Abstract
Cytokines that signal through receptor complexes containing the common γ (γC) chain receptor subunit are central regulators of lymphocyte homeostasis. In this review, we discuss the four major γC cytokines that have proven activity in or potential for immunotherapy: IL-2, IL-7, IL-15 and IL-21. Their shared and unique activities on specific lymphocyte populations suggest therapeutic applications such as enhancing lymphocyte reconstitution, expanding tumor and pathogen-specific lymphocytes, and optimizing vaccines. Because the responsiveness of individual lymphocyte subsets varies under different situations such as lymphopenia and active immune responses, understanding the dynamics of γC-containing receptor expression is important in deciding how to achieve the most desired effect. Current understanding of γC cytokine biology suggests several clinical applications, including their direct administration or use in generation of lymphocytes for adoptive transfer, increasing their endogenous production, and potentiating their activity by complex formation with specific antibodies or their specific receptor-α subunits. Overall, γC cytokines have great potential, through their targeted use alone or in combination, to be an integral part of clinical interventions with enhanced efficacy and decreased toxicity.
Keywords: common gamma chain, lymphocyte homeostasis, cytokine therapy, lymphopenia-induced proliferation, adoptive cell therapy, tumor-infiltrating lymphocytes, regulatory T cells, immune reconstitution, human immunodeficiency virus
Introduction
Cytokines are endogenous proteins that mediate communication between cells, particularly cells of the immune system. The γC family of cytokines consists of IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, all of which signal through specific receptor complexes sharing the common γ receptor chain (γC). As a result of this shared receptor usage, γC cytokines share similar functions while also each possessing unique features. Predominant functions relate to crucial regulation of lymphocyte development, homeostasis, and function, and therefore have the potential to enhance lymphocyte reconstitution, expand tumor and pathogen-specific lymphocytes, and optimize vaccines. The γC cytokine members currently of highest interest to clinical use are IL-2, IL-7, IL-15 and more recently IL-21. Clinical effects of treatment with these cytokines only partially reflect their normal endogenous function or activities in in vitro assays (Table 1). This review will summarize the current understanding of the normal function of these four cytokines, the cytokine receptor expression, their clinical use thus far, and the potential and limitations for future therapeutic applications.
Table 1. Specific in vitro and in vivo activities of γC cytokines in natural and therapeutic settings.
| IL-2 | IL-7 | IL-15 | IL-21 | |
|---|---|---|---|---|
In vitro activities
|
|
Maintains CD4 and CD8 T cell survival |
|
|
In vitro activities
|
|
Induces CD4 and CD8 T cell proliferation |
|
|
| In vivo activities |
|
|
|
|
| Therapeutic uses |
|
|
|
|
IL-2
Normal in vivo functions
IL-2 is the quintessential growth factor for T cells and theoretically would be ideal for expanding T cells due to its high specificity to T cells (Table 1). In vivo, CD4 helper T cells, and to a lesser extent CD8 T cells and DCs, are the source for IL-2, which is used mostly in an autocrine and paracrine manner as a growth factor for nearby T cells. IL-2 mediates its effects by binding to a heterotrimeric receptor complex composed of the IL-2Rα, IL-2Rβ, and γC subunits. Since IL-2 has a low affinity for the IL-2Rβ and γC heterodimeric complex, most in vivo responses require the presence of all three subunits. IL-2Rα (also designated CD25) lacks a cytoplasmic domain and does not contribute to IL-2 signaling; instead, its main function is to increase the affinity of the β/γC complex for IL-2 by approximately 100-fold [1]. Thus, the IL-2 binding specificity is dictated mostly by the expression of the IL-2Rα. IL-2Rα is expressed at a minimal level by naïve T cells and becomes transiently upregulated upon T cell activation, allowing a selective, IL-2 induced expansion of activated effector T cells. Since sustained signaling through the IL-2 receptor complex can result in apoptosis, the transient nature of IL-2Rα expression protects the T cells from this form of over-stimulation. Besides stimulating effector T cells, IL-2 also mediates the development and homeostasis of regulatory T cells (Tregs). Tregs constitutively express elevated levels of IL-2Rα compared to conventional T cells [2] (Figure 1). Accordingly, in a pool of unstimulated T cells, the Tregs are the most sensitive to IL-2 stimulation. Among IL-2Rα-negative cells, IL-2 preferentially stimulates cells with the highest IL-2Rβ expression, i.e. previously activated T cells or T cells with a memory phenotype (Figure 1).
Figure 1. Relationship between cytokine receptor expression and cytokine responsiveness among lymphocyte subsets.
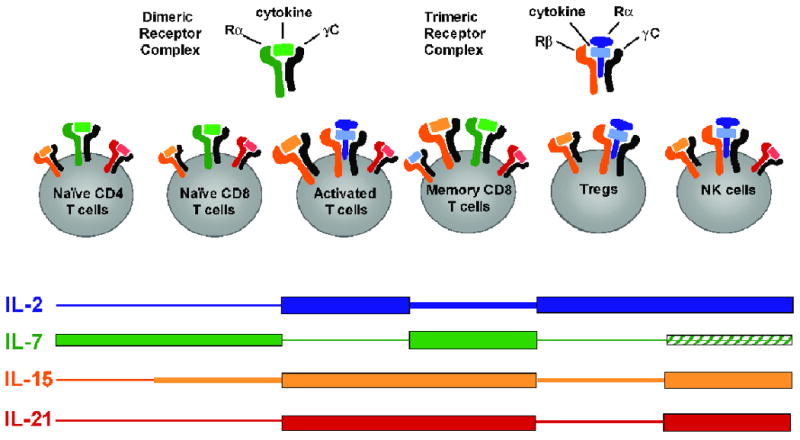
Schematic depiction of relative expression of γC cytokine receptor subunits with bound cytokine on various lymphocyte populations. Black subunits represents the γC, blue=IL-2Rα, green=IL-7Rα, orange=IL-2/15Rβ. Thickness of the colored bars indicates the cells' relative cytokine responsiveness. Size of the receptor complex indicates relative expression level. Hatched bar indicates that some subsets are cytokine responsive.
IL-2 use in clinical setting
HIV
Because of its proliferative effect on T cells, recombinant IL-2 has been tested in a number of clinical trials to increase CD4 T cell counts in HIV-induced CD4 T cell lymphopenia [3-6] (Table 2). Intermittent subcutaneous IL-2 treatment for 5 day cycles increased CD4 T cell counts in HIV patients, irrespective of concurrent highly active antiretroviral (HAART) therapy [4]. Importantly, IL-2 therapy appeared to confer clinical benefit by lowering opportunistic infections and extending patient survival[3,4]. Mechanistically, IL-2 in this setting increased both naïve and memory CD4 T cells, mostly through profound inhibition of their apoptosis [5]. In addition, IL-2 increased numbers of T cells with a classical Treg phenotype (CD4+CD45RO-CD25+foxP3+); however, these cells were not strongly suppressive in direct ex vivo assays, leaving their function in vivo an open question [6]. Currently, the clinical benefit of intermittent IL-2 for HIV patients with low CD4 T cell counts is being rigorously tested in phase III clinical trials.
