl flouride [pH 7.4]) with freshly added Protease Inhibitor Cocktail Set III (Calbiochem, La Jolla, CA). The lysates were collected, cleared by centrifugation, supernatant aliquoted and stored at −80°C. The protein content in the lysates was measured by BCA protein assay (Pierce, Rockford, IL) as per the vendor’s protocol. For Western blot analysis, 25–40 μg protein was resolved over 12% Tris-glycine polyacrylamide gels (Novex, Carlsbad, CA) as described earlier [9].
Transcriptional activity of Survivin and cFLIP
The human survivin promoter activity reporter plasmid (pLuc-Survivin) was a kind gift from Dr. KM Wahidur Rahman (Wayne State University School of Medicine, USA). The human cFLIP reporter plasmid (pGL3-Luc-cFLIP) was a kind gift from Dr. Peter Erb (University of Basel, Basel, Switzerland). E. Coli bacteria with plasmids were used for transformation using an agar media and Maxiprep kit [Qiagen, Valencia, CA]. Cells plated at a density of 5 × 104 cells/well were transfected with the plasmids (1 μg/million cells) for 24 h. Renilla luciferase (50 ng/million cells, pRL-TK; Promega, Madison, WI) was used as an internal control. In addition, the same amounts of empty vectors were transfected in controls cells. After 12 h post-transfection, fresh media was added with Lupeol (1–20 μM) and incubated for 24 h. The cells were then harvested and transcriptional activity was measured in terms of luciferase activity in quadruplicates by using dual-luciferase reporter assay system (Promega, Madison, WI). Relative luciferase activity was calculated with the values from vector alone group with or without Lupeol treated group.
Statistical analyses
Student’s t test for independent analysis was applied to evaluate differences between the treated and untreated cells with respect to the expression of various proteins using S-plus Software (Insightful, Seattle, WA). A p-value of <0.05 was considered to be statistically significant.
Results and discussion
Effect of Lupeol on cell viability of PrEC, LNCaP, CWR22Rν1, PC-3 and DU145 cells
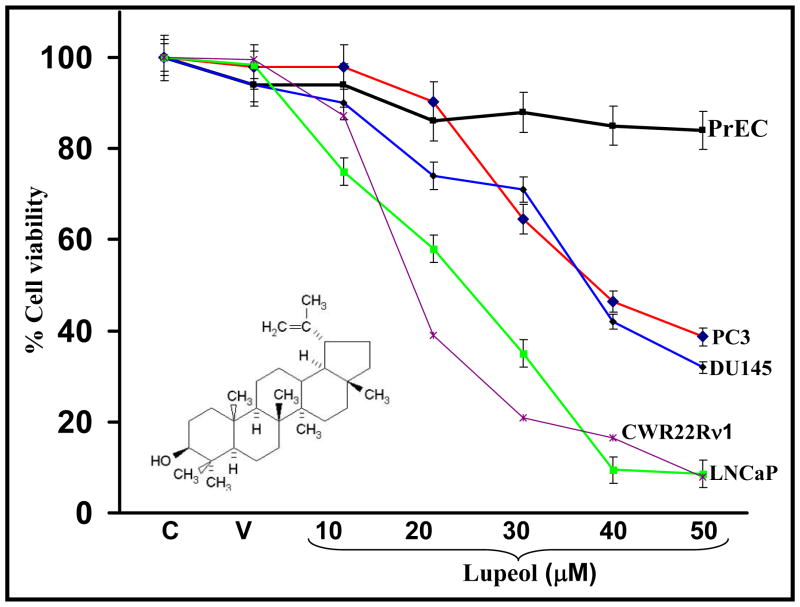
One of the major failures in successfully treating CaP is due to the presence of a mixture of CaP cells [comprising androgen-dependent and androgen-independent cells] as lesions of different androgen-status respond differently to the chemotherapy [10. We investigated the effect of Lupeol (10–50 μM) treatment on the viability of androgen-sensitive (LNCaP, CWR22Rν1), androgen-insensitive (PC-3 and DU145) CaP cells and normal prostate epithelial cell (PrEC). Lupeol treatment caused a dose-dependent decrease in the viability of CaP cells at 48 h post treatment (Fig. 1). However, Lupeol treatment was observed to bear no effect on the viability of PrEC cells at similar doses (Fig. 1). These data is significant because Lupeol caused inhibition in the growth of human CaP cells irrespective of their androgen responsive status and spares normal prostate epithelial cells. In addition, there is an increasing interest and emphasis given by scientists and clinicians on natural diet-based agents which are capable of selective/preferential elimination of cancer cells without affecting normal cells [10].
