Abstract
Objectives
Extended-release niacin effectively lowers plasma TG levels and raises plasma HDL cholesterol levels, but the mechanisms responsible for these effects are unclear.
Methods and Results
We examined the effects of extended-release niacin (2 g/d) and extended-release niacin (2 g/d) plus lovastatin (40 mg/d), relative to placebo, on the kinetics of apolipoprotein (apo) A-I and apoA-II in HDL, apoB-100 in TG-rich lipoproteins (TRL), intermediate-density lipoproteins (IDL) and LDL, and apoB-48 in TRL in five men with combined hyperlipidemia. Niacin significantly increased HDL cholesterol and apoA-I concentrations, associated with a significant increase in apoA-I production rate (PR) and no change in fractional catabolic rate (FCR). Plasma TRL apoB-100 levels were significantly lowered by niacin, accompanied by a trend toward an increase in FCR and no change in PR. Niacin treatment significantly increased TRL apoB-48 FCR but had no effect on apoB-48 PR. No effects of niacin on concentrations or kinetic parameters of IDL and LDL apoB-100 and HDL apoA-II were noted. The addition of lovastatin to niacin promoted a lowering in LDL apoB-100 due to increased LDL apoB-100 FCR.
Conclusion
Niacin treatment was associated with significant increases in HDL apoA-I concentrations and production, as well as enhanced clearance of TRL apoB-100 and apoB-48.
INTRODUCTION
The cholesterol-lowering effect of the vitamin nicotinic acid, or niacin, was first reported by Altschul et al. 1 more than 50 years ago. Since then, treatment with pharmacological doses of niacin has been found to significantly lower the risk of coronary heart disease (CHD) 2, 3. Several trials have also tested the effect of niacin in combination with other lipid-lowering medications on CHD risk, overall showing a beneficial effect 4–6. Niacin primarily decreases plasma triglyceride (TG) levels and very-low-density lipoprotein (VLDL) cholesterol (C) levels and increases plasma HDL-C levels 2, 7. It has been hypothesized that the reduction in TG and VLDL-C is mediated by the niacin-associated inhibition of free-fatty acid (FFA) release from the adipose tissue, which may lead to reduced substrate availability for TG synthesis and secretion in hepatic cells 8. However, a study conducted in one hypertriglyceridemic subject showed faster clearance of autologous 125I-labeled VLDL after niacin treatment 9. Niacin is one of the most potent HDL-C-raising agents currently available. Two previous studies have attempted to elucidate the effect of niacin on HDL metabolism in young, normocholesterolemic subjects 10, 11. The first study was conducted in two subjects and found an increase in HDL-C levels associated with a slower HDL catabolism with niacin 10. The second study, in five young healthy subjects, found a significant increase in plasma HDL-C and apolipoprotein (apo) A-I levels with niacin without significant effects on apoA-I kinetics 11.
To date, very little is known about the mechanism by which niacin affects the metabolism of plasma lipoproteins in subjects with dyslipidemia, who are the ideal targets of niacin treatment. Therefore, the current study was designed to clarify the effects of extended-release niacin, without or with a statin, on the kinetics of apoA-I, apoA-II, apoB-100 and apoB-48 in plasma lipoproteins in subjects with combined hyperlipidemia.
SUBJECTS and METHODS
Subjects
Five male subjects with combined hyperlipidemia were enrolled in this study (age range: 44 to 69 y; BMI range: 24.7 to 33.9 kg/m2). Plasma lipid criteria for enrollment into the study were: TG levels ≥150 mg/dL, LDL-C levels ≥130 mg/dL, and HDL-C levels ≤40 mg/dL. Exclusion criteria were: age <40 years, myocardial infarction in the past 6 months, smoking, thyroid dysfunction, liver or kidney disease, liver cancer, diabetes mellitus, stroke, and current use of medications known to affect lipid metabolism. The study protocol was approved by the Institutional Review Board of Tufts University-New England Medical Center. Study candidates provided written informed consent.
Study design
Subjects were instructed to follow the therapeutic lifestyle changes (TLC) diet (<30% of calories as total fat, <7% saturated fat, <200 mg/day cholesterol)12 throughout the study. The study had a randomized, double-blind, crossover design and consisted of three treatment phases, each lasting 12 weeks: placebo, extended-release niacin (Niaspan®, KOS Pharmaceuticals), and extended-release niacin plus lovastatin (Advicor®, KOS Pharmaceuticals). Niaspan tablets contained 500 mg extended-release niacin. Advicor tablets contained 500 mg extended-release niacin and 10 mg lovastatin. To avoid severe flushing, the dosage of Niacin was titrated according to the following schedule: one tablet during weeks 1–4, two tablets during weeks 5–8, and four tablets (corresponding to 2 g/day of extended-release niacin in the Niaspan phase and 2 g/day extended-release niacin and 40 mg/day lovastatin in the Advicor phase) during weeks 9–12 of each phase. Treatment phases were separated by a 4-week washout period. A 12-hour fast blood sample was obtained for the determination of plasma lipid levels on weeks 11 and 12 of each phase. Blood was centrifuged at 1000 × g for 30 min at +4°C and plasma was stored at −70°C until analyzed.
On week 12 of each phase, subjects underwent a 15-hour primed-constant infusion with 10 μmoles/kg body weight per hour of deuterated leucine (5,5,5-2H3-L-leucine) (C/D/N Isotopes Inc, Pointe-Claire, Canada), as previously described 13, 14. Subjects were fed hourly for 20 hours with small identical meals, whose composition was complying with the TLC diet, starting five hours before and throughout the infusion period. Blood samples were collected into tubes containing EDTA (0.15%) just prior to the infusion (time 0), and at the following times during the infusion: 30, 35, and 45 min, and 1, 1.5, 2, 3, 4, 6, 9, 12, 14, and 15 hour.
