Abstract
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor which requires heterodimerization with the Ah receptor nuclear translocator (Arnt) for function. Arnt is also a dimerization partner of the hypoxia inducible factor-1 alpha (HIF-1α) for the hypoxia signaling. Additionally, Arnt is found to be a potent coactivator of the estrogen receptor (ER) signaling. Thus we examined whether the presence of an increased amount of AhR may suppress both the HIF-1α and ER signaling pathways by sequestering Arnt. We tested our hypothesis using a human AhR construct CΔ553 which is capable of heterodimerizing with Arnt in the absence of a ligand. Transient transfection studies using a corresponding luciferase reporter plasmid in MCF-7 cells showed that CΔ553 effectively suppressed the AhR, HIF-1α, and ER signaling pathways. Reverse transcription/real-time QPCR data showed that CΔ553 blocked the up-regulation of the target genes controlled by AhR (CYP1A1), HIF-1α (VEGF, aldolase C, and LDH-A), and ER (GREB1, pS2, and c-myc) in MCF-7 cells. Since both HIF-1α and ER are highly active in the ERpositive breast cancer, CΔ553 has the potential to be developed as a protein drug to treat breast cancer by blocking these two signaling pathways.
Keywords: estrogen receptor, Ah receptor, Arnt, HIF-1α, GREB1, protein drugs
Introduction
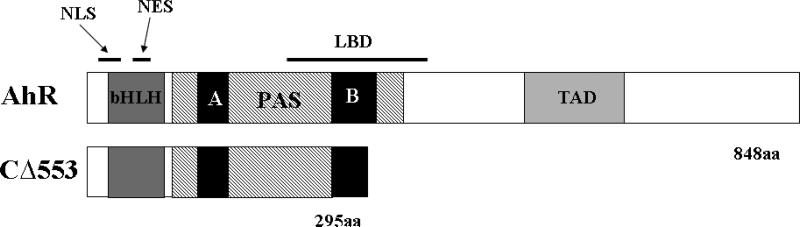
AhR is a transcription factor that is associated with a number of proteins (namely Hsp90, p23, and XAP2) in the cytoplasm. Upon binding of a ligand PAH or HAH, the AhR complex translocates into the nucleus via a mechanism involving p23 and importin. While in the nucleus, AhR heterodimerizes with Arnt and the heterodimer binds to the DRE. This binding triggers the recruitment of coactivators to the promoter and in turn the transcription of target genes such as CYP1A1 is activated.1 CΔ553 is a truncated form of AhR which has previously been shown to associate with Arnt and forms the CΔ553/Arnt/DRE complex in a gel shift assay.2This AhR construct lacks the C-terminal 553 amino acids which harbor the complete TAD and a significant portion of the LBD (Figure 1); the region of LBD is where Hsp90 interacts with AhR and ligand responsiveness is conferred. Without the TAD, CΔ553 cannot recruit coactivators to the promoter so that no activation of gene transcription may occur after the binding of the CΔ553-Arnt heterodimer to the DRE. Lacking a significant portion of the LBD, CΔ553 is capable of forming the CΔ553-Arnt heterodimer in the absence of an AhR ligand.
Figure 1.
The domain structure of human AhR and CΔ553. bHLH, basic-helixloop-helix domains required for DNA binding; PAS, the Per/Arnt/Sim domains for dimeriztion; TAD, transactivation domain necessary for transcription activation; LBD, ligand binding domain. Full-length AhR consisting of 848 amino acids shown to compare with the truncated AhR CΔ553, which lacks part of PAS B, LBD, and TAD.
Solid tumors such as breast cancers have found ways to manipulate their environment to allow for continuous growth. One such way is via the HIF-1α signaling pathway. Similar to AhR, HIF-1α is a dimerization partner of Arnt (also known as HIF-1β). During normoxia, HIF-1α is constantly degraded via the proteasome pathway which requires the oxygen-dependent hydroxylation of the prolyl residues of HIF-1α.3 When the turnover of the HIF-1α protein is inhibited under hypoxia, HIF-1α translocates into the nucleus and dimerizes with Arnt; the heterodimer in turn binds to the HRE to up-regulate gene expression. Activation of HIF-1α in tumors has been found to enhance angiogenesis4-6 and increase glycolytic flux.7-10 Since the growth of many breast cancers is stimulated by estrogen, reduction of the estrogen binding to ER using tamoxifen and letrozole has been the mainstream treatment for the ER-positive breast cancer after mastectomy.11,12
There has been immense interest in developing anticancer drugs that block the HIF-1α signaling; these drugs should be effective in treating cancer such as the ER-positive breast cancer that overexpresses HIF-1α.13 Therefore, many researchers have examined different ways to suppress the HIF-1α pathway, which involve the turnover of HIF-1α,14-17 synthesis of the HIF-1α protein18,19, and binding of the HIF-1α-Arnt heterodimer to the HRE.20 Realizing that the solid tumor core is hypoxic, one would expect that a drug that is only active in the low oxygen environment should be tumor-selective. Tirapazamine is such a drug that is chemically reduced to a DNA cross-linking radical under hypoxic condition.21 This is certainly a promising approach for rational drug design; unfortunately, tirapazamine has not been shown to be effective in a current Phase II trial.22
It has been reported that a cross-talk between AhR and ER exists.23 There have been a number of underlying mechanisms reported and one of them may involve the direct interaction between AhR and ER.23-25 In addition, the liganded AhR-Arnt heterodimer has been shown to bind to the DRE that is located within the promoter region of the E2 responsive target genes such as pS2 and cathepsin D. 26 This binding may act as a physical barrier to inhibit the ER transactivation. 27, 28 Additionally, it has been reported that Arnt is a potent coactivator of the ER signaling in cell culture.29
We postulated that by dimerizing with Arnt, CΔ553 may sequester Arnt so that it is not available to form the HIF-1α-Arnt heterodimer and be a coactivator in the ER signaling. Hence, angiogenesis and the ER signaling may be inhibited and the growth of solid tumors such as the ER-positive breast cancer may be suppressed. Here, we have provided evidence to support our hypothesis that CΔ553 inhibits both the hypoxia and ER signaling pathways. We propose that CΔ553 can be developed as a protein drug for cancer treatment via the existing cross-talks among the signaling pathways of AhR, HIF-1α, and ER.