Figure 1. Effect of Lupeol on viability of normal human prostate epithelial cells [PrEC] androgen-sensitive prostatic cancer cells (LNCaP and CWR22Rν1) and androgen-insensitive prostate cancer cells (PC-3 and DU145).
Cells were treated with specified concentrations of Lupeol for 48 h, and cell viability was determined by MTT assay. Each concentration of Lupeol (10–50 μM) was repeated in ten wells. The values are represented as the percent viable cells where vehicle-treated cells were regarded as 100% viable. Data represents mean value of percent viable cells ± S.E of three independent experiments. C and V represent control and vehicle (alcohol + DMSO) treated cells respectively. The details are described under Materials and Methods. Structure of Lupeol [Lup-20[29]-en-3β-ol], a triterpene found in fruits and vegetables in indicated within the figure.
Effect of Lupeol on cell cycle in CaP cells
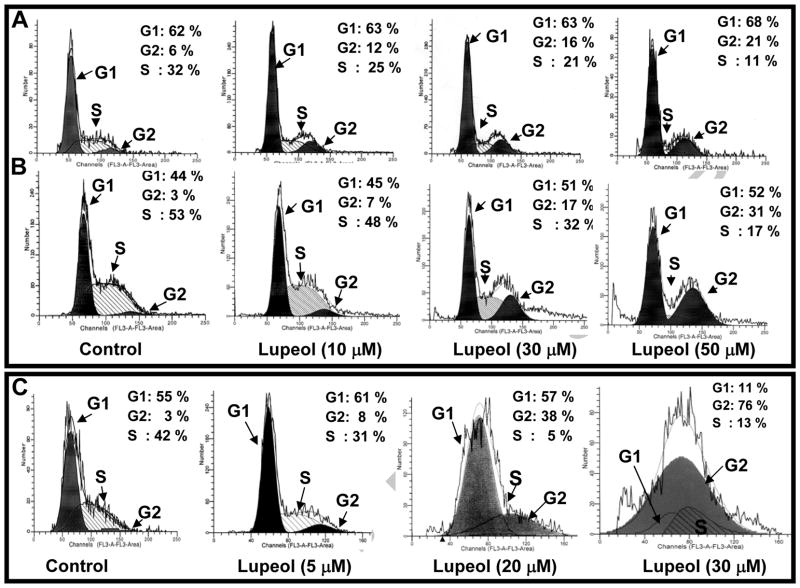
The cell growth and proliferation of mammalian cells are mediated via cell cycle progression [11–12]. In recent years, studies have shown an association between cell cycle regulation and cancer [11–12]. Next, we considered the possibility that inhibition in the growth of CaP cells may involve an arrest of cells at specific check point[s] in the cell cycle followed by apoptosis. We therefore assessed the effect of Lupeol on cell cycle perturbations. As shown in Figure 2A–C, Lupeol treatment resulted in a significant arrest of cells in G2-M phase of cell cycle at 48 h post-treatment. As shown in figure 2A–C, Lupeol treatment significantly increased the number of DU145 (2–3.5 fold), PC-3 (3–10 fold) and LNCaP cells (2–25 fold) in G2 phase with a concomitant decrease in the number of cells in S phase of the cell cycle (Fig. 2A–C). These data suggest that Lupeol treatment significantly induces G2/M cell cycle arrest of both androgen-sensitive and –insensitive CaP cells. Based on cell viability and cell cycle analysis data, we selected LNCaP (5–30 μM) and for DU145 (5–40 μM) cells and a time period of 48 h post-Lupeol treatment for further mechanistic studies.
