Abstract
The glutamate receptor-associated protein Homer2 regulates alcohol-induced neuroplasticity within the nucleus accumbens (NAC), but the precise intracellular signaling cascades involved are not known. This study examined the role for NAC metabotropic glutamate receptor (mGluR)–Homer2–phosphatidylinositol 3-kinase (PI3K) signaling in regulating excessive alcohol consumption within the context of the scheduled high alcohol consumption (SHAC) model of binge alcohol drinking. Repeated bouts of binge drinking (∼1.5 g/kg per 30 min) elevated NAC Homer2a/b expression and increased PI3K activity in this region. Virus-mediated knockdown of NAC Homer2b expression attenuated alcohol intake, as did an intra-NAC infusion of the mGluR5 antagonist MPEP [2-methyl-6-(phenylethynyl)pyridine hydrochloride] (0.1–1 μg/side) and the PI3K antagonist wortmannin (50 ng/side), supporting necessary roles for mGluR5/Homer2/PI3K in binge alcohol drinking. Moreover, when compared with wild-type littermates, transgenic mice with an F1128R point mutation in mGluR5 that markedly reduces Homer binding exhibited a 50% reduction in binge alcohol drinking, which was related to reduced NAC basal PI3K activity. Consistent with the hypothesis that mGluR5–Homer–PI3K signaling may be a mechanism governing excessive alcohol intake, the “anti-binge” effects of MPEP and wortmannin were not additive, nor were they observed in the mGluR5F1128R transgenic mice. Finally, mice genetically selected for a high versus low SHAC phenotype differed in NAC mGluR, Homer2, and PI3K activity, consistent with the hypothesis that augmented NAC mGluR5–Homer2–PI3K signaling predisposes a high binge alcohol-drinking phenotype. Together, these data point to an important role for NAC mGluR5–Homer2–PI3K signaling in regulating binge-like alcohol consumption that has relevance for our understanding of the neurobiology of alcoholism and its pharmacotherapy.
Introduction
Alcoholism is a chronic neuropsychiatric disorder affecting ∼17.6 million people nationwide (National Institute on Alcohol Abuse and Alcoholism, 2007). Alcohol acts as a noncompetitive antagonist at several glutamate receptors (Lovinger, 1996; Minami et al., 1998) and alters limbic glutamate neurotransmission, including that of the nucleus accumbens (NAC), which contributes to the motivation to drink and other properties of alcohol (cf. Krystal et al., 2003; Siggins et al., 2003; De Witte, 2004; Gass and Olive, 2008; Koob and Le Moal, 2008). As observed after repeated alcohol injections (Szumlinski et al., 2005b, 2008b; Kapasova and Szumlinski 2008), binge-like alcohol consumption increases NAC extracellular glutamate levels, and this response sensitizes with repeated bouts of binge-like drinking (Szumlinski et al., 2007). As NAC extracellular glutamate actively regulates alcohol intake (Kapasova and Szumlinski, 2008), molecular candidates contributing to the initiation and maintenance of excessive alcohol drinking are likely those regulating the development of alcohol-induced plasticity at glutamatergic synapses within the NAC.