Plasma lipid and lipoprotein determinations
Lipids were measured both in plasma samples obtained after a 12-hour fast (week 11 and 12) and in non-fasting plasma samples obtained during the infusion (hour 0, 3, and 6 of infusion). Plasma TC and TG levels were measured by automated enzymatic assays 15. Plasma LDL-C and HDL-C concentrations were measured directly with kits from Equal Diagnostics (Exton, PA) and Roche Diagnostics (Indianapolis, IN), respectively.
Plasma apoA-I and apoA-II concentrations were measured using immunoturbidimetric assays, reagents, and calibrators from Wako Diagnostics (Richmond, VA). The concentration of apoB-100 in plasma and in lipoprotein fractions was measured with an enzyme-linked immunosorbent assay (ELISA) 14. TRL apoB-48 was assessed with an ELISA assay (Shibayagi, Japan). Plasma HDL subpopulations were assessed by 2-dimensional gel electrophoresis, as previously described 16. Plasma concentrations of cholesteryl ester transfer protein (CETP) and lecithin:cholesterol acyltransferase (LCAT) were assessed by ELISA (ALPCO Diagnostics, NH). Remnant lipoprotein cholesterol concentrations were measured as previously described 17.
Apolipoprotein isotopic enrichment and kinetic analysis
Five mL of plasma from each infusion time-point were subjected to sequential ultracentrifugation in a Beckman ultracentrifuge (Beckman, Palo Alto, CA) for the isolation of triglyceride-rich lipoprotein (TRL), intermediate-density lipoprotein (IDL), LDL, and HDL fractions, as previously described 18. Lipoprotein fractions were subjected to gradient SDS polyacrylamide gel electrophoresis for separation of apolipoproteins and transferred to a Westran S polyvinylidene difluoride (PVDF) membrane 19. Each apo band was cut and the leucine tracer/tracee ratio (percent) was determined as previously described 14, 20. The Simulation Analysis and Modeling II (SAAM II) program (Seattle, WA) was used to calculate the fractional catabolic rate (FCR) of each apolipoprotein using multicompartmental models previously described 14, 21, 22. Production rates (PR) of these apolipoproteins were determined by the following formula, estimating plasma volume as 4.5% of body weight: PR (mg/kg per day) = [FCR (pools/day) × apo concentration (mg/L) × plasma volume(L)]/body wt (kg).
Biochemical assays
FFA levels in plasma were assessed with a colorimetric assay (Roche Diagnostics, IN). Plasma glycated albumin, insulin, and adiponectin levels were assessed as previously described 23–25.
Plasma concentrations of lathosterol and of the plant sterol β-sitosterol were assessed using agas chromatography method 26.
Statistical analyses
A power calculation was performed and, based on the crossover design of the study, it was determined that 5 subjects were needed to have a >80% probability to detect a treatment difference at a two sided 0.05 significance level, if the difference in HDL-C levels between niacin and placebo is 28% and the standard deviation of the response is 11% 7. The SAS statistical package (SAS version 9.1, Chicago, IL) was used for statistical analyses. For normally distributed variables, means±SD were calculated. Non-normally distributed variables were log-transformed to achieve normality before analysis, and the mean is expressed as geometric mean. The mixed model procedure (PROC MIXED) was used to test for differences in all outcome variables among phases. Analyses were adjusted for treatment sequence (Tukey-Kramer) and a P value ≤0.05 was considered significant.
RESULTS
Treatment with extended-release niacin, relative to placebo, resulted in a significant increase in plasma HDL-C levels and a significant reduction in plasma TG levels, both in the fasted and fed state (Table 1). The combination of extended-release niacin and lovastatin produced a significant reduction in plasma LDL-C levels relative to both placebo and niacin, contributing to significant reductions in plasma TC levels with the combination treatment (Table 1).
Table 1.
Effects of extended-release niacin and a combination of extended-release niacin and lovastatin, relative to placebo, on fasting and non-fasting plasma lipid levels.
| Placebo | Niacin | Niacin+ Lovastatin | Change (1) | Change (2) | Change (3) | |
|---|---|---|---|---|---|---|
| Fasting | mg/dL | mg/dL | mg/dL | |||
| TC | 243±35 | 209±28 | 163±22 | −34±22 (0.01) | −80±30 (0.0001) | −46±13 (0.003) |
| TG * | 343 (221–582) | 174 (90–310) | 164 (111–242) | −176±202 (0.005) | −194±182 (0.006) | −18±88 (0.56) |
| LDL-C | 126±31 | 124±21 | 87±18 | −3±12 (0.44) | −40±17 (0.0001) | −37±11 (0.0001) |
| HDL-C | 34±5 | 46±9 | 46±6 | +12±8 (0.01) | +11±5 (0.01) | 0±5 (0.67) |
| Nonfasting | ||||||
| TC | 224±39 | 199±29 | 157±25 | −25±25 (0.14) | −67±31 (0.01) | −43±22 (0.03) |
| TG * | 382 (260–518) | 259 (127–449) | 243 (209–285) | −115±195 (0.04) | −151±123 (0.05) | −35±128 (0.85) |
| LDL-C | 117±38 | 115±16 | 78±20 | −2±24 (0.59) | −39±22 (0.01) | −37±14 (0.01) |
| HDL-C | 30±6 | 43±9 | 41±6 | +13±7 (0.001) | +11±4 (0.001) | −2±5 (0.11) |
variable was log transformed before analysis and is shown as geometric mean (min-max); all other variables shown as mean±SD;
1: mean±SD of the difference between Niacin and Placebo (P value)
2: mean±SD of the difference between Niacin+Lovastatin and Placebo (P value)
3: mean±SD of the difference between Niacin+Lovastatin and Niacin (P value)
The kinetics of apolipoproteins in different lipoprotein fractions were assessed at the end of each treatment phase. Relative to placebo, extended-release niacin significantly increased plasma apoA-I concentrations (+15%) (Table 2). This was associated with a significant increase in apoA-I PR (+24%), relative to placebo (Table 2). The effect of the combination of lovastatin and niacin on apoA-I concentrations and PR was similar to that of niacin alone. Neither niacin alone nor the combination treatment affected apoA-I FCR. Neither plasma apoA-II concentrations nor ApoA-II kinetic parameters were affected by niacin or the combination treatment, relative to placebo (Table 2). Analysis of the HDL subpopulation profile showed a significant increase in large HDL particle concentrations during niacin, relative to placebo, with significant increases in α1, α2, preα1 and preα2 particles (Table 3). The addition of lovastatin to niacin had non-significant effects on the HDL subpopulation distribution. Plasma CETP and LCAT mass did not change significantly during treatment with niacin or the combination of niacin and lovastatin.