Figure 2. Effect of Lupeol on DNA cell cycle analysis of (A) DU145, (B) PC-3 cells and (C) LNCaP cells as assessed by flow cytometry.
The cells were synchronized in G0 phase by depleting the serum for 12 h [referred as control]. After 12 h of serum starvation, cells were treated with vehicle or Lupeol [5–50 μM for 48 h] in complete serum containing medium and were analyzed by flow cytometry. The percentages of cells in the G0/G1, S, and G2/M phases were calculated using cell fit computer software. Other details are described under Materials and Methods. The data shown here are from a representative experiment repeated three times with similar results.
Effect of Lupeol on cell cycle regulatory proteins in CaP cells
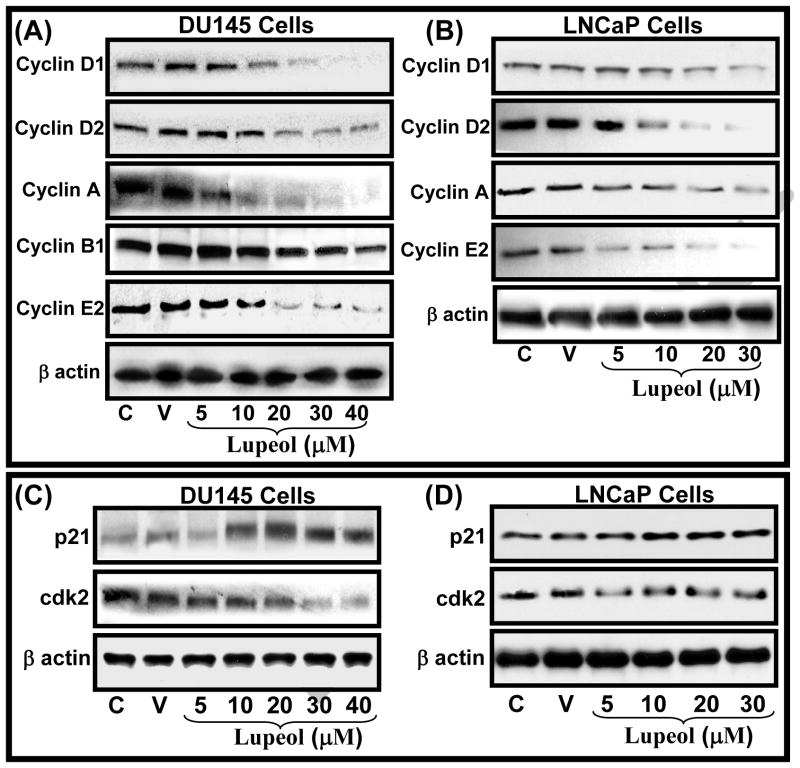
The proliferation of tumor cells is reported to be associated with alterations in cell cycle checkpoints in various cancer types including CaP [11, 13]. The proliferation pathway, which is marked by an increased rate of cells uncontrolled cell divisions in CaP cells, is known to be mediated by activated cyclins such as Cyclin A, D1–D2 and Cyclin E and cyclin-dependent kinases [14]. Therefore repairing the altered cell cycle check points has become an appreciated strategy for the management of cancer. We investigated the effect of Lupeol treatment on the protein levels of the cyclin/cyclin-dependent kinase (cdk) complex which is known to be operative during cell division. Treatment of DU145 cells with Lupeol resulted in a dose-dependent decrease in the protein expression of cyclin D1, cyclin D2, cyclin A, cyclin B1 and cyclin E2 (Fig. 3A). Similarly, Lupeol treatment decreased the protein level of cyclin D1, cyclin D2, cyclin A, cyclin A and cyclin E2, however, no effect was observed on cyclin B1 in LNCaP cells (Fig. 3B).