Experimental Section
Materials
All oligonucleotides (Table I) were ordered from Invitrogen. The plasmid pCMV-CΔ553 was generated by PCR using the primer set OL77 (5′-CGGGATCCATGAACAGCAGCAGCGCC-3′) and OL19 (5′-CCAAGCTTGAAGTCTAGTTTGTGTTTGGTTC-3′) to amplify the N-terminal 885 bp of the full length human AhR cDNA and then cloned it into the Bam HI and Hind III sites of pCMV-Tag4A (Stratagene, La Jolla, CA). The HRE-driven luciferase reporter plasmid pGL3-Epo was generated by using PCR to amplify the hypoxia enhancer region (195 bp) from the 3′ region of the EPO gene using the primer set OL120 (5′-CGGGTACCCTGGGCCCTACGTGCTGTCTC-3′) and OL121 (5′-CGGCTAGCCTCTGGCCTCCCTCTCCTTGATGA-3′) with MCF-7 genomic DNA as the template, followed by cloning into the Kpn I and Nhe I sites of the pGL3 plasmid (Promega, Madison, WI).
Table I.
RT-QPCR primers. Primer sets with oligo names and sequences used for the analysis of various transcripts (aldolase C, c-myc, CYP1A1, GREB1, LDH-A, pS2, VEGF, and the standard 18S) are given with forward (F) and reverse (R) orientations noted. Primer melting temperatures used during PCR analysis are shown along with the fragment sizes generated.
| Aldolase C | OL88: 5′-ATAATGGTGTTCCCTTCGTCCGA-3′ (F) | ||
| (218 bp) | OL89: 5′-TGCAGAGGGTGTACGCTCACTG-3′ (R) | ||
| c-myc | OL230: 5′-GCCCCTCAACGTTAGCTTCA-3′ (F) | ||
| (150 bp) | OL231: 5′-TTCCAGATATCCTCGCTGGG-3′ (R) | ||
| CYP1A1 | OL90: 5′-GGCCACATCCGGGACATCACAGA-3′ (F) | ||
| (336 bp) | OL91: 5′-TGGGGATGGTGAAGGGGACGAA-3′ (R) | ||
| GREB-1 | OL213: 5′-CAAAGAATAACCTGTTGGCCCTGC -3′ (F) | ||
| (172 bp) | OL214: 5′-GACATGCCTGCGCTCTCATACTTA-3′ (R) | ||
| LDH-A | OL86: 5′-GCCCGACGTGCATTCCCGATTCCTT-3′ (F) | ||
| (361 bp) | OL87: 5′-GACGGCTTTCTCCCTCTTGCTGACG-3′ (R) | ||
| pS2 | OL196: 5′-GCCCAGACAGAGACGTGTACA-3′ (F) | ||
| (172 bp) | OL197: 5′-TCACACTCCTCTTCTGGAGGG-3′ (R) | ||
| VEGF | OL118: 5′-GGGGGCTGCTGCAATGACG-3′ (F) | ||
| (441 bp) | OL119: 5′-CGCCTCGGCTTGTCACATCTG-3′ (R) | ||
| 18S | OL96: 5′-CGCCCCCTCGATGCTCTTAG-3′ (F) | ||
| (377 bp) | OL97: 5′-CGGCGGGTCATGGGAATAAC-3′ (R) | ||
Luciferase Assay
MCF-7 cells were grown at 37°C and 5% CO2 in 24-well plates containing Advanced DMEM/F-12 (Gibco, Carlsbad, CA) supplemented with 5% FBS (HyClone, Logan, UT), 2 mM L-glutamate, 100 U/ml of penicillin and 0.1 mg/ml of streptomycin to about 90% confluence prior to transfection. In each well, cells were transfected with 200 μl of Opti-MEM (Gibco, Carlsbad, CA), 1.5 μl of Lipofectamine 2000 (Gibco, Carlsbad, CA) and 0.8 μg or 1.1 μg of the total DNA containing pCMV-CΔ553 or pCMV empty vector, reporter plasmid (GudLuc1.1, pGL3-Epo, or pERE-Luc) and the β-galactosidase plasmid pCH110 (Amersham Pharmacia, Piscataway, NJ). Cells were incubated in this transfection mix for 5 hours at 37°C. Afterwards, the transfection mix was exchanged with fresh complete media before treatment with 3-MC, CoCl2 or E2. Treatment with 3-MC (1 μM) or DMSO occurred 18 hours post-transfection for 6 hours whereas treatment with CoCl2 (100 μM) or water occurred 6 hours post-transfection for 18 hours. For experiments examining the ER signaling, immediately after transfection cells were washed twice with HBSS (Gibco, Carlsbad, CA) preheated to 37°C and replaced with phenol red-free MEM (Gibco, Carlsbad, CA) + 10% charcoal treated FBS (Gemini, West Sacramento, CA) that contained E2 (10 nM) or ethanol/DMSO (4:1) for 18 hours at 37°C. After ligand treatment, cells were washed twice with ice cold PBS and 200 μl of the lysis buffer (100mM potassium phosphate at pH 7.8 containing 1% Trition X-100 and 0.5 mM DTT) was added to each well. Plates were incubated at room temperature for 10 minutes on an orbit shaker at 150 rpm. The resulting cell lysates were centrifuged at 16,000g for 2 minutes at 4°C to obtain the supernatant. The Dual-Light kit (Applied Biosystems, Foster City, CA) was used to perform the luciferase assay. Briefly, 10 μl of the supernatant was added to 25 μl of Dual Light Buffer A prewarmed to room temperature, followed by incubation for 20 seconds. 100 μl of Dual Light Buffer B containing 1 μl of Galacton Plus was then added and immediately analyzed on a Turner Design TD-20/20 luminometer for a four-second duration. Tubes were then removed and incubated at room temperature. After exactly one hour, 100 μl of Accelerator II prewarmed to room temperature was added to each tube and immediately analyzed on the luminometer for a four-second duration to generate β-galactosidase readings. Luciferase activities were normalized by the internal β-galactosidase activities.