The Homer2 member of the Homer family of postsynaptic scaffolding proteins has emerged as critical for NAC glutamate transmission and alcohol-induced neuroplasticity in vivo (cf. Szumlinski et al., 2008a). Through an Ena/VASP1 homology domain, Homers interact with a proline-rich motif (PxxF) located on group 1 metabotropic glutamate receptors (mGluR1/5), as well as on other proteins involved in group 1 mGluR intracellular signaling [including inositol-1,4,5-triphosphate (IP3) receptors and the phosphatidylinositol-3 kinase enhancer-long (PIKE-L) complex] (Tu et al., 1998; Rong et al., 2003). A three-month history of continuous alcohol intake upregulates NAC Homer2 and mGluR1 protein expression in C57BL/6J (B6) mice (Szumlinski et al., 2008b), and virus-mediated NAC Homer2b overexpression augments various aspects of alcohol reward in mice, including ad libitum access alcohol intake and alcohol-induced conditioned place preference (Szumlinski et al. 2005b, 2008b). Conversely, Homer2 deletion in mice or D. Homer deletion in Drosophila results in an alcohol-avoiding phenotype (Szumlinski et al., 2005b; Urizar et al., 2007), which resembles the “anti-alcohol” effects produced by systemic or intra-NAC administration of mGluR1/5 antagonists (Bäckström et al., 2004; Olive et al., 2005; Schroeder et al., 2005; Hodge et al., 2006; Lominac et al., 2006; Bäckström and Hyytiä, 2007; Besheer et al., 2008a,b; Blednov and Adron Harris, 2008). These preclinical data, coupled with reports of associations between single nucleotide polymorphisms (SNPs) in genes encoding glutamate receptors or the p85 regulatory subunit of phosphatidylinositol-3 kinase (PI3K) with risky alcohol-drinking behavior in adolescents (Desrivières et al., 2008; Schumann et al., 2008), have led to the hypothesis that group 1 mGluR–Homer2–PI3K signaling within the NAC is necessary for stable, excessive alcohol consumption. Thus, we used a combination of proteonomic and in vivo pharmacological and genetic approaches to characterize the role for this signaling cascade in regulating sustained, excessive alcohol consumption using the murine scheduled high alcohol consumption (SHAC) model (Finn et al., 2005), one of several rodent models of binge alcohol drinking (for review, see Rhodes et al., 2005). The results of this study provide novel evidence that binge alcohol-drinking-induced increases in NAC mGluR5–Homer2–PI3K pathway activation is necessary for this prevalent form of alcohol-directed behavior and that basal NAC PI3K activity may contribute to genetic variance in binge alcohol-drinking behavior.
Materials and Methods
Subjects
C57BL/6J mice.