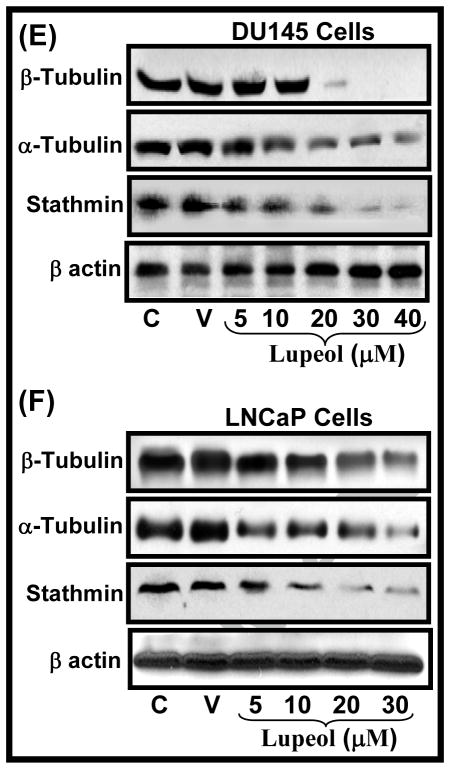
Figure 3. Effect of Lupeol on the protein level of (A–B) cell cycle regulatory molecules, (C) microtubule complex and microtubule regulatory molecules in DU145 and LNCaP cells.
(A–C) Cells were treated with vehicle only or specified concentrations of Lupeol for 48 h and then harvested. The protein expression of (A–B) cyclin D1, cyclin D2, cyclin A, cyclin B1, cyclin E2, cdk2 and WAF1/p21, (C) microtubule proteins (α and β-tubulin), and (C)microtubule regulatory proteins stathmin were determined by Western blot analysis. Equal loading was confirmed by stripping the membrane and reprobing them for β-actin. The immunoblots shown here are representative of three independent experiments with similar results. C stands for untreated and V represents vehicle alone treated cells which is designated as control group.
Next, we determined the effect of Lupeol the protein level of cdk2 in CaP cells. Treatment of DU145 cells with Lupeol resulted in a dose-dependent decrease in cdk2 protein level, (Fig. 3C). Although Lupeol treatment significantly decreased the cdk2 protein levels in LNCaP cells, the effect was not dose dependent (Fig. 3D). Further, we evaluated the effect of Lupeol treatment on the induction of WAF1/p21, which is known to regulate the entry of cells at G1→S transition as well as G2→M checkpoint. Lupeol treatment was observed to result in a significant dose-dependent induction in the expression level of WAF1/p21 protein in both DU145 and LNCaP cells (Fig. 3C–D). Previously, we showed that Lupeol induces apoptosis in CaP cells, however the preceding mechanisms of action of Lupeol were not known [9]. On the basis of these data, we speculate that the net effect of Lupeol treatment [growth inhibition] could be due to the potential of Lupeol to repair the impaired cell cycle regulatory machinery which results in the G2/M cell cycle arrest followed by apoptotic death of CaP cells.
Effect of Lupeol on components of microtubule assembly in CaP cells
G2/M cell cycle phase is reported to be regulated by microtubules in the cells [15]. Microtubules are key components of the cytoskeleton and are composed of heterodimers of α-and β-tubulin proteins [16]. During mitosis, polymerization/depolymerization processes allows the microtubules to grow and assemble into the mitotic spindle [16]. Recent years have shown an emerging trend as anti-microtubule agents being developed for the treatment of cancer [16–18]. Recent studies have shown that G2/M arrest of cells induced by anti-microtubule agents is associated with destabilization of mitotic spindle structure or microtubule assembly [17–18]. Recent studies have showed that cancer cells resistant to anti-microtubule agents contained alterations in the tubulin content such as increased expression of polymerized tubulin and a higher content of the other tubulin isotypes [16 and references therein]. We investigated the effect of Lupeol treatment on two component α and β-tubulin in DU145 and LNCaP cells. Lupeol treatment was observed to decrease the protein level of α and β-tubulin in DU145 and LNCaP cells in a dose-dependent manner (Fig. 3E–F). The effect of Lupeol treatment was much pronounced on β-tubulin than α-tubulin in DU145 cells (Fig. 3E) however, vice versa in LNCaP cells (Fig. 3F).