Reverse transcription/real-time QPCR
MCF-7 cells were grown as mentioned above but in 6-well plates. At about 90% confluence, the cells in each well were transfected with 750 μl of Opti-MEM containing 7.5 μl of Lipofectamine 2000 and 4 μg of pCMV-CΔ553 or pCMV empty vector. Transfection protocol and conditions for induction were essentially the same as mentioned above. After induction, cells were washed with ice cold PBS twice and then dislodged into 1.5 ml of PBS, followed by RNA extraction using the MasterPure RNA Purification kit (Epicentre, Madison, WI) according to the manufacturer's protocol. MMLV reverse transcriptase (Epicentre, Madison, WI) was used to generate the cDNAs using an arbitrary amount of the extracted RNA (3 μl) and 0.5 μg of random primers (Promega, Madison, WI) in a 25 μl reaction. Real-time QPCR was performed using 2 μl of the reverse transcription mix in a 20 μl reaction with either iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) or FailSafe Real-Time PCR kit using 2X Pre- mix G (Epicentre, Madison, WI). PCR conditions (40 cycles) were as follows: 90°C for 30 seconds, primer annealing for 30 seconds (see Table I for annealing temperature), then 72°C for 30 seconds. SYBR green fluorescence readings were taken at 80°C when the fluorescence intensity corresponded solely to the PCR product of interest. The final PCR products were analyzed on an agarose gel to ensure that only one product was amplified. Normalized fold increase of the endogenous transcript was determined by the 2−ΔΔT method using 18S as the standard and the untreated control as the calibrator.30
Statistical analyses
The statistical significance between means (in triplicate) was determined by applying two-tailed t-tests in Excel 2000 software. Single asterisk represents a p value < 0.05 whereas double asterisk represents a p value < 0.005.
Results
Effect of CΔ553 on the enhancer-driven luciferase expression
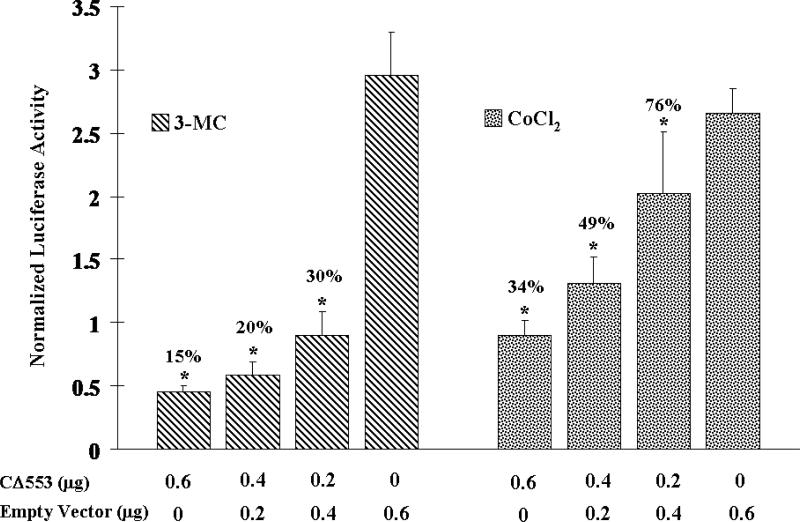
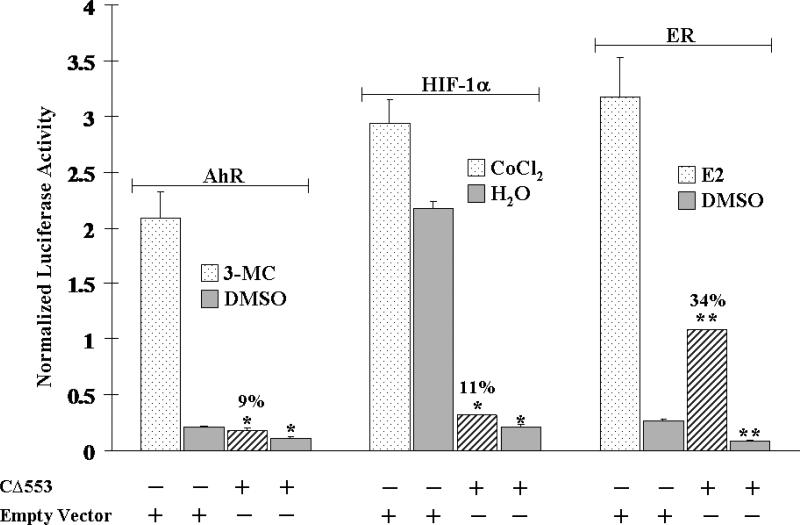
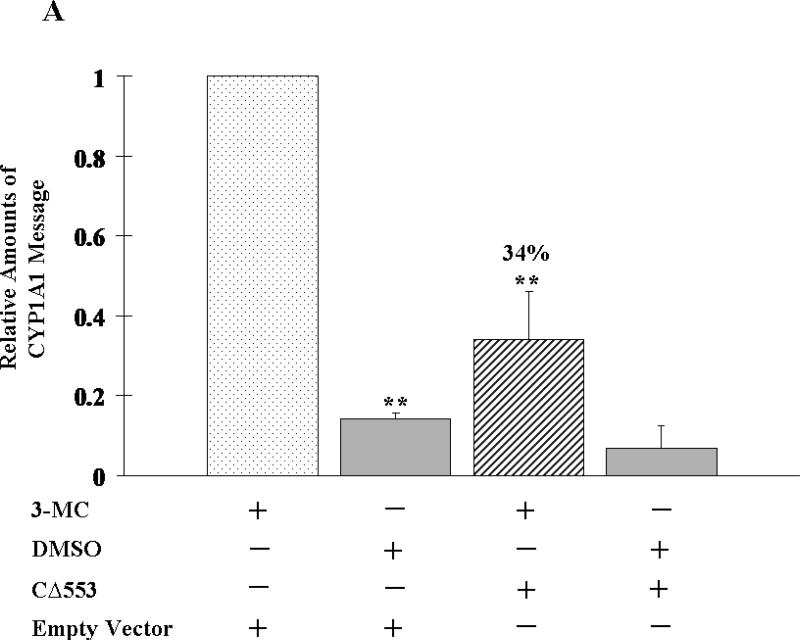
To show the ability of CΔ553 to interfere with the Arnt-dependent signaling in cells, CΔ553 was transiently expressed in MCF-7 cells along with a DRE- or HRE-driven luciferase reporter plasmid. Varying amounts of CΔ553 (0 – 600 ng), which were normalized to 600 ng with the empty vector, showed a concentration-dependent suppression of the 3-MC- and CoCl2-dependent luciferase activities up to 85 and 66%, respectively, in a statistically significant manner (p < 0.05) (Figure 2). Next, we examined the effect of CΔ553 on the inducer-dependent expression of the luciferase gene. We observed that 3-MC (1 μM), CoCl2 (100 μM), and E2 (10 nM) activated the expression of the luciferase gene driven by the DRE, HRE, and ERE, respectively, in MCF-7 cells (Figure 3). Not only did the CΔ553 vector (600 ng) dramatically suppress the inducer 3-MC, CoCl2 and E2-dependent luciferase activities to 9, 11 and 34%, respectively, as compared to the corresponding empty vector plus inducer controls, CΔ553 also suppressed significantly all the luciferase activities without an inducer (p <0.005). In the earlier transfection experiments showing the Arnt-dependent signaling (Figure 2), the HRE plasmid constituted 36% of the total DNA (1.1 μg) transfected. In the latter transfection experiments (Figure 3), the amount of the HRE plasmid was reduced to 23% of the total transfected DNA (0.8 μg) in an effort to increase the transfection efficiency by decreasing the total DNA/lipofectmine ratio. This difference in the protocol probably contributed to the noticeable differences in the overall suppression of the HRE-driven luciferase expression in Figures 2 and 3 (34 versus 11%).