The majority of subjects used in this study were adult male, inbred C57BL/6J (B6) mice (8 weeks of age; 25–30 g; The Jackson Laboratory) that were allowed to acclimate to the colony room for at least 7 d after arrival. For all drinking experiments, animals were single housed in polyethylene cages in a temperature (25°C)- and humidity (71%)-controlled colony room under a 12 h reverse light cycle (lights off at 5:00 A.M.; lights on at 5:00 P.M.); otherwise, mice were housed in groups of four. Food was available ad libitum and water access was restricted in the drinking studies as described below. All experimental protocols were approved by the Institutional Animal Care and Use Committee of our respective institutions and were consistent with the guidelines provided by the National Institute of Health (NIH) Guide for Care and Use of Laboratory Animals (NIH publication number 80-23, revised 1996).
mGluR5F1128R transgenic mouse.
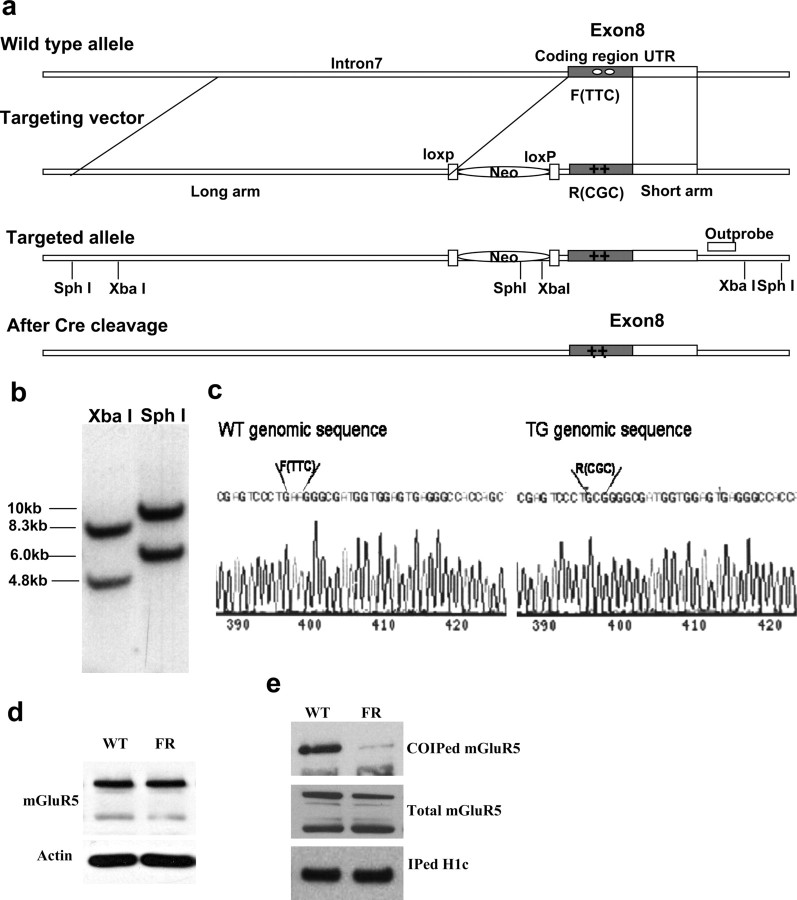
To examine the functional relevance of mGluR5-Homer interactions for binge-drinking behavior and protein expression, a transgenic (TG) mouse with a phenylalanine (F) → arginine (R) point mutation at amino acid position 1128 of mGluR5 was generated using the strategy presented in Figure 1a. The mGluR5F1128R targeting construct was generated based on a 13 kb EcoRV fragment from a DNA bacterial artificial chromosome clone, including mouse mGluR5 gene exon 8. Selective markers were introduced by inserting a lox-p flanked PGK–Neo fragment (including SphI and XbaI restriction sites) in the unique PacI site of exon 8. F1128R mutations were introduced by replacing a 1.2 kb DNA fragment with two point mutations (TTC to CGC) downstream of the PacI site in exon 8 (0.3 kb belonging to intron 7 and 0.9 kb belonging to the exon 8 coding region) (Fig. 1c). Thus, the construct has a 9.8 kb long arm and 2 kb short arm for homologous recombination. The resulting targeting construct was linearized and electroporated into R1 embryonic stem cells. Cells were selected with G-418 for 2 weeks. Clones were picked, screened by PCR, and confirmed by Southern blotting (Fig. 1b). Positive clones were injected into blastocysts, and chimeras were mated to C57BL/6J mice to produce mGluR5F1128R heterozygotes. PGK–Neo was removed by crossing these heterozygotes with actin promoter-driven Cre mice.
Figure 1.
Targeting strategy of mGluR5F1128R knock-in mutant mice and confirmation. a, Schematics of targeting. b, Southern blotting. c, Sequencing result of WT and TG genomic DNA. d, Western blot assay showed that mGluR5 expression is normal in mGluR5F1128R mutant mice. e, IP assays showed that Homer–mGluR5 interaction is disrupted in mGluR5F1126R mutants.