Effect of Lupeol on levels of microtubule regulatory proteins in CaP cells
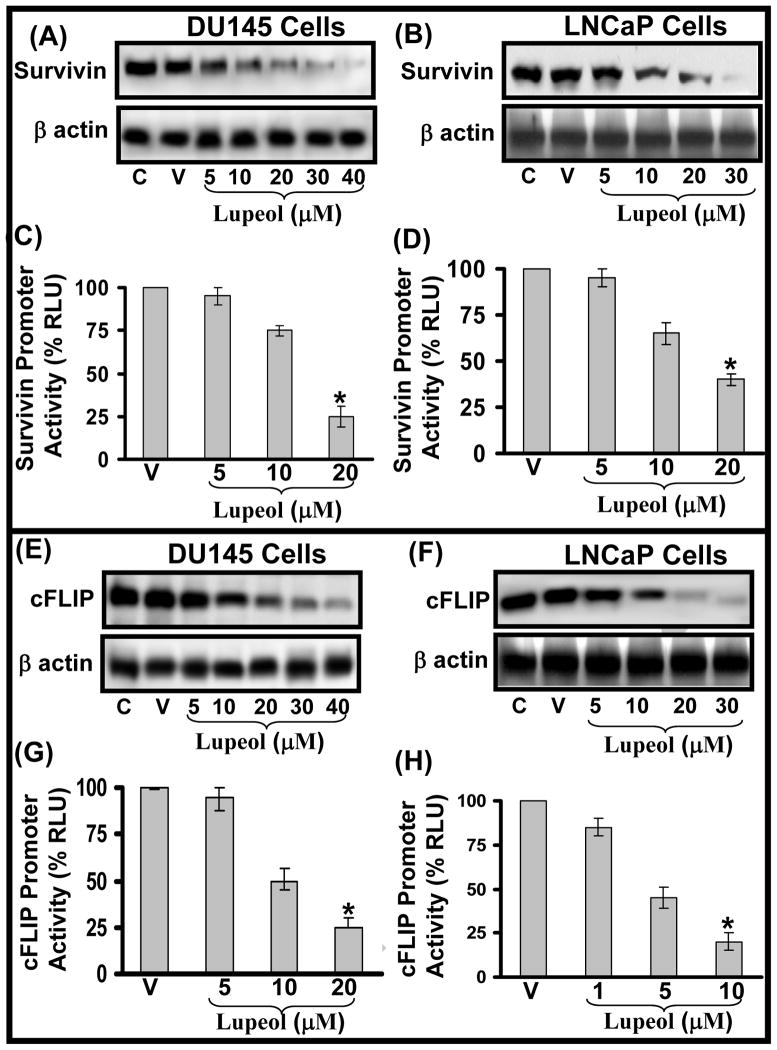
The integrity and function of microtubule in dividing cells is regulated by several proteins including stathmin and survivin [19–22]. Stathmin regulates microtubule dynamics by promoting depolymerization of microtubules and/or preventing polymerization of tubulin heterodimers of microtubules in vitro and in vivo [16, 19–20]. Stathmin is critically important not only for the formation of a normal mitotic spindle upon entry into mitosis but also for the regulation of the function of the mitotic spindle in the later stages of mitosis and for the timely exit from mitosis [16,19–20]. Since stathmin acts as an important regulator of microtubule function, it offers potential as a molecular target for therapeutic agents. We determined the effect of Lupeol treatment on the expression level of stathmin protein in CaP cells. We observed that Lupeol treatment caused a dose-dependent decrease in the protein level of stathmin in DU145 and LNCaP cells (Fig. 3E–F). Survivin has been reported form a component of the chromosome passenger complex, which plays a role in chromosome segregation and cytokinesis in cell division [21–22]. Studies have shown that an increase in the survivin expression is observed in the G2/M phase of cell cycle [21–22]. Survivin has been shown to be required for the maintenance of the spindle assembly checkpoint to allow proper microtubule alignment to ensure cell propagation [21–22]. The expression of survivin is reported to increase during the G2/M phase of the cell cycle [21–22 and references therein]. Survivin is reported to be overexpressed in most human cancers including CaP [21–22 and references therein]. Since survivin has been reported be involved with the regulation of the microtubule dynamics and is increased in CaP cells, we evaluated the effect of Lupeol treatment on survivin protein level in DU145 and LNCaP cells. Lupeol treatment was observed to decrease the level of survivin protein in DU145 and LNCaP cells in a dose-dependent manner (Fig. 4A–B). We next evaluated the effect of Lupeol treatment on the transcriptional activation of survivin gene by employing luciferase reporter assay. Cells transfected with pLuc-survivin were treated with sub-lethal doses (5–20 μM for DU145 and 1–10 μM for LNCaP) of Lupeol and the promoter activity was determined at 24 h post Lupeol treatment by employing luciferase reporter assay. Lupeol treatment significantly decreased the transcriptional activation of survivin in both DU145 and LNCaP cells (Fig. 4C–D). Taken together, our data show that Lupeol effectively modulates microtubule function by targeting it at several levels i.e. by modulating assembly component (α/β tubulin) levels and by targeting microtubule regulatory molecules (stathmin and survivin) in CaP cells.