Figure 2.
CΔ553 inhibited the 3-MC-induced DRE-dependent luciferase expression (left) and CoCl2-induced HRE-dependent luciferase expression (right) in MCF-7 cells. Each condition (well) contained 1.1 μg of DNA (0.4 μg of GudLuc1.1/ pGL3-Epo plus 0.1 μg of pCH110 plus 0-0.6 μg of pCMV-CΔ553 balanced with the empty vector pCMV-Tag4A per 1.5 μl of lipofectamine 2000 plus 1 μM 3-MC or 100 μM CoCl2. Y-axis represents luciferase activity normalized by the β-galactosidase activity. Single asterisk indicates a significant difference (p < 0.05) when compared to the corresponding empty vector control (0.6μg of empty vector). Error bars represent the standard deviation of the means in triplicate (n=3, mean ± SD).
Figure 3.
CΔ553 inhibited the DRE- (left), HRE- (middle), and ERE- (right) driven luciferase expression +/− the corresponding inducer 1 μM 3-MC, 100 μM CoCl2 and 10 nM E2 in MCF-7 cells. 0.8 μg of DNA (0.18 μg reporter plasmid (pGudLuc1.1, pGL3-Epo, or pERE-Luc) plus 0.02 μg of pCH110 plus 0.6 μg of pCMV-CΔ553 or empty vector pCMV-Tag4A) per 1.5 μl of lipofectamine 2000. Y-axis represents luciferase activity normalized by the β-galactosidase activity. Double asterisk shows a significant difference (p < 0.005) when compared to the corresponding empty vector control. Error bars represent the standard deviation of the means in triplicate (n=3, mean ± SD).
Effect of CΔ553 on the expression of the AhR, HIF-1α and ER target genes
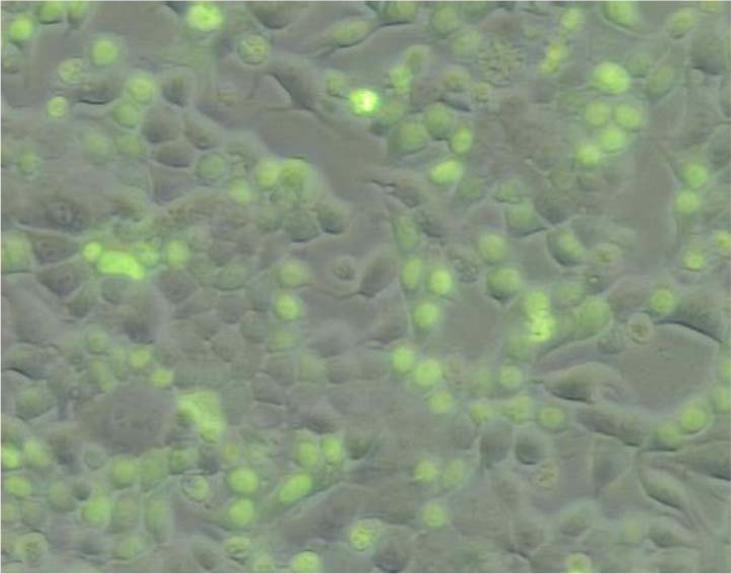
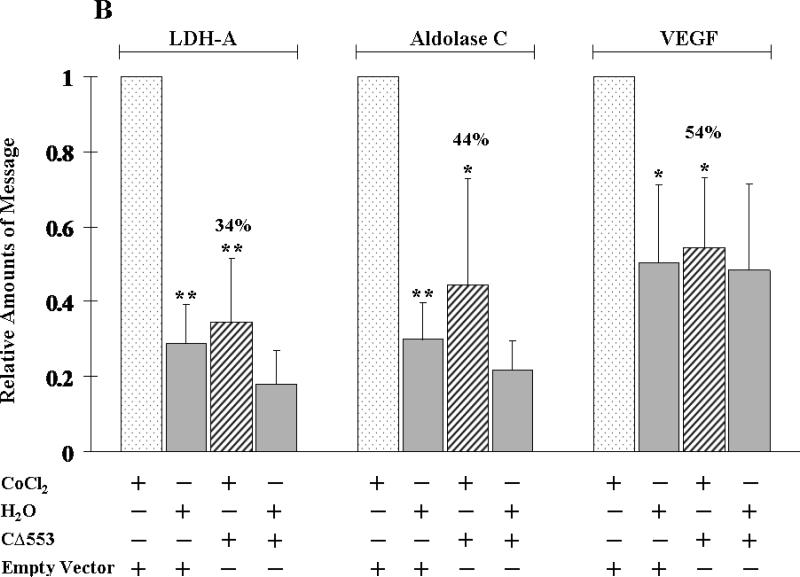
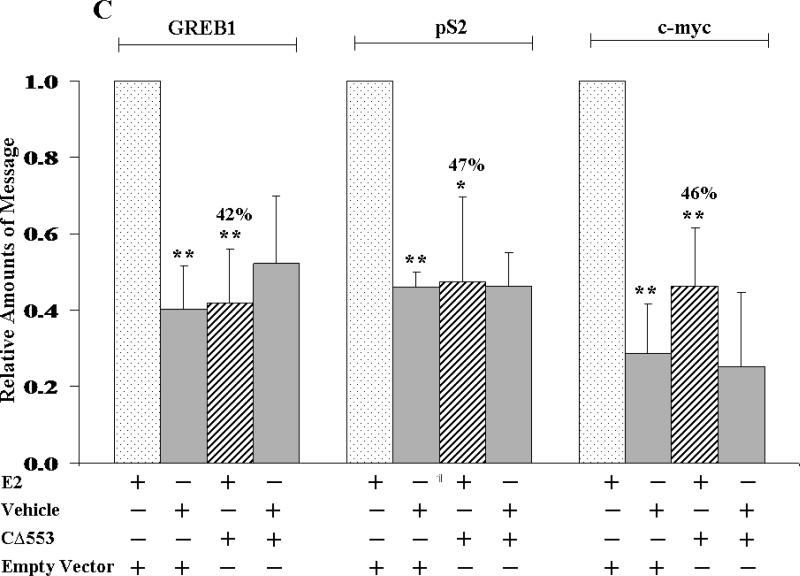
To assess the ability of CΔ553 to disrupt the endogenous gene expression, real-time QPCR was employed to measure the amount of the target gene transcripts generated through the activation of AhR, HIF-1α, or ER in the presence or absence of CΔ553. Our transfection protocol should be sufficient to deliver the CΔ553 plasmid into cells since the transfection efficiency was about 40% when we transfected a plasmid containing the GFP fusion of CΔ553 cDNA into MCF-7 cells using the same protocol (Figure 4). In all cases when we transfected the CΔ553 plasmid, the amount of the CΔ553 message was at least 100-fold higher than the empty vector control, suggesting that CΔ553 was expressed at a higher level as compared to the endogenous AhR (data not shown). Results of the real-time QPCR studies showed that CΔ553 significantly suppressed the up-regulation of the target genes: (1) the up-regulation of the CYP1A1 message by 3-MC was suppressed by 66% (Figure 5A); (2) the up-regulation of the LDH-A, aldolase C and VEGF messages by CoCl2 were suppressed by 66%, 56%, and 46%, respectively (Figure 5B); and (3) the up-regulation of the GREB1, pS2 and c-myc messages by E2 was suppressed by 58%, 53%, and 54%, respectively (Figure 5C). We believe that these suppressions by CΔ553 were not caused by a general transfection effect because we always included a corresponding transfection control which contained an equal amount of DNA (empty vector) and Lipofectamine. Although our transient transfection data showed that CΔ553 suppressed significantly the luciferase activity controlled by DRE, HRE, or ERE even in the absence of an inducer, the endogenous target gene basal levels was basically unaltered by CΔ553, showing that the mechanisms that determine the basal expression of AhR, HIF-1α, and ER target genes tested were not affected by CΔ553. Thus, the ligand-independent suppression observed in our previous luciferase experiments appeared to be biologically irrelevant.