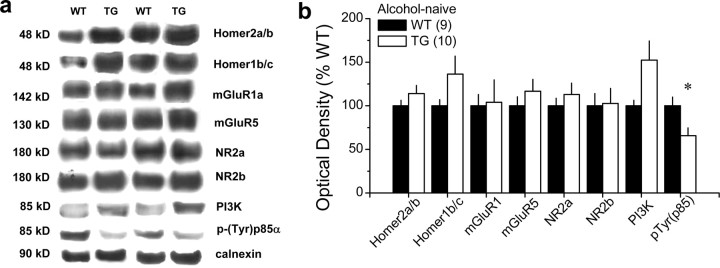
To verify that the F1128R mutation reduced the physical interaction between Homers and mGluR5, coimmunoprecipitation was conducted on brain tissue from wild-type (WT) and mGluR5F1128R TG mice. Whole brains were dissected and sonicated in an immunoprecipitation (IP) buffer (1× PBS, pH 7.4, with 5 mm EDTA, 5 mm EGTA, 1 mm Na3VO4, 10 mm sodium pyrophosphate, 50 mm NaF, and 1% Triton X-100) containing Complete EDTA-Free protease inhibitors. Samples were centrifuged at 13,000 rpm for 30 min at 4°C. The supernatant (300 ml) was then mixed with 0.5–2 μg of the appropriate antibody for 3 h at 4°C. Then 50 ml of 1:1 protein A- or protein G-Sepharose slurry (GE Healthcare) was added for an additional 1 h. The protein beads were washed three times with IP buffer containing 1% Triton X-100. The protein samples were separated electrophoretically using NuPAGE 4–12% Bis-Tris gels (Invitrogen) and transferred to an Immobilon-P polyvinylidene difluoride (PVDF) membrane (Millipore). The membrane was blocked with TBST (50 mm Tris, pH 7.5, with 150 mm NaCl and 0.1% Tween 20) containing 5% nonfat milk for 1 h at room temperature, followed by incubation with primary antibody in TBST buffer overnight at 4°C. After three washes with TBST buffer, membranes were incubated with HRP-conjugated anti-rabbit antibody in TBST for another hour. After three washes with TBST buffer, the membrane was treated with SuperSignal ECL substrate (Pierce) according to the protocol of the manufacturer. Using this procedure, immunoblotting verified that the point mutation interfered with the capacity of Homers to bind to mGluR5 (Fig. 1e), without affecting total mGluR5 expression (Fig. 1d) (Student's t test, p = 0.13; n = 4). A follow-up examination of accumbens tissue also confirmed that the point mutation did not significantly affect total mGluR5 expression, nor did it alter the expression level of either Homer1b/c or Homer2a/b protein (see Fig. 8).
Figure 8.
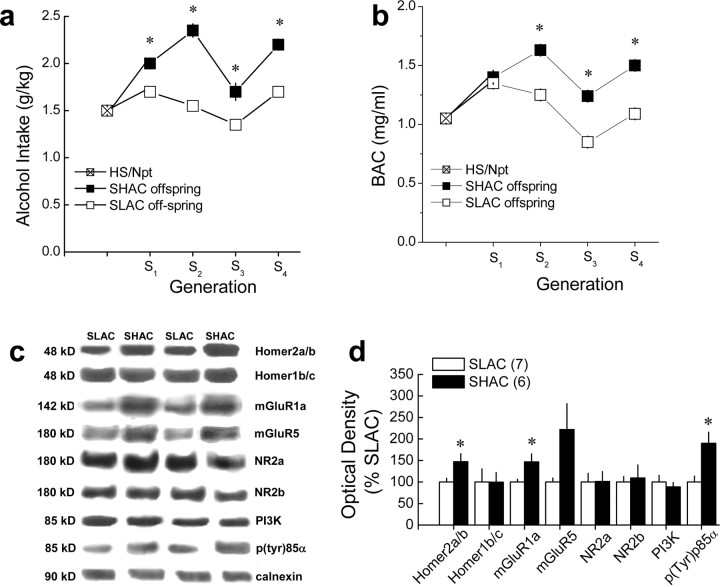
Line differences in NAC Homer2 and PI3K activity in selectively bred SHAC and SLAC mice. a, b, Summary of the average alcohol dose consumed (grams per kilogram; a) and BACs (b) attained after the second session of 30 min alcohol drinking (selection phenotype) in the genetically heterogeneous HS/Npt mice (foundation population for the selection) and in the SHAC and SHAC offspring, across four generations of selective breeding. Data in a and b represent the mean ± SEM of 80–104 mice per line per generation; *p < 0.05, SHAC offspring versus SLAC offspring. c, Representative immunoblots for the total protein levels of Homer2a/b, Homer1b/c, mGluR1, mGluR5, NR2a, NR2b, PI3K, p(Tyr)p85α PI3K binding motif [p(Try)p85α], and calnexin (loading control) in the NAC of fourth generation (S4) selectively bred SHAC and SLAC mice, at 3 months after their second 30 min alcohol-drinking session. d, Summary of the line differences in protein expression, expressed as a percentage of average levels of SLAC animals. Data in d represent the mean ± SEM of the number of mice indicated in the figure. *p < 0.05 versus SLAC (t tests).