Figure 4. Effect of Lupeol on the protein levels and transcriptional activation of Survivin and cFLIP in DU145 and LNCaP cells.
(A–B) LNCaP and DU145 cells were treated with vehicle only or specified concentrations of Lupeol for 48 h and then harvested. The protein expression of survivin was determined by Western blot analysis. Equal loading was confirmed by stripping the membrane and reprobing them for β-actin. The immunoblots shown here are representative of three independent experiments with similar results. C stands for untreated and V represents vehicle alone treated cells which is designated as control group. (C–D). Histograms represent the effect of Lupeol treatment on the transcriptional activation of survivin in DU145 and LNCaP cells respectively. Cells were transfected with the pLuc-survivin for 24 h. Renilla luciferase was used as an internal control. In addition controls were transfected with the same amount of empty vectors. Twelve hours post-transfection, fresh media was added with Lupeol and incubated for 24 h. The cells were then harvested and transcriptional activity was measured in quadruplicates as described under Materials and Methods. (E–F) LNCaP and DU145 cells were treated with vehicle only or specified concentrations of Lupeol for 48 h and then harvested. The protein expression of survivin was determined by Western blot analysis. Equal loading was confirmed by stripping the membrane and reprobing them for β-actin. The immunoblots shown here are representative of three independent experiments with similar results. C stands for untreated and V represents vehicle alone treated cells which is designated as control group. (D–H). Histograms represent the effect of Lupeol treatment on the transcriptional activation of survivin in DU145 and LNCaP cells respectively. Cells were transfected with the pGL3-Luc-cFLIP for 24 h. Renilla luciferase was used as an internal control. In addition controls were transfected with the same amount of empty vectors. Twelve hours post-transfection, fresh media was added with Lupeol and incubated for 24 h. The cells were then harvested and transcriptional activity was measured in quadruplicates as described under Materials and Methods.
Effect of Lupeol on the protein levels and transcriptional activation of apoptosis inhibitor cFLIP in CaP cells
The characteristics of chemoresistance and survival acquired by cancer cells including CaP cells have been associated with the impairment of apoptotic machinery such as inactivation of caspases by survivin and cFLIP in cancerous cells [16, 23]. cFLIP is known to impair apoptotic signaling by competing with caspase-8 for death receptor thus slowing down the apoptosis, increasing the tumor cell survival and inhibiting cells to respond to chemotherapeutic stimuli [23–25 and references therein]. cFLIP is reported to interrupt apoptotic signaling by disrupting the interaction between FADD and caspase-8 in cancer cells [23–25 and references therein]. Elevated expression of cFLIP has been found in various types of tumor cells which are often resistant to death-receptor-mediated apoptosis and numerous reports have shown that down-regulation of cFLIP results in sensitizing a various types of resistant tumor cells including CaP [23–25 and references therein]. Taken together, these studies imply that cFLIP may be an attractive therapeutic target for the treatment of chemoresistant cancers [23–25 and references therein]. We next evaluated the effect of Lupeol treatment on cFLIP protein levels in DU145 and LNCaP cells. Lupeol treatment was observed to significantly cause a decrease in the expression level of cFLIP protein in both DU145 and LNCaP cells (Fig. 4E–F). Lupeol treatment [at sub-lethal doses] was also observed to significantly inhibit the transcriptional activation of CaP cells (Fig. 4G–H). Previously, we showed that Lupeol induces the Fas/FADD/Caspase-8 machinery in CaP cells and sensitize CaP cells for anti-Fas-antibody therapy [9]. However the query as to how Lupeol activates Caspase-8 (which mostly remains inactive during CaP development) remained unresolved. Based on these observations, we suggest the possibility of the activation of Fas/FADD/Caspase-8 machinery in CaP cells might be facilitated by the inactivation of cFLIP by Lupeol treatment in CaP cells.