Figure 4.
Fluorescence image showing the transfection efficiency of a CΔ553 plasmid. The plasmid pEGFP-CΔ553 was generated by cloning the N-terminal 885 bp of the full length human AhR cDNA into the Xho I and Hind III sites of pEGFPC2 (Clontech). This cloned plasmid was transfected into MCF-7 cells in a 6-well plate under the same condition for RT/real-time QPCR studies. Eighteen hours after transfection, cells were visualized on a Nikon Eclipse TE200 microscope with a 10X/0.3 NA objective with use of the FITC filter block for EGFP fluorescence. Images were captured by Optronics DEI-470 camera and processed with Image Pro 5.0 software. About 40% of transfected cells showed fluorescent staining caused by the expression of the GFP-CΔ553 fusion protein.
Figure 5.
Effect of CΔ553 on up-regulation of target gene expression in MCF-7 cells. A, 1 μM 3-MC or DMSO for 6 hours; B, 100 μM CoCl2 or water for 6 hours and C, 10 nM E2 or ethanol/DMSO (4:1) for 18 hours. Each condition (well) contained 4 μg of pCMV-CΔ553 or empty vector pCMV-Tag4A per 7.5 μl of Lipofectamine 2000. RT/real-time QPCR results showing the amount of messages from various conditions normalized to the amount of messages in the standard (minus CΔ553 plus inducer) which is arbitrarily set to one in all cases (first left column in each panel). Y-axis represents the normalized fold increase using the 2−ΔΔCT method. Error bars represent the standard deviation of the means in triplicate (n=3, mean ± SD).
Discussion
Overexpression of HIF-1α in solid tumors has been associated with poor prognosis,31, 32 resistance to radiation treatment and chemotherapy21, and cancer 12 progression and metastasis notably in breast cancer.33 Thus it is not surprising that many researchers have proposed to develop HIF-1α inhibitors for cancer treatment.13 34 35 Other researchers and this corresponding author have reported that AhR cross-talks with the HIF-1α pathway.36-40 Although the mechanism for the cross-talk remains controversial, the fact that both AhR and HIF-1α share the same Arnt partner suggests an idea that if Arnt is somehow sequestered during hypoxia, the HIF-1α function might be blocked. This idea is supported by the observation that the hypoxia-mediated induction of VEGF was significantly reduced in Arnt deficient Hepa c4 cells as compared to the wild-type Hepa-1 cells.41 We are interested in exploring the possibility that an Arnt-interacting protein may be used to inhibit the HIF-1α function via an Arnt-sequestering mechanism. Therefore, we used the human AhR construct CΔ553 to test whether it is possible to inhibit the HIF-1α function via this mechanism. CΔ553 was chosen in this case because it is capable of dimerizing with Arnt in the absence of an AhR ligand and is transcriptionally silent in the AhR signaling.