Table 2. Selected human clinical trials with γC cytokines.
| Patient population |
# of patients |
cytokine | dose | concurrent treatments |
immunological effect of cytokine |
clinical outcome |
dose-limiting cytokine toxicity |
remarks | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| HIV infected | 35 | IL-2 | variable: 1-18 mIU daily for 5 days for up to 28 cyles over 6-7 years | none | ↑ CD4 T naïve/Tcm/“Treg” | not reported | not reported | ↑ “Treg” are foxP3int:IL-2- induced Teff? | [5,6] |
| HIV infected | 204 | IL-2 | 9 mIU (i.v.) daily or 7.5 mIU (s.c) twice daily for 5 days every 8 weeks up to 9 weeks | Highly Active Antiretroviral Therapy | ↑ CD4 T naïve and Teff/Tmem, ↔ CD8T | ↓ new AIDS-defining event in IL-2 treatment arms | nausea, rash, fatigue, fever | [87] | |
| melanoma | 182 | IL-2 | 2 cycles of 720,000 IU/kg (i.v.) every 8 hours up to 5 days | none | lymphocytosis | 6.6% CR, 8.2% PR | hypotension, mental status changes, renal impairment, oliguria, respiratory events | toxicities rapidly reversed | [88] |
| renal cell carcinoma | 227 | IL-2 | 2 cycles of 720,000 IU/kg (i.v.) every 8 hours up to 5 days | none | lymphocytosis | 9.3% CR, 9.7% PR | hypotension, mental status changes, renal impairment, oliguria, respiratory events | toxicities rapidly reversed | [7,88,89] |
| melanoma | 31 | IL-2 | 2 cycles of 720,000 IU/kg (i.v.) every 8 hours up to 5 days | gp100.209_2M peptide/IFA vaccination | ↓ vaccine- induced CD8 T | 3.2% CR, 39% PR | typical IL-2 toxicity; not specified | updated retrospective response rate for vaccine + IL-2 at 22% vs 13% for IL-2 alone | [7,19-21,88,89] |
| melanoma | 43 | IL-2 | 2 cycles of 720,000 IU/kg (i.v.) every 8 hours up to 5 days | lymphodepletio n regimen 1, adoptive T cell transfer | unclear; presumably proliferation/maintenance of transferred T cells | 9.3% CR, 40% PR | typical IL-2 toxicity; not specified | [90,91] | |
| melanoma | 25 | IL-2 | 2 cycles of 720,000 IU/kg (i.v.) every 8 hours up to 5 days | lymphodepletio n regimen 2, adoptive T cell transfer | unclear; presumably proliferation/maintenance of transferred T cells | 16% CR, 56% PR | typical IL-2 toxicity; not specified | [23] | |
| melanoma | 11 | IL-7 | 3-60 μg/kg (s.c.) every 3 days for 8 doses | gp100.209_2M and MART-1.26_2L peptide/IFA vaccination | ↑ CD4 and CD8T, ↓ Treg | no objective responses | none | no evidence of vaccine enhancement by IL-7 | [41] |
| non-hematolog ic, non-lymphoid cancer | 16 | IL-7 | 3-60 μg/kg (s.c.) every 2 days for 8 doses | none | ↑ naïve and Tcm but not Teff CD4 and CD8 T, ↓ Treg, broadened TCR repertoire | not reported: future publication | 1 hypertension, 1 ↑ aminotransferase | [42] | |
| melanoma | 29 | IL-21 | 1-100 μg/kg thrice weekly for 6 weeks or 3 cycles of daily for 5 days, 9 days rest | none | transient lymphopenia; ↑ soluble CD25 | 3.4% PR | ↑ aminotransferase, neutropenia, thrombocytopenia, lightheadedness, fatigue | [92,93] | |
| melanoma, renal cell carcinoma | 24 | IL-21 | 3-100 μg/kg days 1-5 and 15-19 (i.v); additional cycles optional | none | transient lymphopenia; ↑ soluble CD25, ↑ perforin-1 and granzyme B mRNA in CD8 T | 4.2% CR (melanoma); 21% PR (renal cell carcinoma) | “grade 3 laboratory abnormalities” | [94] |
Melanoma and Renal cancer
Early after its discovery as a T cell growth factor, IL-2 was hypothesized to boost the immune system's natural abilities to eliminate tumors. After promising results in animal models followed by clinical trials in a variety of cancers, IL-2 was approved for the treatment of metastatic melanoma and renal cancer (Table 2). High-dose IL-2 therapy results in a modest clinical response rate of 14% [7]. IL-2 therapy is of continued interests in part because about one-third of the clinical responses are complete, durable remissions. The reason for the selectivity of this response among patients is not known and emphasizes a need to better understand the mechanism underlying IL-2-induced tumor regression. Animal studies and some observations in patients point to a predominant role for CD8 T cells and possibly NK cells in IL-2-mediated tumor regression.
Despite its modest success, there are number of drawbacks in the use of IL-2 therapy, the most notable being its high toxicity. In particular, high dose IL-2 induces a vascular leak syndrome resulting in multiple organ edema, blood flow perturbations, and hypotension [8]. Unfortunately, less toxic lower-dose regimens resulted in lower response rates [9]. The mechanism for the vascular symptoms is not completely clear and may involve direct effects on endothelial cells [10-12]. In contrast, mouse studies have implicated IL-2-mediated effects on NK cells as a contributing factor [13]. Interestingly, while effects on T cells are believed to be important for IL-2 responses, T cells become less abundant in the peripheral circulation of patients receiving IL-2. This temporary T cell loss is thought to occur because IL-2-responsive cells leave the circulation and/or alternatively undergo activation-induced cell death [11,12,14]. IL-2 tends to drive CD8 T cells to a terminally differentiated effector stage, in which they possess a high capacity for cytolysis and effector cytokine secretion and preference for peripheral, (non-lymphoid) tissues, but also a greatly reduced capacity to proliferate and a high propensity towards apoptosis [15]. This is another drawback of IL-2 treatment, as these T cell characteristics are not ideal for long-term tumor control and protection against recurrence. Lastly, as in mice, IL-2 treatment promotes the accumulation of Tregs in humans [16,17].