Table 2.
Plasma concentrations (C), fractional catabolic rate (FCR), and production rate (PR) of apoA-I and apoA-II during the placebo, extended-release niacin and a combination of extended-release niacin and lovastatin phases.
| ApoA-I | ApoA-II | |||||
|---|---|---|---|---|---|---|
| Phase/subject | C, mg/dL | FCR, pools/d | PR, mg/kg d−1 | C, m/dL | FCR, pools/d | PR, mg/kg d− |
| Placebo | ||||||
| 1 | 90 | 0.252 | 10.3 | 22 | 0.154 | 1.51 |
| 2 | 92 | 0.198 | 8.2 | 24 | 0.144 | 1.53 |
| 3 | 104 | 0.159 | 7.5 | 24 | 0.122 | 1.33 |
| 4 | 117 | 0.204 | 10.7 | 24 | 0.146 | 1.61 |
| 5 | 111 | 0.213 | 10.6 | 29 | 0.108 | 1.40 |
| Mean±SD | 103±11 | 0.205±0.033 | 9.5±1.5 | 25±3 | 0.135±0.019 | 1.48±0.11 |
| Niacin | ||||||
| 1 | 101 | 0.267 | 12.2 | 27 | 0.154 | 1.84 |
| 2 | 116 | 0.188 | 9.8 | 22 | 0.125 | 1.26 |
| 3 | 125 | 0.220 | 12.4 | 23 | 0.143 | 1.47 |
| 4 | 123 | 0.208 | 11.5 | 27 | 0.119 | 1.44 |
| 5 | 127 | 0.204 | 11.6 | 31 | 0.094 | 1.31 |
| Mean±SD | 118±5 | 0.217±0.030 | 11.5±1.0 | 26±4 | 0.127±0.023 | 1.46±0.23 |
| Niacin+Lovastatin | ||||||
| 1 | 103 | 0.220 | 10.2 | 25 | 0.148 | 1.67 |
| 2 | 110 | 0.224 | 11.1 | 23 | 0.175 | 1.81 |
| 3 | 124 | 0.243 | 13.6 | 25 | 0.166 | 1.87 |
| 4 | 126 | 0.190 | 10.8 | 26 | 0.125 | 1.46 |
| 5 | 133 | 0.225 | 13.4 | 31 | 0.146 | 2.01 |
| Mean±SD | 1199±5 | 0.220±0.019 | 11.8±1.6 | 26±3 | 0.152±0.019 | 1.76±0.21 |
| Change (P value)1 | +16±7 (0.001) | +0.012±0.029 (0.47) | +2.0±1.7 (0.04) | +1±3 (0.47) | −0.008±0.019 (0.79) | −0.01±0.24 (0.99) |
| Change (P value)2 | +16±6 (0.001) | +0.015±0.045 (0.41) | +2.4±2.5 (0.02) | +1±2 (0.32) | +0.017±0.028 (0.20) | +0.28±0.30 (0.06) |
| Change (P value)3 | +1±4 (0.97) | +0.003±0.034 (0.99) | +0.3±1.6 (0.99) | 0±2 (0.99) | +0.025±0.026 (0.10) | +0.30±0.37(0.08) |
1: mean±SD of the difference between Niacin and Placebo (P value)
2: mean±SD of the difference between Niacin+Lovastatin and Placebo (P value)
3: mean±SD of the difference between Niacin+Lovastatin and Niacin (P value)
Table 3.
Effects of extended-release niacin and a combination of extended-release niacin and lovastatin, relative to placebo, on apoA-I-containing HDL subpopulation concentrations in the non-fasting state.