To test out our hypothesis that CΔ553 may compete with the Arnt partner for heterodimerization with Arnt, we examined whether increasing concentrations of CΔ553 could suppress the AhR and HIF-1α signaling. Our initial transient transfection experiments utilized the luciferase reporter plasmid containing either the 13 mouse CYP1A1 promoter (pGudLuc1.1) or the Epo 3′ enhancer region (pGL3-Epo). We found that CΔ553 clearly reduced the 3-MC- and CoCl2-induced luciferase expressions in a concentration-dependent manner, confirming our prediction that the transfected CΔ553 is capable of suppressing both the AhR and HIF-1α signaling pathways. Next, we determined whether this suppression by CΔ553 could be observed in the endogenous gene expressions controlled by AhR and HIF-1α. Indeed, induction of the target genes of both AhR (CYP1A1) and HIF-1α (VEGF, aldolase C and LDH-A) was effectively suppressed by CΔ553, suggesting that adequate concentrations of the CΔ553 protein could be established via transient transfection to competitively inhibit the formation of the corresponding Arnt complexes. Since CΔ553 lacks the TAD, its ability to activate gene transcription (such as in the case of CYP1A1) is lost, even though the endogenous Arnt contains a C-terminal TAD. Our data are consistent with the literature showing that only the AhR TAD, but not the Arnt TAD, is required for the CYP1A1 gene transcription in Hepa-1 cells.42
We believed that this reduction of signaling should be attributed to the CΔ553's ability to compete with the endogenous AhR and HIF-1α in three possible ways. First, CΔ553 may compete with the endogenous AhR and HIF-1α for heterodimerization with Arnt and therefore lesser amounts of the endogenous 14 heterodimers are formed. Second, the CΔ553-Arnt complex may outcompete the AhR-Arnt heterodimer for binding to the DRE. Third, binding of the CΔ553-Arnt heterodimer to the DREs (if present) in the hypoxia responsive gene promoters may interfere with the binding of the HIF-1α-Arnt heterodimer to the HRE. This last mechanism may seem less likely but certainly conceivable because five DREs had been found in the Epo gene promoter37 and echinomycin effectively suppressed the hypoxia-induced VEGF expression by inhibiting the binding of the HIF-1α-Arnt heterodimer to the HRE.20
We also addressed whether the ER signaling pathway can be inhibited by the formation of the CΔ553-Arnt heterodimer. We suspected this to be possible because Arnt has been shown to be a potent coactivator in the ER signaling in T47D cells29 and silencing of the Arnt gene expression using the antisense Arnt suppressed the E2-dependent CAT expression in MCF-7 cells.28 We found that CΔ553 dramatically decreased the ER-dependent luciferase expression in MCF-7 cells. This effect should not be caused by a direct interaction between CΔ553 and ER since the ER interaction domain within AhR (the P/S/T region of the TAD) is absent in CΔ553.25 Furthermore, our reporter plasmid contained only the repeated ERE sequence in tandem; the observed effect should therefore involve the binding of the ER homodimer to the ERE in the presence of CΔ553. It is unlikely that recruitment of coactivators, other than Arnt, was affected since CΔ553 does not contain the TAD 15 that is responsible for interactions with coactivators. In an effort to examine whether this suppressive effect would translate into the endogenous gene regulation, we monitored the change of three ER target genes GREB1, pS2 and c-myc in the presence or absence of CΔ553. We chose these three target genes because (1) these genes are well known to be E2 responsive in breast cancer cells; (2) GREB1 and c-myc have been shown to be responsible for the estrogen-dependent breast cancer growth in cell culture43 44; and (3) the induction of the GREB1 message in MCF-7 cells is Arnt-dependent (our unpublished results). We purposely exclude two other well known ER target genes cathepsin D and cyclin D1 because we were not able to detect the E2-dependent transcription of these genes using our MCF-7 cells (data not shown). Once again, CΔ553 suppressed the GREB1, pS2 and c-myc messages induced by E2, showing that CΔ553 is capable of down-regulating the endogenous ER signaling in MCF-7 cells. Additionally, we were also able to reproduce these suppressions in another breast cancer cell line T47D (data not shown), further confirming our finding that CΔ553 suppresses the E2-dependent gene regulation.
Here we presented data showing that CΔ553 inhibits both the HIF-1α- and ER-dependent gene expressions. From a therapeutic standpoint, the potential of reducing the amount of gene products that appear to play an important role in the development of the ER-positive breast cancer by CΔ553 is particularly attractive – VEGF is essential for angiogenesis, LDH-A is involved in glucose metabolism 16 during hypoxia, and GREB1 and c-myc are essential for the estrogen-dependent cancer growth. As predicted, CΔ553 also inhibits the AhR signaling. This inhibition may seem to be undesirable in breast cancer cells since the constitutive function of AhR may involve suppression of the breast cancer growth by inhibiting the cell cycle progression from G0/G1 to S phase.45 On the contrary, AhR appears to stimulate growth in the ER-negative breast cancer cells Hs578T, suggesting that cell cycle suppression by AhR may be controlled by the ER responsiveness. The endogenous relevance of this AhR-induced breast cancer cell growth is unclear because inhibition of the AhR function in Hs578T cells by either the AhR antagonist 1α-naphthoflavone or the AhR repressor protein did not affect cell proliferation.46All in all, the role of AhR on breast cancer cell growth is not well understood and the implication of the suppression of the AhR signaling by CΔ553 on cell proliferation is not apparent.
This inhibition of the hypoxia and ER signaling pathways by CΔ553 is somewhat unique because CΔ553 is a truncated protein rather than a small organic molecule. But using a protein to achieve therapeutic outcome is certainly workable – some researchers recently used an adenoviral system to deliver a plasmid containing a truncated HIF-1α cDNA to block the HIF-1α function in cell culture and in turn reversed the resistance to etoposide.47 It is understandably desirable to have protein molecules, such as this HIF-1α construct, to be used as therapeutic 17 agents to combat drug resistance found in HIF-1α expressing cancers. The idea of using a therapeutic protein for cancer treatment is attractive and becoming increasingly feasible. This kind of protein therapy was tested using a TAT fusion of Casp3 and was shown to be effective in growth inhibition in cell culture and tumor shrinkage in a xenograft mouse model.48 Additionally, there are a number of peptides that have been shown to suppress cancer growth by interfering with the intracellular signaling mechanisms.49 These peptides were fused to a protein transduction domain (e.g. TAT and a stretch of arginine) so that they are capable of penetrating into cells directly and can be developed into anticancer drugs. For example, overexpression of XIAP, which is anti-apoptotic because it inhibits caspases, contributes to the chemoresistance observed in human non-small cell lung cancer NCI-H460 cells. The N-terminal region of Smac (SmacN7) interacts with XIAP and the interaction was hypothesized to suppress XIAP's anti-apoptotic property. This hypothesis was proven to be the case when polyarginine-fusion of SmacN7 effectively reversed the chemoresistance in H460 cells.50 Renal cell carcinoma contains a mutated VHL that inhibits the IGF-I signaling and is responsible for the degradation of the HIF-1α protein under normoxia. When the VHL region (amino acid 104-123) that interacts with IGF-I and subsequently abrogates the IGF-I signaling is fused to TAT, this TAT-VHL fusion protein suppressed the renal tumor growth in a rat tumor model.51 Uveal melanoma 18 expresses abundant amounts of HDM2 which may contribute to the carcinogenic process by inhibiting the p53 function. The interaction domain of p53 was mapped to a 12 amino acid region and this region, when fused to TAT, was capable of killing tumor cells by apoptosis when delivered directly into the rabbit eyes.52 Currently we are developing the TAT fusion of CΔ553 and its derivatives to study their protein transduction potential and their anticancer properties in cell lines and a mouse tumor model.