For the present experiments, male WT and homozygous mGluR5F1128R TG littermate mice were generated from heterozygous breeder pairs (F10–F12; C57BL/6J × 129Xi/SvJ), housed under conditions described for the B6 mice above. All testing for behavior or for changes in protein expression commenced when mice were 7–8 weeks of age.
Selectively bred scheduled high and low alcohol consumption lines of mice.
“Binge” alcohol drinking is defined by NIH as alcohol consumption that brings blood alcohol concentrations (BACs) to 0.8 mg/ml within an ∼2 h drinking period (National Institute on Alcohol Abuse and Alcoholism, 2004) and is the most prevalent pattern of heavy alcohol consumption exhibited within the United States (National Institute on Alcohol Abuse and Alcoholism, 2007). Given this, we chose to conduct selective breeding to optimize the genetic contribution to the “binge-drinking” phenotype (see subsection below) and produce lines of mice that differed in their limited-access alcohol intake. One advantage of selected lines is that they can be used to identify genetically correlated traits. That is, the appearance of a significant difference in the selection phenotype, as well as in another trait, implies the existence of pleiotropically acting genes. Additionally, the selection pressure alters the allele frequencies of genes that are relevant to the trait of interest, making selected lines a powerful tool to explore mechanisms underlying the selected trait. Many of the characteristics of selected lines for use in evaluating genetic correlation, as well as the theoretical considerations involved in their construction and evaluation of practical/theoretical tradeoffs, have been discussed previously (Crabbe et al., 1990; Crabbe, 1999). To examine whether or not the NAC expression of glutamate receptors Homers and PI3K activity were genetically correlated with a binge-drinking phenotype, genetically heterogeneous mice (HS/Npt), created by Dr. Robert Hitzemann (Oregon Health and Science University, Portland, OR) and maintained at Oregon Health and Science University, served as the founding population for the generation of SHAC and scheduled low alcohol consumption (SLAC) selected lines. The selection of the SHAC and SLAC selected lines began with testing 121 HS/Npt mice (59 male and 62 female) on the selection version of the SHAC procedure. Briefly, after acclimation to individual housing, mice had access to 4 h of fluid per day. On days 1 and 2 and days 4 and 5, mice had access to tap water for 4 h. On day 3, mice initially had access to a 5% alcohol solution for 30 min, followed by access to tap water for 3.5 h. Because binge alcohol drinking is defined in terms of BACs attained after limited-access drinking (National Institute on Alcohol Abuse and Alcoholism, 2007), on day 6, a retro-orbital blood sample was taken to assess BAC immediately after the 30 min alcohol session (Finn et al., 2007). The behavioral experiment was terminated at this point, and animals were maintained on ad libitum food and water until decisions on breeders were made (i.e., after all animals had been tested). It was decided that the selection phenotype for the SHAC and SLAC lines would be based on the BAC on day 6, because pilot studies determined that BAC and alcohol dose consumed on day 6 were positively correlated with these dependent variables on day 21 (when animals had 10 h of total fluid availability), indicating that the alcohol intake patterns on day 6 were representative of intake patterns when animals were no longer fluid restricted.