| Placebo | Niacin | Niacin+Lovastatin | |
|---|---|---|---|
| preβ1 | 18.1±5.5 | 16.3±5.4 | 18.5±5.8 |
| preβ2 | 2.7±1.1 | 3.0±1.0 | 3.2±1.6 |
| α1 | 7.1±3.5 | 14.1±5.9* | 13.5±3.7* |
| α2 | 27.0±5.6 | 33.5±2.2† | 36.9±4.1* |
| α3 | 25.4±5.9 | 22.5±6.9 | 21.5±4.2 |
| α4 | 11.8±1.7 | 10.2±2.0 | 9.1±0.7 |
| preα1 | 2.4±2.1 | 7.8±5.1† | 6.0±3.1 |
| preα2 | 4.1±1.0 | 6.8±2.1† | 6.6±2.0† |
| preα3 | 2.9±0.9 | 2.8±0.7 | 2.6±0.3 |
| preα4 | 1.5±0.6 | 1.3±0.4 | 1.3±0.2 |
| CETP | 0.98±0.15 | 0.91±0.31 | 0.78±0.19 |
| LCAT | 11.0±1.5 | 9.6±1.5 | 10.2±1.8 |
HDL subpopulations expressed as mg/dL of apoA-I; CETP and LCAT mass expressed as μg/ml Values are mean±SD;
P<0.01 vs placebo;
P<0.03 vs placebo
The TRL apoB-100 concentration was significantly lowered (−28%) by niacin, relative to placebo, accompanied by a trend toward an increase in TRL apoB-100 FCR (+94%, P=0.06) (Table 4 and Figure 1). No significant changes in the conversion of TRL apoB-100 to IDL (45% vs 53%, P=0.39) were observed. Niacin did not affect TRL apoB-100 PR (Table 4). In addition, niacin did not affect the plasma concentration or the kinetic parameters of apoB-100 in IDL and LDL. The addition of lovastatin to niacin resulted in a significant reduction in IDL apoB-100 concentrations accompanied by a significant increase in IDL apoB-100 FCR, and in a significant reduction in LDL apoB-100 concentrations with a significant increase in LDL apoB-100 FCR, relative to placebo (Table 4 and Figure 1). The significant effects of the combination treatment on LDL apoB-100 kinetic parameters were also maintained relative to niacin alone (Table 4). Similar to TRL apoB-100, treatment with niacin resulted in lower plasma TRL apoB-48 concentrations, accompanied by a significant increase in apoB-48 FCR and no change in PR (Table 5 and Figure 1). A trend towards an increase in TRL apoB-48 FCR was observed with the combination treatment.
Table 4.
Plasma concentrations (C), fractional catabolic rate (FCR), and production rate (PR) of apoB-100 in TRL, IDL, and LDL during the placebo, extended-release niacin, or extended-release niacin plus lovastatin phases.
| TRL apoB-100 | IDL apoB-100 | LDL apoB-100 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Phase/subject | C* mg/dL |
FCR* pools/d |
PR* mg/kg d−1 |
C* mg |
FCR* pools/d |
PR* mg/kg d−1 |
C* mg/dL |
FCR* pools/d |
PR* mg/kg d−1 |
| Placebo | |||||||||
| 1 | 9.4 | 2.75 | 11.6 | 2.6 | 3.15 | 3.66 | 70 | 0.274 | 8.7 |
| 2 | 12.5 | 1.99 | 11.2 | 2.9 | 4.44 | 5.88 | 68 | 0.270 | 8.3 |
| 3 | 19.6 | 1.20 | 10.6 | 6.3 | 1.15 | 3.99 | 60 | 0.391 | 10.6 |
| 4 | 11.5 | 3.76 | 19.5 | 4.1 | 4.85 | 9.00 | 101 | 0.245 | 11.1 |
| 5 | 11.1 | 3.38 | 16.9 | 4.5 | 4.90 | 9.81 | 108 | 0.263 | 12.8 |
| Geometric mean | 12.4 | 2.42 | 13.5 | 3.9 | 3.42 | 5.96 | 79 | 0.285 | 10.2 |
| Niacin | |||||||||
| 1 | 7.6 | 3.79 | 13.0 | 3.2 | 4.92 | 7.08 | 74 | 0.294 | 9.8 |
| 2 | 6.2 | 2.77 | 7.7 | 3.1 | 4.05 | 5.64 | 69 | 0.234 | 7.3 |
| 3 | 4.7 | 6.24 | 13.3 | 2.9 | 2.99 | 3.95 | 66 | 0.301 | 8.9 |
| 4 | 7.5 | 3.88 | 13.1 | 2.4 | 3.89 | 5.88 | 73 | 0.214 | 7.0 |
| 5 | 15.4 | 2.30 | 15.9 | 5.3 | 4.18 | 9.89 | 82 | 0.327 | 12.1 |
| Geometric mean | 7.6 | 3.58 | 12.3 | 3.5 | 3.96 | 6.20 | 73 | 0.271 | 8.9 |
| Niacin+Lovastatin | |||||||||
| 1 | 7.4 | 4.11 | 13.7 | 1.5 | 5.98 | 3.93 | 57 | 0.319 | 8.1 |
| 2 | 7.8 | 3.92 | 13.7 | 1.5 | 4.46 | 3.01 | 44 | 0.367 | 7.2 |
| 3 | 6.5 | 4.44 | 12.9 | 1.7 | 3.43 | 2.55 | 45 | 0.346 | 7.0 |
| 4 | 12.3 | 2.73 | 15.4 | 4.3 | 3.97 | 7.59 | 66 | 0.331 | 9.8 |
| 5 | 8.3 | 5.53 | 20.7 | 2.4 | 9.75 | 10.40 | 74 | 0.129 | 14.24 |
| Geometric mean | 8.2 | 4.04 | 15.0 | 2.1 | 5.13 | 4.73 | 56 | 0.356 | 8.9 |
| Change (P value)1 | −4.5±6.9 (0.01) | +1.2±2.3 (0.06) | −1.3±3.7 (0.78) | −0.5±1.7 (0.23) | +0.3±1.3 (0.77) | 0±2.3 (0.87) | −9±17 (0.92) | 0±0.06 (0.15) | −1.3±1.9 (0.30) |
| Change (P value)2 | −4.4±5.3 (0.05) | +1.5±1.6 (0.06) | +1.3±3.1(0.35) | −1.8±1.8 (0.01) | +1.8±2.3 (0.03) | −1.0±1.4 (0.27) | −25±10 (0.001) | +0.1±0.08(0.05 | −1.0±1.8 (0.35) |
| Change (P value)3 | +0.2±4.4 (0.27) | +0.3±2.0 (0.94) | +2.7±2.7(0.16) | −1.3±1.4 (0.06) | +1.5±2.3 (0.10) | −1.0±2.1 (0.17) | −16±8 (0.001) | +0.1±0.05(0.01 | +0.2±2.2 (0.93) |
variable was log-transformed before analysis
1: mean±SD of the difference between Niacin and Placebo (P value)
2: mean±SD of the difference between Niacin+Lovastatin and Placebo (P value)
3: mean±SD of the difference between Niacin+Lovastatin and Niacin (P value)
Figure 1.