Acknowledgement
We thank Dr. Jesika Faridi (University of the Pacific) for the ERE-driven luciferase reporter plasmid pERE-Luc and MCF-7 cells and Dr. Mike Denison (UC Davis) for the DRE-driven luciferase reporter plasmid pGudLuc1.1. This work is supported in part by a grant from the National Institutes of Health (ES09794).
Abbreviations
- PAH
polycyclic aromatic hydrocarbon
- HAH
halogenated aromatic hydrocarbon
- AhR
aryl hydrocarbon receptor
- CΔ553
the human AhR construct (aa 1-295) with the C-terminal 553 amino acids deleted
- Arnt
AhR nuclear translocator
- HIF-1α
hypoxia inducible factor 1α
- ER
estrogen receptor
- 3-MC
3-methylcholanthrene
- CoCl2
cobalt chloride
- E2
17β-estradiol
- PAS
Per/Arnt/Sim
- bHLH
basic-helixloop-helix
- TAD
transactivation domain
- LBD
ligand binding domain
- DRE
dioxin response element
- HRE
hypoxia response element
- ERE
estrogen response element
- CYP1A1
cytochrome P450 1A1 gene
- LDH-A
lactose dehydrogenase-A
- VEGF
vascular endothelial growth factor
- GREB1
gene regulated by estrogen in breast cancer protein
- Epo
erythropoietin
References
- 1.Petrulis JR, Perdew GH. The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem Biol Interact. 2002;141:25–40. doi: 10.1016/s0009-2797(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 2.Delucchi AB, Jensen KA, Chan WK. Synthesis of 32P-labelled protein probes using a modified thioredoxin fusion protein expression system in Escherichia coli. Biomol. Eng. 2003;20:1–5. doi: 10.1016/s1389-0344(02)00050-3. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J. Appl. Physiol. 2004;96:1173–1177. doi: 10.1152/japplphysiol.00770.2003. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 5.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kietzmann T, Roth U, Jungermann K. Induction of the plasminogen activator inhibitor-1 gene expression by mild hypoxia via a hypoxia response element binding the hypoxia-inducible factor-1 in rat hepatocytes. Blood. 1999;94:4177–4185. [PubMed] [Google Scholar]
- 7.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 8.Firth JD, Ebert BL, Ratcliffe PJ. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J. Biol. Chem. 1995;270:21021–21027. doi: 10.1074/jbc.270.36.21021. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 10.Graven KK, Yu Q, Pan D, Roncarati JS, Farber HW. Identification of an oxygen responsive enhancer element in the glyceraldehyde-3-phosphate dehydrogenase gene. Biochim. Biophys. Acta. 1999;1447:208–218. doi: 10.1016/s0167-4781(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 11.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N. Engl. J. Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 12.Ganz PA. Breast cancer, menopause, and long-term survivorship: critical issues for the 21st century. Am. J. Med. 2005;118:136–141. doi: 10.1016/j.amjmed.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 13.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol. Med. 2002;8:S62–67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 14.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol. Med. 2002;8:S55–61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 16.Mabjeesh NJ, Post DE, Willard MT, Kaur B, Van Meir EG, Simons JW, Zhong H. Geldanamycin induces degradation of hypoxiainducible factor 1alpha protein via the proteosome pathway in prostate cancer cells. Cancer Res. 2002;62:2478–2482. [PubMed] [Google Scholar]
- 17.Escuin D, Kline ER, Giannakakou P. Both microtubule-stabilizing and microtubule-destabilizing drugs inhibit hypoxia-inducible factor-1alpha accumulation and activity by disrupting microtubule function. Cancer Res. 2005;65:9021–9028. doi: 10.1158/0008-5472.CAN-04-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rapisarda A, Uranchimeg B, Sordet O, Pommier Y, Shoemaker RH, Melillo G. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 2004;64:1475–1482. doi: 10.1158/0008-5472.can-03-3139. [DOI] [PubMed] [Google Scholar]
- 19.Tan C, de Noronha RG, Roecker AJ, Pyrzynska B, Khwaja F, Zhang Z, Zhang H, Teng Q, Nicholson AC, Giannakakou P, Zhou W, Olson JJ, Pereira MM, Nicolaou KC, Van Meir EG. Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway. Cancer Res. 2005;65:605–612. [PubMed] [Google Scholar]
- 20.Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, Fisher RJ, Shoemaker RH, Melillo G. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- 21.Brown JM. The hypoxic cell: a target for selective cancer therapy--eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1999;59:5863–5870. [PubMed] [Google Scholar]
- 22.Le QT, Taira A, Budenz S, Jo Dorie M, Goffinet DR, Fee WE, Goode R, Bloch D, Koong A, Martin Brown J, Pinto HA. Mature results from a randomized Phase II trial of cisplatin plus 5-fluorouracil and radiotherapy with or without tirapazamine in patients with resectable Stage IV head and neck squamous cell carcinomas. Cancer. 2006;106:1940–1949. doi: 10.1002/cncr.21785. [DOI] [PubMed] [Google Scholar]
- 23.Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, Yanagisawa J, Fujii-Kuriyama Y, Kato S. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 24.Klinge CM, Kaur K, Swanson HI. The aryl hydrocarbon receptor interacts with estrogen receptor alpha and orphan receptors COUP-TFI and ERRalpha1. Arch. Biochem. Biophys. 2000;373:163–174. doi: 10.1006/abbi.1999.1552. [DOI] [PubMed] [Google Scholar]
- 25.Beischlag TV, Perdew GH. ERalpha -AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin inducible gene transcription. J. Biol. Chem. 2005;280:21607–21611. doi: 10.1074/jbc.C500090200. [DOI] [PubMed] [Google Scholar]
- 26.Safe S. Molecular biology of the Ah receptor and its role in carcinogenesis. Toxicol. Lett. 2001;120:1–7. doi: 10.1016/s0378-4274(01)00301-0. [DOI] [PubMed] [Google Scholar]