While IL-2 treatment enhances the anti-tumor effects of endogenous CD8 T cells and NK cells, this ability of IL-2 has also been utilized to expand tumor-specific T cells induced by vaccination (Table 2). Indeed, since cancer vaccine-induced T cell responses in humans tend to be weak, members of the γC cytokine family have been investigated for their ability to enhance the generation of Ag-specific effector and memory cells in mouse models with some encouraging results [18]. Currently, IL-2 and IL-7 are the only γC cytokines yet tested in humans for this purpose. The findings on IL-2 were reported a decade ago in a study demonstrating that the addition of peptide-based vaccination against the melanoma antigen, gp100, doubled the clinical response rate to IL-2 therapy [19]. This finding was recently substantiated by a large retrospective study showing an increased response rate from 13 to 22% by the addition of gp100 vaccination to high-dose IL-2 therapy while gp100 vaccination by itself was essentially ineffective [20]. Interestingly, although that IL-2 treatment specifically lowered the number of vaccine-induced CD8 T cells in the circulation, it still synergized with peptide vaccination to induce tumor regression [19,21]. Overall, the modest success of IL-2 used in combination with vaccination has led to further studies. As such, a large randomized, prospective multi-center trial is almost completed to determine the exact clinical benefit of gp100 peptide vaccination as an addition to IL-2 therapy in metastatic melanoma.
Ex vivo uses
Adoptive cell therapy (ACT)
In addition to their in vivo use, cytokines can be used ex vivo to expand or differentiate specific immune cells. Ex vivo expansion can circumvent complex immunoregulatory mechanisms present in vivo and allows more precise control over the specific functions of the cells prepared for infusion. For this procedure, tumor-infiltrating lymphocytes (TIL) are isolated from a surgically removed melanoma lesion, grown short-term in the presence of IL-2, and then subjected to a rapid expansion with anti-CD3 antibody to mimic antigenic stimulation through the TCR, PBMC as feeder cells, and IL-2. This typically results in a 1000-fold expansion of the initial culture, yielding 10-100 billion cells. Meanwhile, the patient is conditioned with a short-term, non-myeloablative fludarabine/cyclophosphamide-based regimen to temporarily deplete endogenous lymphocytes. The cultured TIL are then infused and patients receive high-dose IL-2. In a cohort of 35 patients, this regimen resulted in a response rate of 51% [22] (Table 2). Considering that the only two currently FDA-approved treatments for metastatic melanoma, high dose IL-2 and the cytotoxic agent dacarbazine, both have a response rate of approximately 15%, the results with ACT have generated a strong interest in further improvement of this regimen.
A more recent study showed that the addition of whole-body irradiation to further reduce endogenous lymphocytes further increased response rates to 72% [23]. Lymphodepletion presumably enhances the response by removing cell that compete for γC cytokines that mediate homeostasis. The resultant increased level of γC cytokines actually induces a form of T cell proliferation called lymphopenia-induced proliferation (LIP), which is discussed in more detail below. Lymphodepletion, besides removing competition for cytokines, possibly functions by depleting Tregs that hinder the anti-tumor function of the transferred TIL, especially in a setting of concurrent IL-2 administration which promotes Treg proliferation. Due to this strong capacity to preferentially expand Tregs, IL-2 may not be the most favorable γC cytokine for the support of transferred T cells.
As mentioned above, another potential downside of IL-2 is its tendency to favor the generation of T cells with an effector memory phenotype. Although effector memory T cells have strong cytolytic effector function and tend to preferentially locate to non-lymphoid tissues including tumors, their more limited capacity for long-term persistence may prevent them from completely eliminating the tumor and providing long term immune surveillance against recurrence. This notion is supported by the observed correlation between persistence of transferred T cells and favorable clinical outcome [24]. Therefore, therapy generating a mixture of effectors with strong immediate anti-tumor activity along with more long-lived memory cells for continued tumor surveillance may be ideal, but it is currently unclear how best to generate such a mixture. It is clear, however, that various γC cytokines differentially induce and expand these different T cell subsets, offering an avenue for their specific therapeutic use in the generation of tailored T cell populations for adoptive transfer (Figure 1).
The ex vivo expansion of T cells for adoptive therapy is not limited to the use of tumor-specific effector T cells. Adoptive immunotherapy is also being employed to control infections, restore immunity, and treat autoimmunity. Ex vivo expanded, donor-derived CMV and EBV-specific CD8 T cells have successfully been used to help control established infections in patients receiving stem cell transplants during a time when patients are most susceptible to infections [25,26]; this strategy is also being attempted for HIV [27]. In attempts to restore CD4 T cell numbers in HIV, one study expanded patients' isolated CD4 T cells with IL-2 in the presence of antiviral agents followed by retroviral transduction with a gene that confers HIV resistance giving the CD4 T cells a selective growth advantage[28]. A year after adoptive transfer of these modified cells, CD4 T cell numbers were increased, correlating with the presence of gene-modified T cells. In many types of autoimmune conditions, the presence of functional Tregs inversely correlates with severity of disease; hence there is a great interest in increasing Treg numbers in vivo [29,30]. As with tumor-specific T cells, culture conditions for the optimal ex vivo expansion of functional, antigen-specific Tregs are incompletely defined. Currently, the attempts to expand human Tregs ex vivo have utilized IL-2 [31]. Determining whether these ex vivo generated Tregs have clinical efficacy in inhibiting autoimmunity and GVHD is ongoing.
IL-7
Normal functions in vivo
Under normal circumstances, IL-7 is primarily produced by stromal cells of the thymus and BM and mediates the development and homeostasis of naïve and memory T cells. As IL-7 is involved in the survival of very early thymocytes, its absence results in a profound T cell deficiency [32,33]. In the periphery, IL-7 promotes the survival of both naïve and memory T cells [34,35]. Therefore, IL-7 may be utilized to boost de novo T cell production during T cell deficiency caused by immune system disorders, diseases such as HIV infection, or clinical interventions such as chemotherapy (Table 1).
Under baseline conditions, the level of constitutively produced IL-7 is appropriate to maintain a normal pool of T cells; however, when T cell numbers decrease because of disease or treatment (i.e. infection with HIV and some other viruses, irradiation or chemotherapy) the amount of IL-7 available per T cell increases. This results in an enhanced survival signal through the IL-7R complex and, if sufficiently strong, also a proliferation signal. Therefore, T cells transferred into a lymphopenic environment undergo a unique type of proliferation called lymphopenia-induced proliferation (LIP) [36]. This is one of the few situations where naive T cells undergo proliferation in the absence of overt antigen stimulation. Actually, IL-7 likely lowers the threshold of T cell activation allowing their stimulation by self-antigen/MHC[37]. Along with this proliferation, the T cells acquire some phenotypical characteristics of activation and/or memory and thus are more responsive to antigens than naïve T cells. Thus, in addition to enhancing thymic T cell output, IL-7 can increase T cell numbers in a thymus-independent manner.