Conclusion
This is the first report providing evidence that Lupeol is a novel microtubule targeting agent. In addition, findings of this study demonstrate the anticancer efficacy of Lupeol, with mechanistic rationale, against androgen-sensitive as well as androgen-insensitive human CaP cells. We suggest that Lupeol alone or as an adjuvant to other microtubule agents could be developed as a potential agent for the treatment of human CaP. These observations warrant further in vivo efficacy studies in models that mimic progressive forms of human prostatic disease. The positive outcomes of such an in vivo study could form a strong basis for the development of Lupeol as a novel agent for human CaP prevention and/or intervention.
Acknowledgments
This work was supported by United States PHS grant R03 CA130064 to Mohammad Saleem, Bhat. Author (Imtiyaz Murtaza) was recipient of Overseas Associateship Award (BT/IN/BTON/Nich/09/2007) from Department of Biotechnology, Ministry of Science and Technology, Government of India. We thank Dr. KM Wahidur Rahman (Department of Pathology, Wayne State University School of Medicine, USA) for providing pLuc-Survivin plasmid. We thank Dr. Peter Erb (University of Basel, Basel, Switzerland) for providing pGL3-Luc-cFLIP construct.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;16:2193–2203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 2.Bradford PG, Awad AB. Phytosterols as anticancer compounds. Mol Nutr Food Res. 2007;51:161–170. doi: 10.1002/mnfr.200600164. [DOI] [PubMed] [Google Scholar]
- 3.Beveridge TH, Li TS, Drover JC. Phytosterol content in American ginseng seed oil. J Agric Food Chem. 2002;50:744–750. doi: 10.1021/jf010701v. [DOI] [PubMed] [Google Scholar]
- 4.Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.04.033. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saleem M, Alam A, Arifin S, Shah MS, Ahmed B, Sultana S. Lupeol, a triterpene, inhibits early responses of tumor promotion induced by benzoyl peroxide in murine skin. Pharmacol Res. 2001;43:127–134. doi: 10.1006/phrs.2000.0710. [DOI] [PubMed] [Google Scholar]
- 6.Murtaza I, Saleem M, Adhami VM, Hafeez BB, Mukhtar H. Suppression of cFLIP by lupeol, a dietary triterpene, is sufficient to overcome resistance to TRAIL-mediated apoptosis in chemoresistant human pancreatic cancer cells. Cancer Res. 2009;69:1156–1165. doi: 10.1158/0008-5472.CAN-08-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saleem M, Maddodi N, Abu Zaid M, Khan N, Hafeez BB, Asim M, Suh Y, Yun JM, Setaluri V, Mukhtar H. Lupeol inhibits growth of highly aggressive human metastatic melanoma cells in vitro and in vivo by inducing apoptosis. Clin Cancer Res. 2008;14:2119–2127. doi: 10.1158/1078-0432.CCR-07-4413. [DOI] [PubMed] [Google Scholar]
- 8.Lee TK, Poon RT, Wo JY, Ma S, Guan XY, Myers JN, Altevogt P, Yuen AP. Lupeol suppresses cisplatin-induced nuclear factor-kappa B activation in head and neck squamous cell carcinoma and inhibits local invasion and nodal metastasis in an orthotopic nude mouse model. Cancer Res. 2007;67:8800–8809. doi: 10.1158/0008-5472.CAN-07-0801. [DOI] [PubMed] [Google Scholar]