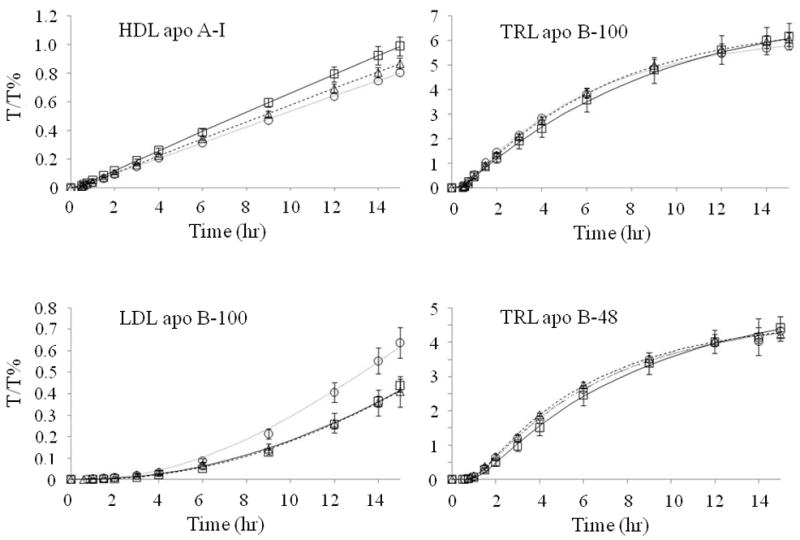
Leucine tracer/tracee ratios (T/T %) (mean±SD) of HDL apoA-I, TRL apoB-100, LDL apoB-100, and TRL apoB-48 during the placebo (square), extended-release niacin (triangle), and extended-release niacin and lovastatin (circle) phases. Lines represent the model-predicted values (placebo: continuous line; extended-release niacin: broken line; extended-release niacin plus lovastatin: dotted line).
Table 5.
Kinetics of apoB-48 in TRL during the placebo, extended-release niacin, or extended-release niacin plus lovastatin phases.
| TRL apoB-48 | |||
|---|---|---|---|
| Phase/subject | C *mg/dL | FCR*pools/d | PR*mg/kg d−1 |
| Placebo | |||
| 1 | 1.7 | 2.76 | 2.11 |
| 2 | 1.6 | 2.83 | 2.07 |
| 3 | 2.3 | 1.83 | 1.90 |
| 4 | 0.8 | 5.03 | 1.81 |
| 5 | 0.8 | 2.16 | 0.80 |
| Geometric mean | 1.33 | 2.74 | 1.64 |
| Niacin | |||
| 1 | 0.9 | 4.34 | 1.7 |
| 2 | 1.4 | 3.98 | 2.51 |
| 3 | 0.7 | 5.40 | 1.63 |
| 4 | 0.9 | 4.48 | 1.82 |
| 5 | 1.1 | 2.44 | 1.24 |
| Geometric mean | 0.96 | 4.00 | 1.73 |
| Niacin+Lovastatin | |||
| 1 | 0.7 | 4.95 | 1.56 |
| 2 | 0.8 | 3.57 | 1.33 |
| 3 | 1.0 | 3.42 | 1.49 |
| 4 | 1.1 | 3.22 | 1.59 |
| 5 | 0.5 | 4.31 | 1.03 |
| Geometric mean | 0.80 | 3.84 | 1.38 |
| Change (P value)1 | −0.45±0.78 (0.06) | +1.21±1.55 (0.04) | +0.04±0.39 (0.99) |
| Change (P value)2 | −0.62±0.63 (0.03) | +0.97±1.66 (0.10) | −0.34±0.37(0.36) |
| Change (P value)3 | −0.17±0.42 (0.97) | −0.23±1.52 (0.52) | −0.38±0.45 (0.35) |
variable was log-transformed before analysis
1: mean±SD of the difference between Niacin and Placebo (P value)
2: mean±SD of the difference between Niacin+Lovastatin and Placebo (P value)
3: mean±SD of the difference between Niacin+Lovastatin and Niacin (P value)
Plasma remnant lipoprotein cholesterol concentrations were significantly lowered and plasma insulin and adiponectin levels were significantly increased by niacin, relative to placebo (Table I), No effect of niacin on plasma FFA levels and markers of cholesterol homeostasis was observed (Table I). In contrast, lovastatin had a significant and independent effect on cholesterol homeostasis by lowering plasma lathosterol, a marker of cholesterol synthesis, and increasing plasma β-sitosterol, a marker of cholesterol absorption, relative to both placebo and niacin alone (Table I).