IL-7 is an attractive cytokine for immunotherapy as it acts on a broad array of T cells. Expression of IL-7Rα is similar among CD4 and CD8 T cells and among naive and memory T cells, with some reports showing slightly higher levels on memory T cells than on naive T cells [34,35,38] (Figure 1). Upon T cell activation, IL-7Rα is downregulated and then regained during the differentiation into memory T cells [34] (Figure 1). Since T cell death is a major event concurrent with the generation of memory T cells and IL-7 can enhance T cell survival, IL-7 treatment during this phase has been tested for its ability to over-ride T cell death and increase the generation of memory T cells. Whereas exogenous IL-7 can boost the generation of memory T cells, these effects are often short-lived, apparently due to an inability of IL-7 to alter the programming of events set in motion earlier in the immune response [39]. Similar to activated T cells, Tregs constitutively have decreased IL-7Rα and as such undergo minimal expansion in lymphopenic conditions or after IL-7 administration [40]. This may contribute to the increased incidence of autoimmune phenomena after disease or treatment-induced lymphopenia.
IL-7 use in clinical setting
Due to its function during thymic development and peripheral T cell homeostasis, IL-7 has potential to enhance T cell reconstitution, ACT, and vaccination efficacy. To date, only the effects of IL-7 in altering T cell populations in humans with complete immune systems have been studied; these results have been documented in two recent reports [41,42] (Table 2). In the first study, four cohorts of patients (3 each) were each given different doses of IL-7 every 3 days for 21 days [41]. Absolute numbers of CD4 and CD8 T cells increased in a dose-dependent manner over the treatment period, without significant changes in the numbers of B cells, NK cells, CD4+Foxp3+ Tregs, neutrophils, eosinophils, and basophils. At the highest dose, the expression of the IL-7Rα chain (CD127) on T cells tended to be decreased, likely because of previously described feedback downregulation [43]. B cell progenitor levels in bone marrow were increased but did not translate in increased peripheral B cell levels. Toxicity of IL-7 was mild, including transient fevers and mild erythema at the injection site.
The second study used a similar protocol and more extensively examined the effects of IL-7 on T cell subsets and TCR repertoire [42]. As T cell expansion during LIP favors T cells with an increased affinity to self-peptide [37], it was important to determine whether administration of IL-7 to patients skew the T cell repertoire towards self-reactivity. In addition, it was speculated that IL-7 might have a limited ability to boost T cell production from the thymus in an elderly population due to thymus atrophy. Interestingly, IL-7 treatment in humans induced a preferential expansion of naïve T cells resulting in a broader T cell repertoire than before treatment, and this effect was independent of age [42]. IL-7 administration increased both CD4+ and CD8 T cell counts at a similar rate, maintaining the CD4/CD8 T cell ratio. This increase correlated with an increase in T cell proliferation as well as increased levels of the anti-apoptotic bcl-2 protein [42]. There was a trend toward preferential increase in naïve (CD45RA+) T cells over memory (CD45RO+) T cells. These T cells were functional; in fact IL-7 treatment increased their capacity to proliferate upon in vitro stimulation by up to 10-fold. In this same study, a comparison was also done with IL-2 treatment. While IL-7 selectively promoted accumulation of CD8 T cells with a more naïve phenotype, IL-2 markedly boosted the absolute and relative number of Tregs and CD8 T cells with effector functions.
As a part of this study, the melanoma patients received a gp100 and MART-1 peptide vaccine along with the IL-7 treatment [41]. This showed no evidence of enhanced gp100 or MART-1-specific immunity, possibly because unlike IL-2, IL-7 favors the expansion of naïve CD8 T cells over effector CD8 T cells which downregulate IL-7Rα expression after activation by antigen [43].
While IL-7 had mild side effects, five out of 12 patients showed low-level induction of IL-7 specific antibodies [41], possibly due to the lack of glycosylation of this bacterially produced form of the IL-7 protein. The antibodies had low neutralizing activity in vitro and did not appear to inhibit endogenous IL-7 levels in the patients, as none of them experienced a post-treatment decrease in lymphocyte counts as might be expected if endogenous IL-7 were neutralized [41]. Nevertheless, a fully glycosylated IL-7 protein is currently being manufactured for use in future studies.
The ability of IL-7 to induce proliferation in normally non-proliferating T cells may also be of use in the treatment of HIV patients. One problem with HIV is its ability to remain sequestered inside T cells, which are normally not proliferating. Because it induces T cell proliferation, IL-7 also upregulates latent HIV replication [44]. Therefore, IL-7 induced virus purging by concurrent retroviral treatment may help reduce viral burden in these patients.
IL-7 may also be of use in the setting of ACT in cancer or viral disease. Here, the ability of IL-7 to maintain the survival of antigen-experienced, memory-type T cells may be of use in the later phase of ACT. Since re-expression of IL-7Rα does not occur until after cessation of TCR stimulation, allowing ex vivo expanded T cells to rest without TCR stimulation before their adoptive transfer may increase their responsiveness to endogenous or exogenous IL-7. The limited expansion of Tregs by IL-7 are a further argument for inducing lymphopenia prior to infusion of tumor-specific T cells and/or administration of IL-7 to support infused cells after ACT. Indeed, IL-7Rα expression on adoptively transferred, melanoma-specific CD8 T cells directly correlated with their ability to proliferate and persist in vivo in an animal model of ACT (Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8 T cells [45].
IL-15
Normal in vivo functions
IL-15 shares many functions with IL-2, largely because both cytokines signal through two of the same receptor subunits, i.e. IL-2/15Rβ, and γC. Despite the sharing of two receptor subunits, IL-15 has distinct functions in vivo. In mouse studies, IL-15 has been shown to be important for the development and homeostasis of memory CD8 T cells, NK cells, NK T cells, and certain subsets of intestinal CD8 T cells [46,47]. Cell proliferation and survival induced by IL-15 are major components by which IL-15 mediates it developmental and homeostatic effects. The activities of IL-2 and IL-15 appear more similar in vitro than they are in vivo due to the nature of the culture conditions used, including cytokine concentrations, use of homogenous cell populations, and overriding mechanisms that confer cytokine specificity in vivo. Similar to IL-2Rα, the IL-15Rα contributes if any signals associated with IL-15-mediated functions and instead mainly functions to confer specificity [48]. Unlike IL-2Rα, the IL-15Rα has a very high affinity for IL-15 that is not further increased in the presence of the IL-2/15Rβ/γC complex. The affinity of IL-15 for the IL-2/15Rβ/γC complex is moderate and allows IL-15 to signal through this heterodimeric complex in the absence of IL-15Rα. Whereas this can occur in vitro by stimulating with soluble IL-15, it likely does not normally occur in vivo as soluble IL-15 is rarely found in the circulation or tissue fluids.