The starting stock of HS mice that was tested initially (S0) contained representative animals from 44 of the 45 families that comprised the HS/Npt mice. The alcohol intake and BAC on day 6 in all animals was 1.50 g/kg and 1.05 mg/ml, respectively. Because we had proposed to start with mass selection, we chose 12 primary and three alternate breeding pairs to begin propagating the SHAC line, representing 20 families. The animals chosen were those with the highest BACs. Alcohol intake and BAC was 1.97 g/kg and 1.59 mg/ml in the breeders for the SHAC line. Likewise, we chose 12 primary and three alternate breeding pairs (mice with the lowest BACs) to begin propagating the SLAC line, representing 22 families. Alcohol intake and BAC was 0.924 g/kg and 0.531 mg/ml in the breeders for the SLAC line. After choices for S1 breeders were determined, the breeding strategy was switched to within-family selection. A within-family selection breeding strategy reduces the rate of inbreeding to approximately one-half of that seen with mass selection by systematically reducing the (already small) degree of genetic relatedness of each pair of mice mated to produce the next generation (for detailed discussion, see Falconer and Mackay, 1996; Crabbe, 1999). Beginning with testing of the S1 offspring, choices for S2 breeders were made by picking one male and female from each family of the SHAC line (with the highest BAC on day 6) for the SHAC breeders and one male and female animal from each family of the SLAC line (with the lowest BAC on day 6) for the SLAC breeders. A similar strategy was used to produce each subsequent generation. The SHAC and SLAC selected lines were maintained in reproductive isolation from each other starting with S1. A rotational breeding scheme was used so that mating pairs were constructed to exclude littermates, as well as common grandparents, whenever possible. The proportion of animals selected to serve as breeders from the S0 generation was 25%, and the breeder proportion did not exceed 16% in subsequent generations (i.e., 15 breeding pairs for each line, testing ∼90–100 offspring per line). Additional consideration was given to the relationship between BAC and alcohol dose on day 6 (to ensure that high intake corresponded to high BAC and visa versa), to the relationship between alcohol dose on days 3 and 6 (to ensure that consumption had not dropped on day 6), as well as to significant (±20%) changes in body weight. There was never a circumstance in which we had to substitute another breeder for our first choices of SHAC and SLAC mating pairs, based on these additional considerations. Scheduled water consumption was not considered as a potential confounding variable, because we had previously observed that water consumption under a similar fluid restriction schedule did not differ markedly between animals on the SHAC procedure or similarly treated water controls (Cronise et al., 2005).
Because of financial limitations, the selection study was terminated after testing of the S4 offspring was completed. Three months after the offspring were tested for intake, the S4 SHAC and SLAC mice were killed. Whole brain was removed from a subset of the S4 offspring from each selected line and shipped frozen to the University of California at Santa Barbara for subsequent immunoblotting procedures. As described in Results and depicted in Figure 9b, the significant divergence in BAC in the SHAC and SLAC lines beginning in the S2 generation provides support for the use of these lines as a genetic animal models of “binge drinking” and “low binge drinking,” respectively.
Extended SHAC procedures
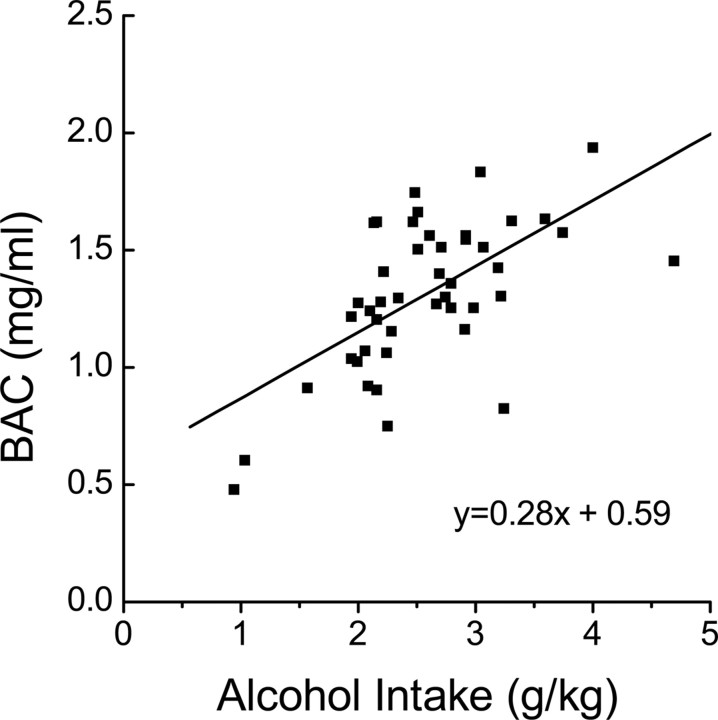