DISCUSSION
Treatment with extended-release niacin proved very effective in lowering high plasma TG levels and increasing low plasma HDL-C levels, consistent with the known effect of this medication 2, 7, 27. These changes were associated with significant reductions in remnant lipoproteins and a shift toward larger HDL subpopulation particles and point to a marked overall beneficial effect of this medication on the TG-HDL metabolism.
Our study indicates that the effect of extended-release niacin on plasma HDL-C concentrations is mediated in part by an increase in HDL apoA-I secretion. Our findings are different from the results of two previous studies 10, 11. Blum et al. 10 studied the effect of 1g of niacin/three times daily on apoA-I kinetics in two young normolipidemic subjects (a male and a female) using 125I-labeled autologous HDL and a multicompartmental model, and showed a slower catabolism of HDL particles 10. Shepherd et al. 11 studied five young normolipidemic subjects (three males and two females) treated with niacin 1 g/three times daily. Autologous HDL were labeled with 131I-apoA-I and 125I-apoA-II and the kinetic parameters of these apolipoprotein were calculated by mathematical models. Plasma apoA-I concentrations were increased by 7%, but no significant changes in apoA-I PR or FCR were observed 11. Previous in vitro studies in HepG2 cells have shown niacin to increase apoA-I concentration in the media and reduce apoA-I hepatic uptake without affecting apoA-I gene expression, suggesting that a reduction in apoA-I catabolism is the main mechanism in the regulation of HDL-C levels 28. In our study, extended-release niacin treatment significantly raised plasma apoA-I levels, mostly due to an increase in apoA-I PR. The discrepancy in the results between our study and the two previous kinetic studies may be explained by differences in the characteristics of the selected subjects and in the study methodology. In our study, subjects with abnormal plasma TG and HDL-C levels, ideal targets for niacin treatment, were selected. The metabolism of TG-rich lipoproteins and HDL may be affected differently by niacin in subjects who are young, healthy, and normolipidemic. In addition, in our study, apolipoproteins were endogenously labeled with a stable isotope, a method which has the advantages of labeling nascent particles and conserving the structure, metabolism, and binding characteristics of lipoproteins 29, 30. The molecular mechanism that mediates the niacin-associated increase in apoA-I production is not know, however niacin can activate both the mitogen activated protein (MAP) kinase pathway and the peroxisome proliferator activated receptors (PPAR) transcription factors 31. Both MAP kinase and PPAR have been shown to affect hepatic apoA-I secretion 32, 33.
The analysis of HDL subpopulations by 2-dimensional gel electrophoresis suggests that niacin promotes the maturation of HDL into large particles, such as α1 and α2 and their corresponding preα particles. The mechanism that mediates this effect is not known, but a reduction in CETP activity, caused by the lowering in TG and TRL particle concentrations, may play a role. A niacin-associated change in HDL subpopulations to larger particles has been described previously with other methodologies 11, 27. In the HATS study, the increase in plasma α1 particle levels associated with niacin plus simvastatin treatment was significantly related with slower coronary disease progression 34.
Lovastatin had no significant independent effect on apoA-I kinetics, consistent with some previous reports of a lack of effect of statins on apoA-I kinetics 14, 35.
The plasma concentrations and kinetic parameters of apoA-II in HDL were not affected by treatment with niacin. This is in contrast with the report by Shepherd et al, 11 where a significant reduction in plasma apoA-II levels, mostly explained by a reduction in apoA-II PR, was observed.
The reduction in plasma TG levels with niacin has been previously attributed to an inhibition of adipose tissue FFA release by this medication 36: the reduced availability of fatty acids for hepatic TG synthesis would lead to an impaired hepatic VLDL assembly and reduced secretion. It has been shown, however, that the inhibition of FFA release by niacin lasts only a few hours and is followed by a marked rebound in FFA release that is already detectable 4 hours after extended-release niacin administration 37, 38. In our study, a modest and non-significant increase in plasma FFA levels was observed approximately 9 hours after administration, consistent with a rebound phase. Previously, Wang et al. 38 have reported in normolipidemic women that the production of VLDL-TG was lowered by niacin, but the reduction was not fully explained by the effect of niacin on FFA levels. In vitro experiments have also suggested that niacin inhibits the activity of diglycerol acyl-transferase-2, the enzyme involved in TG synthesis in liver cells 39. In our study, niacin reduced the plasma concentration of TRL apoB-100. However, this reduction was not explained by TRL apoB-100 synthesis, but was mostly due to an almost significant increase in FCR. The same effect was observed for TRL apoB-48, where a significant increase in FCR was observed with niacin. This is consistent with the observation in one hypertriglyceridemic subject that autologous 125I-labeled VLDL underwent faster clearance after niacin treatment 9. The mechanism for the faster clearance of TRL is not clear. It is likely that it does not involve an increased expression of the LDL receptor, since niacin was not observed to affect the clearance of IDL and LDL apoB-100. Lipolysis may play a small role, as there was a slight trend toward an increased conversion of apoB-100 VLDL to IDL. The statin-induced effect on apoB-100 kinetics is consistent with several previous statin studies 14.
In conclusion, in male subjects with elevated TG and low HDL-C levels, extended-release niacin induces beneficial changes in lipid and lipoprotein levels. The increase in HDL-C levels achieved with extended-release niacin is mediated in part by an increased production of apoA-I, while the reduction in plasma TG levels is mostly mediated by an increased clearance of both hepatic and intestinal TRL.