Whether IL-15 utilizes the three receptor subunits as a heterotrimeric complex is questionable. It now appears that IL-15Rα binds and then presents or delivers IL-15 to target cells, a mechanism called trans-presentation [49]. A trans-presenting cell expresses both IL-15 and IL-15Rα, which associates intracellulary and are shuttled to the cell surface where the complex stimulates neighboring cells through their IL-2/15Rβ/γC receptor complex. Therefore, due to the high affinity of IL-15Rα for IL-15, soluble IL-15 given in vivo would likely be bound up by empty IL-15Rα that could then be delivered via trans-presentation. Because of this unique mechanism utilized by IL-15, the expression of IL-15Rα does not correlate to cell responsiveness and rather is an indicator of a cell capable of presenting IL-15 to neighboring cells [50-52]. As IL-15 signals are mediated through the IL-2/15Rβ/γC complex, the expression level of IL-2/15Rβ is a good indicator of responsiveness to IL-15 [53] (Figure 1). All lymphocytes express both the IL-2/15Rβ and γC, but CD8 T cells, particularly memory CD8 T cells, NKT cells, and NK cells express the highest levels of IL-2/15Rβ chain making these cells particularly sensitive to IL-15 [53] (Figure 1).
As the model of trans-presentation would imply, coordinate expression of both IL-15 and IL-15Rα is required for a cell to trans-present IL-15 and this appears to be true based on functional studies using mouse models [54,55]. Since both the amount of IL-15 produced by cells and the reagents available for its detection are limited, the precise identity of the cells providing IL-15 in vivo remains under investigation. Many cell types express IL-15 transcripts, but detection of protein has been difficult. This difficulty relates to both the tight regulation of IL-15 protein production along with the fact that the predominant form of IL-15 is bound to cell surface IL-15Rα [49,56]. As IL-15 impacts lymphocyte development and homeostasis during the steady state, IL-15 protein is probably constitutively produced at low levels, similar to IL-7. Upregulation of IL-15 mRNA and protein is induced by many pathogens via TLR stimulation and type I IFNs in DCs, monocytes, and macrophages [53,57,58]. As a consequence, these are also the cell types thought to be the main producers of IL-15.
In vivo treatment
To date, in vivo studies of IL-15 have been limited to animals (Table 1). This may seem surprising considering human IL-15 was cloned in 1994 [59] and has shown strong therapeutic activity in multiple settings in animal models of human disease including cancer [60]. The lack of human trails is partially due to difficulties in producing sufficient quantities of IL-15 protein and to restricted commercial licensing [61]. In mice, in vivo treatment with rIL-15 has many of the same effects as rIL-2 in that it induces the proliferation of T cells, with a preference towards memory CD8 T cells and NK cells (Table 1). Although similar to IL-2 in stimulating CD8 T cells, IL-15 could have advantages as it is predicted not to boost Treg levels as observed for IL-2 (Table1). In addition, although the half-life of IL-15 in the circulation is shorter than that of IL-2, the functional activities of IL-15 in vivo may be more sustained because it is stabilized upon binding to IL-15Rα and thus maintained on the cell surface. Once production and licensing hurdles are overcome, treatment with IL-15 may be used similar to IL-2 with possibly increased efficacy and reduced side effects. Indeed, rIL-15 is currently being produced at the NCI for the purpose of human clinical trials [62].
IL-21
IL-21 is another immunomodulator in the γC family primarily produced by CD4 T cells, particularly TH17 CD4 T cells and NKT cells. IL-21 signals through a heterodimeric complex that is composed of the IL-21R and the γC[63]. IL-21R is present on many cells types such as CD4 T cells, CD8 T cells, NK cells, B cells, DC, macrophages and epithelial cells [63,64] and its expression tends to be upregulated upon activation (Figure 1). It does not appear to play a major role in the development of immune cells as IL-21R deficient mice do not display major immune cell deficiencies. They do, however, have reduced levels of IgG1 and IgE [65], highlighting its ability to modulate B cell functions. In general, IL-21 appears to primarily modulate the function of mature lymphocytes. For CD8 T cells, IL-21 has little effect on in vitro T cell proliferation, but can synergize with IL-15 or IL-7 [61,66]. The synergistic effect of IL-21 with other γC cytokines has also been observed with other cell types and highlights the limited understanding of the underlying mechanism. IL-21 is unusual in that it has been reported to have biphasic effects. For example, at low doses it promotes, but a high doses inhibits NK cell proliferation [67].
Although IL-21 can act on many immune cells, the predominant effect of IL-21 administration is a potent anti-tumor effect mediated by NK cells and CD8 T cells (Table 1). Interestingly, in one study, the anti-tumor effects of IL-21 were predominately mediated through NK cells while in other studies, the effect was mediated by CD8 T cells [39,68-71]. These discrepancies could be due to differences in cytokine delivery and/or particular tumor type and/or its localization. In studies comparing the anti-tumor effects of IL-2, IL-15, and IL-21, IL-21 had the most dramatic effect in enhancing animal survival. Although IL-21 treatment predominately affects CD8 T cells and NK cells, the recently recognized ability of IL-21 to skew T helper responses toward inflammatory TH17 will surely be an important consideration for future clinical trials. In multiple studies, the differentiation of TH17 cells over Tregs promoted by IL-21 has been implicated in inflammatory diseases such as Systemic Lupus Erythematosus, Multiple Sclerosis, Celiac disease, Crohn's disease, and type I Diabetes [72,73].
There are currently 2 published reports on the clinical use of rIL-21: a phase I study in melanoma patients and one in metastatic melanoma and renal cell carcinoma patients [74,75]. Both reports show some efficacy in both melanoma (1/24 complete response and 1/29 partial response) and renal cell carcinoma (4/19 partial response). The most prominent toxicities were flu-like symptoms and rash similar to those observed in patients treated with other cytokines such as rIL-2, rIL-12 and rIL-18 [7,76-78]. A transient lymphopenia was also common similar to what has been observed in human studies using IL-2 and mouse studies using IL-15. Altogether, these results suggest that the highest dose of rIL-21 (30 μg/kg) is well-tolerated and has some anti-tumor activity in melanoma and renal cell carcinoma. The overall response rate in this population was slightly lower than the ∼15% response rate routinely observed with high-dose rIL-2[7,9]. Considering these were phase I dose-escalation trials and most patients received a suboptimal dose of rIL-21, the response rate may increase as more patients are treated with higher doses.
Complexed cytokines and cytokine agonists
During investigations dissecting the mechanism of IL-15 trans-presentation, it was discovered that the complex of IL-15 bound to the IL-15Rα induced a robust proliferation in CD8 T cells, NK cells, and NKT cells. In addition, it induced naïve CD8 T cell activation, effector differentiation, and memory development [79,80]. In addition to inducing a surge in CD8 T cells and NK cells, IL-15/IL-15Rα complexes promoted tumor destruction by boosting the proliferation of tumor-resident CD8 T cells [80]. Increasing doses of free IL-15 could only induce a fraction of the response that the complexed IL-15 induced, likely due to increased stability of the complexed cytokine. In addition, compared to free IL-15, the IL-15/IL-15Rα complex has a 20-fold higher affinity for the IL-2/15Rβ/γC complex [81]. While the effect was most dramatic for IL-15, enhanced in vivo activity of IL-2 and IL-7 on T cell proliferation was also observed when these cytokines were allowed to form complexes to their respective Rα subunits (K.S. Schluns, T. Stoklasek, and L. Lefrancois, unpublished observations). These findings open up a new avenue of exploration that may yield improved cytokine reagents that may achieve an enhanced response with a reduced amount of cytokine.