- 9.Saleem M, Kweon MH, Yun JM, Adhami VM, Khan N, Syed DN, Mukhtar H. A novel dietary triterpene Lupeol induces fas-mediated apoptotic death of androgen-sensitive prostate cancer cells and inhibits tumor growth in a xenograft model. Cancer Res. 2005;65:11203–11213. doi: 10.1158/0008-5472.CAN-05-1965. [DOI] [PubMed] [Google Scholar]
- 10.Revelos K, Petraki C, Scorilas A, Stefanakis S, Malovrouvas D, Alevizopoulos N, Kanellis G, Halapas A, Koutsilieris M. Correlation of androgen receptor status, neuroendocrine differentiation and angiogenesis with time-to-biochemical failure after radical prostatectomy in clinically localized prostate cancer. Anticancer Res. 2007;27:651–660. [PubMed] [Google Scholar]
- 11.Scholey JM, Brust-Mascher I, Mogilner A. Cell division. Nature. 2003;422:746–752. doi: 10.1038/nature01599. [DOI] [PubMed] [Google Scholar]
- 12.DiPaola RS. To arrest or not to G [2]-M Cell-cycle arrest. Clin Cancer Res. 2002;8:3512–3519. [PubMed] [Google Scholar]
- 13.Buolamwini JK. Cell cycle molecular targets in novel anticancer drug discovery. Curr Pharm Des. 2000;6:379–392. doi: 10.2174/1381612003400948. [DOI] [PubMed] [Google Scholar]
- 14.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, Fang K, Fang G. Mechanism, function and regulation of microtubule-dependent microtubule amplification in mitosis. Mol Cells. 2009;27:1–3. doi: 10.1007/s10059-009-0014-2. [DOI] [PubMed] [Google Scholar]
- 16.Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–9086. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- 17.Islam MN, Iskander MN. Microtubulin binding sites as target for developing anticancer agents. Mini Rev Med Chem. 2004;4:1077–1104. doi: 10.2174/1389557043402946. [DOI] [PubMed] [Google Scholar]
- 18.Cabral F. Factors determining cellular mechanisms of resistance to antimitotic drugs. Drug Resist Updat. 2001;4:3–8. doi: 10.1054/drup.2000.0172. [DOI] [PubMed] [Google Scholar]
- 19.Gavet O, Ozon S, Manceau V, Lawler S, Curmi P, Sobel A. The stathmin phosphoprotein family: intracellular localization and effects on the microtubule network. J Cell Sci. 1998;111:3333–3346. doi: 10.1242/jcs.111.22.3333. [DOI] [PubMed] [Google Scholar]
- 20.Rana S, Maples PB, Senzer N, Nemunaitis J. Stathmin 1: a novel therapeutic target for anticancer activity. Expert Rev Anticancer Ther. 2008;8:1461–1470. doi: 10.1586/14737140.8.9.1461. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto H, Ngan CY, Monden M. Cancer cells survive with survivin. Cancer Sci. 2008;99:1709–1714. doi: 10.1111/j.1349-7006.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;4:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 23.Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, Los M. Apoptosis and cancer: mutations within Caspase genes. J Med Genet. 2009;46:497–510. doi: 10.1136/jmg.2009.066944. [DOI] [PubMed] [Google Scholar]
- 24.Kataoka T. The caspase-8 modulator c-FLIP. Crit Rev Immunol. 2005;25:31–58. doi: 10.1615/critrevimmunol.v25.i1.30. [DOI] [PubMed] [Google Scholar]
- 25.Wajant H. Targeting the FLICE Inhibitory Protein (FLIP) in cancer therapy. Mol Interv. 2003;3:124–127. doi: 10.1124/mi.3.3.124. [DOI] [PubMed] [Google Scholar]