Acknowledgments
Sources of Funding
This work was supported by an investigator-initiated research grant from KOS Pharmaceuticals to Dr. Ernst. Schaefer, and by the U.S. Department of Agriculture under agreement No. 58-1950-4-401. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture. Support was also provided by grant M01 RR00054 to Tufts Medical Center General Clinical Research Center, funded by the National Center for the Research Resources of the NIH. Dr. P. Hugh R. Barrett is a senior research fellow of the National Health and Medical Research Council of Australia and is supported in part by the NIH (National Institute of Biomedical Imaging and Bioengineering grant P41 EB-00195).
Footnotes
Disclosure
Dr. Ernst Schaefer has received grant support from KOS Pharmaceuticals, now part of Abbott, and from Abbott. He has been a consultant and in the Speakers’ Bureau for KOS Pharmaceutical and Abbott. Dr. Bela Asztalos has received grant support from Abbott.
Reference List
- 1.Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem. 1955;54:558–559. doi: 10.1016/0003-9861(55)90070-9. [DOI] [PubMed] [Google Scholar]
- 2.The Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. JAMA. 1975;231:360–381. [PubMed] [Google Scholar]
- 3.Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald WT. Fifteen year mortality in Coronary Drug Project patients: long-term benefits with niacin. J Am Coll Cardiol. 1986;8:1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 4.Brown G, Albers JJ, Fisher L, Schaefer SM, Lin JT, Kaplan C, Zhao XQ, Bisson BD, Fitzpatrick VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 5.Brown B, Zhao X, Chait A, Fisher L, Cheung M, Morse J, Dowdy A, Marino E, Bolson E, Alaupovic P, Frohlich J, Albers J. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 6.Taylor AJ, Sullenberger LE, Lee HJ, Lee J, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 7.Capuzzi DM, Guyton JR, Morgan J, Goldberg AC, Kreisberg RA, Brusco OA, Brody J. Efficacy and safety of an extended-release niacin (Niaspan): a long-term study. Am J Cardiol. 1998;82:74U–81U. doi: 10.1016/s0002-9149(98)00731-0. [DOI] [PubMed] [Google Scholar]
- 8.Carlson LA, Oro L. The effect of nicotinic acid on the plasma free fatty acids. Acta Med Scand. 1962;172:641–645. doi: 10.1111/j.0954-6820.1962.tb07203.x. [DOI] [PubMed] [Google Scholar]
- 9.Kushwaha RS, Haffner S, Foster DM, Hazzard WR. Compositional and metabolic heterogeneity of apha2 and beta-very-low-density lipoproteins in subjects with broad beta disease and endogenous hypertriglyceridemia. Metabolism. 1985;34:1029–38. doi: 10.1016/0026-0495(85)90075-7. [DOI] [PubMed] [Google Scholar]
- 10.Blum CB, Levy RI, Eisenberg S, Hall M, III, Goebel RH, Berman M. High density lipoprotein metabolism in man. J Clin Invest. 1977;60:795–807. doi: 10.1172/JCI108833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepherd J, Packard CJ, Patsch JR, Gotto AM, Taunton OD. Effects of nicotinic acid therapy on plasma high density lipoprotein subfraction distribution and composition and on apolipoprotein A metabolism. J Clin Invest. 1979;63:858–867. doi: 10.1172/JCI109385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Expert Panel. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 13.Cohn J, Wagner D, Cohn S, Millar J, Schaefer EJ. Measurement of very low density and low density lipoprotein apolipoprotein (apo) B-100 and high density lipoprotein apo A-I production in human subjects using deuterated leucine. Effects of fasting and feeding. J Clin Invest. 1990;85:804–811. doi: 10.1172/JCI114507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamon-Fava S, Diffenderfer M, Barrett PH, Buchsbaum A, Matthan N, Lichtenstein AH, Dolnikowski GG, Horvath KV, Asztalos BF, Zago V, Schaefer EJ. Effects of different doses of atorvastatin on human apolipoprotein B-100, B-48, and A-I metabolism. J Lipid Res. 2007;48:1746–1753. doi: 10.1194/jlr.M700067-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 16.Asztalos BF, Sloop CH, Wong L, Roheim PS. Two-dimensional electrophoresis of plasma lipoproteins: recognition of new apo A-I-containing subpopulations. Biochim Biophys Acta. 1993;1169:291–300. doi: 10.1016/0005-2760(93)90253-6. [DOI] [PubMed] [Google Scholar]
- 17.Miyauchi K, Kayahara N, Ishigami M, Kuwata H, Mori H, Sugiuchi H, Irie T, Tanaka A, Yamashita S, Yamamura T. Development of a homogeneous assay to measure remnant lipoprotein cholesterol. Clin Chem. 2007;53:2128–2135. doi: 10.1373/clinchem.2007.092296. [DOI] [PubMed] [Google Scholar]
- 18.Havel RJ, Eder H, Bragdon J. The distribution and chemical composition of ultracentrifugally separated lipoprotein in human serum. J Clin Invest. 1955;34:1345–1363. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dwyer KP, Barrett PH, Chan D, Foo JI, Watts GF, Croft KD. Oxazolinone derivative of leucine for GC-MS: a sensitive and robust method for stable isotope kinetic studies of lipoproteins. J Lipid Res. 2002;43:344–349. [PubMed] [Google Scholar]
- 20.Cobelli C, Toffolo G, Bier D, Nosadini R. Models to interpret kinetic data in stable isotope tracer studies. Am J Physiol. 1987;253:E551–E564. doi: 10.1152/ajpendo.1987.253.5.E551. [DOI] [PubMed] [Google Scholar]