- 27.Gillesby BE, Stanostefano M, Porter W, Safe S, Wu ZF, Zacharewski TR. Identification of a motif within the 5′ regulatory region of pS2 which is responsible for AP-1 binding and TCDD-mediated suppression. Biochemistry. 1997;36:6080–6089. doi: 10.1021/bi962131b. [DOI] [PubMed] [Google Scholar]
- 28.Wang F, Hoivik D, Pollenz R, Safe S. Functional and physical interactions between the estrogen receptor Sp1 and nuclear aryl hydrocarbon receptor complexes. Nucleic Acids Res. 1998;26:3044–3052. doi: 10.1093/nar/26.12.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunnberg S, Pettersson K, Rydin E, Matthews J, Hanberg A, Pongratz I. The basic helix-loop-helix-PAS protein ARNT functions as a potent coactivator of estrogen receptor-dependent transcription. Proc. Natl. Acad. Sci. USA. 2003;100:6517–6522. doi: 10.1073/pnas.1136688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693–4696. [PubMed] [Google Scholar]
- 32.Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin. Cancer Res. 2002;8:1831–1837. [PubMed] [Google Scholar]
- 33.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 34.Mazure NM, Brahimi-Horn MC, Berta MA, Benizri E, Bilton RL, Dayan F, Ginouves A, Berra E, Pouyssegur J. HIF-1: master and commander of the hypoxic world. A pharmacological approach to its regulation by siRNAs. Biochem. Pharmacol. 2004;68:971–980. doi: 10.1016/j.bcp.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Hewitson KS, Schofield CJ. The HIF pathway as a therapeutic target. Drug Discov. Today. 2004;9:704–711. doi: 10.1016/S1359-6446(04)03202-7. [DOI] [PubMed] [Google Scholar]
- 36.Gassmann M, Kvietikova I, Rolfs A, Wenger RH. Oxygen- and dioxinregulated gene expression in mouse hepatoma cells. Kidney Int. 1997;51:567–574. doi: 10.1038/ki.1997.81. [DOI] [PubMed] [Google Scholar]
- 37.Chan WK, Yao G, Gu YZ, Bradfield CA. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. J. Biol. Chem. 1999;274:12115–12123. doi: 10.1074/jbc.274.17.12115. [DOI] [PubMed] [Google Scholar]
- 38.Pollenz RS, Davarinos NA, Shearer TP. Analysis of aryl hydrocarbon receptor-mediated signaling during physiological hypoxia reveals lack of competition for the aryl hydrocarbon nuclear translocator transcription factor. Mol. Pharmacol. 1999;56:1127–1137. doi: 10.1124/mol.56.6.1127. [DOI] [PubMed] [Google Scholar]
- 39.Nie M, Blankenship AL, Giesy JP. Interactions between aryl hydrocarbon receptor (AhR) and hypoxia signaling pathways. Environ. Toxicol. Pharmacol. 2001;10:17–27. doi: 10.1016/s1382-6689(01)00065-5. [DOI] [PubMed] [Google Scholar]
- 40.Prasch AL, Andreasen EA, Peterson RE, Heideman W. Interactions between 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and hypoxia signaling pathways in zebrafish: hypoxia decreases responses to TCDD in zebrafish embryos. Toxicol. Sci. 2004;78:68–77. doi: 10.1093/toxsci/kfh053. [DOI] [PubMed] [Google Scholar]
- 41.Wood SM, Gleadle JM, Pugh CW, Hankinson O, Ratcliffe PJ. The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. Studies in ARNT-deficient cells. J. Biol. Chem. 1996;271:15117–15123. doi: 10.1074/jbc.271.25.15117. [DOI] [PubMed] [Google Scholar]
- 42.Ko HP, Okino ST, Ma Q, Whitlock JP., Jr Dioxin-induced CYP1A1 transcription in vivo: the aromatic hydrocarbon receptor mediates transactivation, enhancer-promoter communication, and changes in chromatin structure. Mol. Cell. Biol. 1996;16:430–436. doi: 10.1128/mcb.16.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson PH, Pon RT, Shiu RP. Inhibition of c-myc expression by phosphorothioate antisense oligonucleotide identifies a critical role for c-myc in the growth of human breast cancer. Cancer Res. 1991;51:3996–4000. [PubMed] [Google Scholar]
- 44.Rae JM, Johnson MD, Scheys JO, Cordero KE, Larios JM, Lippman ME. GREB 1 is a critical regulator of hormone dependent breast cancer growth. Breast Cancer Res Treat. 2005;92:141–149. doi: 10.1007/s10549-005-1483-4. [DOI] [PubMed] [Google Scholar]
- 45.Abdelrahim M, Smith R, 3rd, Safe S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ahresponsiveness in MCF-7 and HepG2 cancer cells. Mol. Pharmacol. 2003;63:1373–1381. doi: 10.1124/mol.63.6.1373. [DOI] [PubMed] [Google Scholar]
- 46.Murray TJ, Yang X, Sherr DH. Growth of a human mammary tumor cell line is blocked by galangin, a naturally occurring bioflavonoid, and is accompanied by down-regulation of cyclins D3, E, and A. Breast Cancer Res. 2006;8:R17. doi: 10.1186/bcr1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown LM, Cowen RL, Debray C, Eustace A, Erler JT, Sheppard FC, Parker CA, Stratford IJ, Williams KJ. Reversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting hypoxia inducible factor-1. Mol. Pharmacol. 2006;69:411–418. doi: 10.1124/mol.105.015743. [DOI] [PubMed] [Google Scholar]
- 48.Harada H, Hiraoka M, Kizaka-Kondoh S. Antitumor effect of TAT-oxygendependent degradation-caspase-3 fusion protein specifically stabilized and activated in hypoxic tumor cells. Cancer Res. 2002;62:2013–2018. [PubMed] [Google Scholar]
- 49.Harada H, Kizaka-Kondoh S, Hiraoka M. Antitumor protein therapy; application of the protein transduction domain to the development of a protein drug for cancer treatment. Breast Cancer. 2006;13:16–26. doi: 10.2325/jbcs.13.16. [DOI] [PubMed] [Google Scholar]
- 50.Yang L, Mashima T, Sato S, Mochizuki M, Sakamoto H, Yamori T, Oh-Hara T, Tsuruo T. Predominant suppression of apoptosome by inhibitor of apoptosis protein in non-small cell lung cancer H460 cells: therapeutic effect of a novel polyarginine-conjugated Smac peptide. Cancer Res. 2003;63:831–837. [PubMed] [Google Scholar]
- 51.Datta K, Sundberg C, Karumanchi SA, Mukhopadhyay D. The 104-123 amino acid sequence of the beta-domain of von Hippel-Lindau gene product is sufficient to inhibit renal tumor growth and invasion. Cancer Res. 2001;61:1768–1775. [PubMed] [Google Scholar]
- 52.Harbour JW, Worley L, Ma D, Cohen M. Transducible peptide therapy for uveal melanoma and retinoblastoma. Arch. Ophthalmol. 2002;120:1341–1346. doi: 10.1001/archopht.120.10.1341. [DOI] [PubMed] [Google Scholar]