Similarly, antibodies that form complexes with cytokines have been shown to enhance cytokine activity. Treatment with anti-IL-2 antibodies in vivo was found to increase proliferation of memory-phenotype CD8 T cells. While originally it was believed that this paradoxical effect was due to IL-2 deprivation of Tregs, it was more recently found that the antibody binds endogenous IL-2, forming a cytokine/antibody complex that directly stimulates CD8 T cells and NK cells. Specifically, a 100-fold increase in CD8 T cells and a 20-fold increase in NK cells was observed [82]. While the complex preferentially stimulated T cells with a memory-phenotype, naïve CD8 T cells also responded by becoming activated, obtaining effector functions, and differentiating into memory T cells. As this effect on CD8 T cells was similar to that observed with IL-15/IL-15Rα complex [80,83], it suggests a comparable mechanism of stimulation. The increase in effector function was relevant as the CD8 T cells provided protection against Listeria infection [83] and enhanced the numbers of vaccine-induced CD8 T cells [84]. Interestingly, not all IL-2 antibodies had this stimulatory effect on CD8 T cells and NK cells: one specific anti-IL-2 antibody instead stimulated Tregs. This particular antibody is thought form a complex that preferentially promotes binding of IL-2 to the IL-2Rα/β/γC complex, favoring CD25hi Tregs, while the other IL-2 antibodies promote binding to the IL-2Rβ/γC complex independent of the IL-2Rα, favoring non-Tregs. As such, depending on the binding specificity, IL-2 antibodies appear to utilize endogenous IL-2 to form a complex that can behave as either a more potent version of IL-2 or as IL-15.
An IL-7 antibody that forms complexes with IL-7 has also been identified; these complexes were shown to have a 100-fold increased activity compared to free IL-7 [85]. In vivo, this complex increased thymopoeisis homeostatic proliferation of both naïve and memory CD4 and CD8 T cells [85]. The observation that three of the γC cytokines have increased activity upon complexing implies that this phenomenon may also occur upon complexing other γC cytokines or even other non-γC cytokines. The finding that γC cytokines complexed to receptor subunits or specific antibodies enhances the activity of these proteins can surely benefit immunotherapy by reducing cytokine production burden and/or enhancing endogenous cytokine responses.
Optimizing the clinical use of specific cytokines
Understanding the normal functions of the γC cytokine members and the relative responsiveness to them of specific cell types (Figure 1, Table 1), allows the rational design of better cytokine-based immunotherapies. While there is some overlap in functions among the γC cytokines, therapeutic fine-tuning may be achieved by choosing the appropriate cytokines based on knowledge of cytokine receptor expression, the timing of cytokine delivery, and the use of cytokine combinations. For instance, if the goal is to generate anti-tumor responses, stimulation of CD8 T cells and NK cells with IL-15 or IL-15/IL-15Rα complexes may be the best choice. To reconstitute lymphopenic patients after chemotherapy or HIV infection, IL-7 therapy would seem ideal as it preferentially promotes and supports urgently needed naïve CD4 and CD8 T cells. Furthermore, the addition of IL-2 to that regiment could also restore Tregs, thus reducing the chance of developing autoimmunity-like conditions although possibly also interfering with immune-mediated viral control. As IL-21 tends to have synergistic effects with most other γC cytokines, its addition could potentiate a γC cytokine treatment. These are examples of how combinations of cytokines may provide additional benefits over monotherapy. Optimal timing of cytokine administration is particularly relevant when the activation of T cells is involved as expression of individual cytokine receptors changes over the course of T cell differentiation. While IL-2 clearly enhances the expansion of T cells, continued stimulation with IL-2 is counter-productive as it can induce activation-induced cell death [86]. While Ag and IL-2 are used to expand Ag-specific T cells, it is becoming increasingly clear that a rest from Ag stimulation is important to prevent exhaustion and allow differentiation into memory-like T cells that have greatly enhanced capacity to proliferate and survive. Thus, after expansion of the desired population of T cells, they could be maintained by a secondary in vitro stimulation with IL-7 and/or IL-15 that would help promote the survival of the T cell clones by promoting their differentiation into long-lived memory cells.
In considering cytokine therapies, it is also important to take into consideration how cytokine responses differ in specific environments. Interestingly, IL-7 administration is most likely to work in an IL-7 deficient environment. Studies have shown that administration of supraphysiological levels of IL-7 in steady state conditions has little impact on thymopoiesis or enhancing Ag-specific memory T cells despite the expression of IL-7Rα by T cells; this indicates that other homeostatic mechanisms must be in place. The advantages of IL-15 and IL-2 lie in their ability to preferentially enhance previously-activated CD8 T cells and NK cell numbers that can provide effective anti-tumor responses. However, one should keep in mind that since IL-2/15Rβ is expressed at similar levels on activated and memory CD8 T cells, T cells targeted in vaccine regiments or stimulated for ACT will have no competitive advantages over endogenous memory-phenotype CD8 T cells and NK cells. Other manipulations may be needed to enhance their competition.