- 21.Lamon-Fava S, Postfai B, Diffenderfer M, deLuca C, O’Connor J, Jr, Welty FK, Dolnikowski GG, Barrett P, Schaefer EJ. Role of the estrogen and progestin in hormonal replacement therapy on apolipoprotein A-I kinetics in postmenopausal women. Arterioscler Thromb Vasc Biol. 2006;26:385–391. doi: 10.1161/01.ATV.0000199248.53590.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parhofer K, Barrett P, Bier D, Schonfeld G. Determination of kinetic parameters od apolipoprotein B metabolism using amino acids labeled with stable isotopes. J Lipid Res. 1991;8:1311–1323. [PubMed] [Google Scholar]
- 23.Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta. 2002;324:61–71. doi: 10.1016/s0009-8981(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 24.Kamei T, Tuji N, Taketani K, Nakamoto M, Yamaguchi F, Sasaki A, Nabiki J, Ikeda M, Tabata N, Kudo T, Harano Y. Evaluation and clinical significance of latex immunoassay (LIA) of insulin using the routine biochemical autoanalyzer. Clin Chem (Japan) 2006;35:48–53. [Google Scholar]
- 25.Nishimura A, Sawai T. Determination of adiponectin in serum using a latex particle-enhanced turbidimetric immunoassay with an autoanalyzer. Clin Chim Acta. 2006;371:163–168. doi: 10.1016/j.cca.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Matthan N, Giovanni A, Schaefer EJ, Brown B, Lichtenstein AH. Impact of simvastatin, niacin, and/or antioxidants on cholesterol metabolism in CAD patients with low HDL. J Lipid Res. 2003;44:800–806. doi: 10.1194/jlr.M200439-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Kuvin JT, Dave DM, Sliney KA, Mooney P, Patel AR, Kimmelstiel CD, Karas R. Effects of extended-release niacin on lipoprotein particle size, distribution, and inflammatory markers in patients with coronary artery disease. Am J Cardiol. 2006;98:743–745. doi: 10.1016/j.amjcard.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Jin FY, Kamanna VS, Kashyap ML. Niacin decreases removal of high-density lipoprotein apolipoprotein A-I but not cholesterol ester by Hep G2 cells. Arterioscler Thromb Vasc Biol. 1997;17:2020–2028. doi: 10.1161/01.atv.17.10.2020. [DOI] [PubMed] [Google Scholar]
- 29.Marsh J, Welty FK, Schaefer EJ. Stable isotope turnover of apolipoproteins of high-density lipoproteins in humans. Curr Opin Lipidol. 2000;11:261–266. doi: 10.1097/00041433-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Osborne JC, Schaefer EJ, Powell GM, Lee NS, Zech LA. Molecular properties of radioiodinated apolipoprotein A-I. J Biol Chem. 1984;259:347–353. [PubMed] [Google Scholar]
- 31.Watt MJ, Southgate RJ, Holmes AG, Febbraio MA. Suppression of plasma free fatty acids upregulates peroxisome proliferator-activated receptor (PPAR) alpha and delta and PPAR coactivator 1alpha in human skeletal muscle, but not lipid regulatory genes. J Mol Endocrinol. 2004;33:533–544. doi: 10.1677/jme.1.01499. [DOI] [PubMed] [Google Scholar]
- 32.Lamon-Fava S, Micherone D. Regulation of apo A-I gene expression: mechanism of action of estrogen and genistein. J Lipid Res. 2004;45:106–112. doi: 10.1194/jlr.M300179-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Pandey NR, Renwick J, Misquith A, Sokoll K, Sparks DL. Linoleic acid-enriched phospholipids act through peroxisome proliferator-activated receptors alpha to stimulate hepatic apolipoprotein secretion. Biochemistry. 2008;47:1579–1587. doi: 10.1021/bi702148f. [DOI] [PubMed] [Google Scholar]
- 34.Asztalos BF, Batista M, Horvath KV, Cox CE, Dallal G, Morse JS, Brown GB, Schaefer EJ. Change in alpha1 HDL concentration predicts progression in coronary artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:847–582. doi: 10.1161/01.ATV.0000066133.32063.BB. [DOI] [PubMed] [Google Scholar]
- 35.Chan D, Watts GF, Nguyen MN, Barrett P. Factorial study of the effect of n-3 fatty acid supplementation and atorvastatin on the kinetics of HDL apolipoproteins A-I and A-II in men with abdominal obesity. Am J Clin Nutr. 2006;84:37–43. doi: 10.1093/ajcn/84.1.37. [DOI] [PubMed] [Google Scholar]
- 36.Carlson LA, Ostman J. Inhibition of the mobilization of free fatty acids form adipose tissue in diabetes. II. Effect of nicotinic acid and acetylsalicylate on blood glucose in human diabetics. Acta Med Scand. 1965;178:71–79. doi: 10.1111/j.0954-6820.1965.tb04252.x. [DOI] [PubMed] [Google Scholar]
- 37.Vega GL, Cater NB, Meguro S, Grundy SM. Influence of extended-release nicotinic acid on nonesterified fatty acid flux in the metabolic syndrome with atherogenic dyslipidemia. Am J Cardiol. 2005;95:1309–1313. doi: 10.1016/j.amjcard.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Basinger A, Neese RA, Shane B, Myong S-A, Christiansen M, Hellerstein MK. Effect of nicotinic acid administration on hepatic very low-desnity lipoprotein-triglyceride production. Am J Physiol Endocrinol Metab. 2001;43:E540–E547. doi: 10.1152/ajpendo.2001.280.3.E540. [DOI] [PubMed] [Google Scholar]
- 39.Ganji SH, Tavintharan S, Zhu D, Xing Y, Kamanna VS, Kashyap ML. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J Lipid Res. 2004;45:1835–1845. doi: 10.1194/jlr.M300403-JLR200. [DOI] [PubMed] [Google Scholar]