The last few years have seen a great increase in our understanding of the specific properties of the members of the γC cytokine family, including their the expression patterns of their specific receptors and their differential in vivo activities on specific immune cell subsets. Altogether, these insights make clear that the γC cytokines should not be considered as largely redundant factors, but as individual potential therapeutics with specific clinical applications. Now that IL-2, IL-7, and IL-21 have been proven clinically safe and biologically-active, there is great opportunity, through their targeted use alone or in combination, to implement immunotherapies with enhanced efficacy and decreased toxicity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Boyman O, Surh CD, Sprent J. Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Expert Opin Biol Ther. 2006;6:1323–1331. doi: 10.1517/14712598.6.12.1323. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emery S, Capra WB, Cooper DA, Mitsuyasu RT, Kovacs JA, Vig P, Smolskis M, Saravolatz LD, Lane HC, Fyfe GA, Curtin PT. Pooled analysis of 3 randomized, controlled trials of interleukin-2 therapy in adult human immunodeficiency virus type 1 disease. J Infect Dis. 2000;182:428–434. doi: 10.1086/315736. [DOI] [PubMed] [Google Scholar]
- 4.Marchetti G, Meroni L, Varchetta S, Terzieva V, Bandera A, Manganaro D, Molteni C, Trabattoni D, Fossati S, Clerici M, Galli M, Moroni M, Franzetti F, Gori A. Low-dose prolonged intermittent interleukin-2 adjuvant therapy: results of a randomized trial among human immunodeficiency virus-positive patients with advanced immune impairment. J Infect Dis. 2002;186:606–616. doi: 10.1086/342479. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs JA, Lempicki RA, Sidorov IA, Adelsberger JW, Sereti I, Sachau W, Kelly G, Metcalf JA, Davey RT, Jr, Falloon J, Polis MA, Tavel J, Stevens R, Lambert L, Hosack DA, Bosche M, Issaq HJ, Fox SD, Leitman S, Baseler MW, Masur H, Di Mascio M, Dimitrov DS, Lane HC. Induction of prolonged survival of CD4+ T lymphocytes by intermittent IL-2 therapy in HIV-infected patients. J Clin Invest. 2005;115:2139–2148. doi: 10.1172/JCI23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sereti I, Imamichi H, Natarajan V, Imamichi T, Ramchandani MS, Badralmaa Y, Berg SC, Metcalf JA, Hahn BK, Shen JM, Powers A, Davey RT, Kovacs JA, Shevach EM, Lane HC. In vivo expansion of CD4CD45RO-CD25 T cells expressing foxP3 in IL-2-treated HIV-infected patients. J Clin Invest. 2005;115:1839–1847. doi: 10.1172/JCI24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 8.Rosenstein M, Ettinghausen SE, Rosenberg SA. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986;137:1735–1742. [PubMed] [Google Scholar]
- 9.Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, Liewehr DJ, Merino MJ, Rosenberg SA. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotran RS, Pober JS, Gimbrone MA, Jr, Springer TA, Wiebke EA, Gaspari AA, Rosenberg SA, Lotze MT. Endothelial activation during interleukin 2 immunotherapy. A possible mechanism for the vascular leak syndrome. J Immunol. 1988;140:1883–1888. [PubMed] [Google Scholar]
- 11.Kornfeld H, Berman JS, Beer DJ, Center DM. Induction of human T lymphocyte motility by interleukin 2. J Immunol. 1985;134:3887–3890. [PubMed] [Google Scholar]
- 12.Vyth-Dreese FA, Dellemijn TA, Frijhoff A, van KY, Figdor CG. Role of LFA-1/ICAM-1 in interleukin-2-stimulated lymphocyte proliferation. Eur J Immunol. 1993;23:3292–3299. doi: 10.1002/eji.1830231235. [DOI] [PubMed] [Google Scholar]
- 13.Gately MK, Anderson TD, Hayes TJ. Role of asialo-GM1-positive lymphoid cells in mediating the toxic effects of recombinant IL-2 in mice. J Immunol. 1988;141:189–200. [PubMed] [Google Scholar]
- 14.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 15.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, Long LM, Bernstein D, Hill BJ, Douek DC, Berzofsky JA, Carter CS, Read EJ, Helman LJ, Mackall CL. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 17.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, Wunderlich JR, Parkhurst MR, Kawakami Y, Seipp CA, Einhorn JH, White DE. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, White DE, Steinberg SM, Rosenberg SA. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14:5610–5618. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Sznol M, Schwarz SL, Spiess PJ, Wunderlich JR, Seipp CA, Einhorn JH, Rogers-Freezer L, White DE. Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J Immunol. 1999;163:1690–1695. [PMC free article] [PubMed] [Google Scholar]
- 22.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 26.Bollard CM, Kuehnle I, Leen A, Rooney CM, Heslop HE. Adoptive immunotherapy for posttransplantation viral infections. Biol Blood Marrow Transplant. 2004;10:143–155. doi: 10.1016/j.bbmt.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Joseph A, Zheng JH, Follenzi A, Dilorenzo T, Sango K, Hyman J, Chen K, Piechocka-Trocha A, Brander C, Hooijberg E, Vignali DA, Walker BD, Goldstein H. Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo HIV-1-specific inhibitory activity. J Virol. 2008;82:3078–3089. doi: 10.1128/JVI.01812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Lunzen J, Glaunsinger T, Stahmer I, von B, V, Baum C, Schilz A, Kuehlcke K, Naundorf S, Martinius H, Hermann F, Giroglou T, Newrzela S, Muller I, Brauer F, Brandenburg G, Alexandrov A, von Laer D. Transfer of autologous gene-modified T cells in HIV-infected patients with advanced immunodeficiency and drug-resistant virus. Mol Ther. 2007;15:1024–1033. doi: 10.1038/mt.sj.6300124. [DOI] [PubMed] [Google Scholar]
- 29.Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells--ex vivo expansion and therapeutic potential. Semin Immunol. 2006;18:103–110. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 31.Allan SE, Broady R, Gregori S, Himmel ME, Locke N, Roncarolo MG, Bacchetta R, Levings MK. CD4+ T-regulatory cells: toward therapy for human diseases. Immunol Rev. 2008;223:391–421. doi: 10.1111/j.1600-065X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 32.Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nature Immunology. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 35.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rocha B, Dautigny N, Pereira P. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur J Immunol. 1989;19:905–911. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- 37.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swainson L, Verhoeyen E, Cosset FL, Taylor N. IL-7R alpha gene expression is inversely correlated with cell cycle progression in IL-7-stimulated T lymphocytes. J Immunol. 2006;176:6702–6708. doi: 10.4049/jimmunol.176.11.6702. [DOI] [PubMed] [Google Scholar]
- 39.Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol. 2004;172:2048–2058. doi: 10.4049/jimmunol.172.4.2048. [DOI] [PubMed] [Google Scholar]
- 40.Banham AH. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3(+) regulatory T cells. Trends Immunol. 2006;27:541–544. doi: 10.1016/j.it.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, Buffet R, Mackall CL, Gress RE. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 44.Smithgall MD, Wong JG, Critchett KE, Haffar OK. IL-7 up-regulates HIV-1 replication in naturally infected peripheral blood mononuclear cells. J Immunol. 1996;156:2324–2330. [PubMed] [Google Scholar]
- 45.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 48.Anderson DM, Kumaki S, Ahdieh M, Bertles J, Tometsko M, Loomis A, Giri J, Copeland NG, Gilbert DJ, Jenkins NA. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15RA and IL2RA genes. J Biol Chem. 1995;270:29862–29869. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 49.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 50.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5616–5621. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schluns KS, Klonowski KD, Lefrancois L. Transregulation of memory CD8 T-cell proliferation by IL-15R alpha(+) bone marrow-derived cells. Blood. 2004;103:988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 52.Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, Boone DL. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proc Natl Acad Sci USA. 2003;100:4724–4729. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 54.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate Expression and Trans Presentation of Interleukin (IL)-15R{alpha} and IL-15 Supports Natural Killer Cell and Memory CD8+ T Cell Homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J Immunol. 2004;173:6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 56.Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J Immunol. 1998;160:4418–4426. [PubMed] [Google Scholar]
- 57.Neely GG, Robbins SM, Amankwah EK, Epelman S, Wong H, Spurrell JC, Jandu KK, Zhu W, Fogg DK, Brown CB, Mody CH. Lipopolysaccharide-stimulated or granulocyte-macrophage colony-stimulating factor-stimulated monocytes rapidly express biologically active IL-15 on their cell surface independent of new protein synthesis. J Immunol. 2001;167:5011–5017. doi: 10.4049/jimmunol.167.9.5011. [DOI] [PubMed] [Google Scholar]
- 58.Musso T, Calosso L, Zucca M, Millesimo M, Ravarino D, Giovarelli M, Malavasi F, Ponzi AN, Paus R, Bulfone-Paus S. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 1999;93:3531–3539. [PubMed] [Google Scholar]
- 59.Anderson DM, Johnson L, Glaccum MB, Copeland NG, Gilbert DJ, Jenkins NA, Valentine V, Kirstein MN, Shapiro DN, Morris SW. Chromosomal assignment and genomic structure of Il15. Genomics. 1995;25:701–706. doi: 10.1016/0888-7543(95)80013-c. [DOI] [PubMed] [Google Scholar]
- 60.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenberg SA. Secrecy in medical research. N Engl J Med. 1996;334:392–394. doi: 10.1056/NEJM199602083340610. [DOI] [PubMed] [Google Scholar]
- 62.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 63.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci USA. 2000;97:11439–11444. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 65.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, III, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 66.Liu S, Lizee G, Lou Y, Liu C, Overwijk WW, Wang G, Hwu P. IL-21 synergizes with IL-7 to augment expansion and anti-tumor function of cytotoxic T cells. Int Immunol. 2007;19:1213–1221. doi: 10.1093/intimm/dxm093. [DOI] [PubMed] [Google Scholar]
- 67.Toomey JA, Gays F, Foster D, Brooks CG. Cytokine requirements for the growth and development of mouse NK cells in vitro. J Leukoc Biol. 2003;74:233–242. doi: 10.1189/jlb.0303097. [DOI] [PubMed] [Google Scholar]
- 68.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang G, Tschoi M, Spolski R, Lou Y, Ozaki K, Feng C, Kim G, Leonard WJ, Hwu P. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016–9022. [PubMed] [Google Scholar]
- 70.Ma HL, Whitters MJ, Konz RF, Senices M, Young DA, Grusby MJ, Collins M, Dunussi-Joannopoulos K. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J Immunol. 2003;171:608–615. doi: 10.4049/jimmunol.171.2.608. [DOI] [PubMed] [Google Scholar]
- 71.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 72.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 73.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gamma delta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172:4151–4158. doi: 10.4049/jimmunol.172.7.4151. [DOI] [PubMed] [Google Scholar]
- 75.Tsuchiya T, Fukuda S, Hamada H, Nakamura A, Kohama Y, Ishikawa H, Tsujikawa K, Yamamoto H. Role of gamma delta T cells in the inflammatory response of experimental colitis mice. J Immunol. 2003;171:5507–5513. doi: 10.4049/jimmunol.171.10.5507. [DOI] [PubMed] [Google Scholar]
- 76.Tesselaar K, Xiao Y, Arens R, van Schijndel GM, Schuurhuis DH, Mebius RE, Borst J, van Lier RA. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J Immunol. 2003;170:33–40. doi: 10.4049/jimmunol.170.1.33. [DOI] [PubMed] [Google Scholar]
- 77.Robertson MJ, Kirkwood JM, Logan TF, Koch KM, Kathman S, Kirby LC, Bell WN, Thurmond LM, Weisenbach J, Dar MM. A dose-escalation study of recombinant human interleukin-18 using two different schedules of administration in patients with cancer. Clin Cancer Res. 2008;14:3462–3469. doi: 10.1158/1078-0432.CCR-07-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robertson MJ, Mier JW, Logan T, Atkins M, Koon H, Koch KM, Kathman S, Pandite LN, Oei C, Kirby LC, Jewell RC, Bell WN, Thurmond LM, Weisenbach J, Roberts S, Dar MM. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer. Clin Cancer Res. 2006;12:4265–4273. doi: 10.1158/1078-0432.CCR-06-0121. [DOI] [PubMed] [Google Scholar]
- 79.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci USA. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, Plet A, Jacques Y. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 × IL-15R alpha fusion proteins. J Biol Chem. 2006;281:1612–1619. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 82.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 83.Kamimura D, Bevan MJ. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J Exp Med. 2007;204:1803–1812. doi: 10.1084/jem.20070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mostbock S, Lutsiak ME, Milenic DE, Baidoo K, Schlom J, Sabzevari H. IL-2/anti-IL-2 antibody complex enhances vaccine-mediated antigen-specific CD8(+) T cell responses and increases the ratio of effector/memory CD8(+) T cells to regulatory T cells. J Immunol. 2008;180:5118–5129. doi: 10.4049/jimmunol.180.7.5118. [DOI] [PubMed] [Google Scholar]
- 85.Boyman O, Ramsey C, Kim DM, Sprent J, Surh CD. IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T Cell expansion without lymphopenia. J Immunol. 2008;180:7265–7275. doi: 10.4049/jimmunol.180.11.7265. [DOI] [PubMed] [Google Scholar]
- 86.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 87.Mitsuyasu R, Gelman R, Cherng DW, Landay A, Fahey J, Reichman R, Erice A, Bucy RP, Kilby JM, Lederman MM, Hamilton CD, Lertora J, White BL, Tebas P, Duliege AM, Pollard RB. The virologic, immunologic, and clinical effects of interleukin 2 with potent antiretroviral therapy in patients with moderately advanced human immunodeficiency virus infection: a randomized controlled clinical trial--AIDS Clinical Trials Group 328. Arch Intern Med. 2007;167:597–605. doi: 10.1001/archinte.167.6.597. [DOI] [PubMed] [Google Scholar]
- 88.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–319. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fyfe GA, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1996;14:2410–2411. doi: 10.1200/JCO.1996.14.8.2410. [DOI] [PubMed] [Google Scholar]
- 90.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci USA. 2004;101 2:14639–14645. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frederiksen KS, Lundsgaard D, Freeman JA, Hughes SD, Holm TL, Skrumsager BK, Petri A, Hansen LT, McArthur GA, Davis ID, Skak K. IL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1439–1449. doi: 10.1007/s00262-008-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davis ID, Skrumsager BK, Cebon J, Nicholaou T, Barlow JW, Moller NP, Skak K, Lundsgaard D, Frederiksen KS, Thygesen P, McArthur GA. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res. 2007;13:3630–3636. doi: 10.1158/1078-0432.CCR-07-0410. [DOI] [PubMed] [Google Scholar]
- 94.Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, Sievers EL, Hughes SD, DeVries TA, Hausman DF. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26:2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]