One of the major criticisms of the majority of existing rodent models of alcohol drinking is that rarely do the animals achieve pharmacologically relevant blood alcohol concentrations during alcohol access (i.e., BAC >0.8 mg/ml), nor do they maintain pharmacologically significant BACs over extended periods of alcohol access (Dole and Gentry, 1984; Lê et al., 2001; Grahame and Grose, 2003). Although previous data (Szumlinski et al., 2005b, 2008b) indicated an important role for NAC Homer2 function in moderate alcohol intake under both operant and non-operant alcohol self-administration procedures, the role for group 1 mGluRs and the intracellular mediators of mGluR5/Homer2 activation in maintaining excessive, binge-like alcohol drinking is not known. To address this issue, the present study used a variation of the murine SHAC model of excessive, binge-like alcohol drinking, originally described by Finn et al. (2005). The SHAC procedures used were identical to those described recently by Szumlinski et al. (2007) and involved the presentation of a single 50 ml sipper tube containing either water or a 5% (v/v) alcohol solution for 30 min, starting at 3 h into the circadian dark cycle. Alcohol presentation occurred every 3 d, with water presentation on intervening days. After each 30 min drinking session, the home-cage water bottle was returned, and mice were allowed to consume water for an additional 9.5 h (total fluid availability per day is 10 h). At this time (6:00 P.M.), the home-cage water bottle was removed. The amount of water and/or alcohol consumed during the 30 min session was determined daily by bottle weight before and after each 30 min drinking session, and alcohol/water intake was calculated, respectively, on a gram per kilogram and milliliter per kilogram body weight basis. Because mice consume the majority of their daily fluid intake during the circadian dark period (Middaugh et al., 1999), our modified SHAC procedures involved fluid restriction throughout the circadian light period into the first 3 h of the circadian dark phase (Szumlinski et al., 2007). Despite eliciting high levels of alcohol intake (1.3–1.9 g/kg per 30 min) and correspondingly high BACs (>1.0 mg/ml) (Szumlinski et al., 2007), this amount of fluid restriction is considered minimal because it does not promote excessive water intake during a 30 min presentation of a sipper tube containing water as assessed previously in pilot experiments (non-water restricted, 9.9 ± 3.62 ml/kg vs water restricted, 15.41 ± 6.05 ml/kg; n = 11; t(10) = 1.12; p > 0.05). Our data for water intake in this extended SHAC procedure are consistent with previous SHAC studies using longer periods of fluid deprivation (Cronise et al., 2005; Finn et al., 2005), as well as other data (Toth and Gardiner, 2000) indicating that fluid-restricted mice adapt and do not exhibit a compensatory increase in fluid consumption or other detrimental effects, such as a reduction in feeding or weight loss. Because binge alcohol drinking is defined by a BAC >0.8 mg/ml within a 2 h period (National Institute on Alcohol Abuse and Alcoholism, 2007), we conducted a regression analysis on the relationship between alcohol intake during the seventh 30-min drinking session and the BACs attained by male B6 mice (n = 45) (Fig. 2). As reported previously (Finn et al., 2005), this analysis indicated a relationship between these two variables that was significantly different from 0 (F(1,43) = 26.39; p < 0.0001), and the resulting equation, BAC = 0.28 * (intake) + 0.59, was used to determine whether or not our transgenic and pharmacological manipulations, by definition, prevented binge-drinking behavior in our mice. Additionally, the goodness of fit (r2) of the regression line was 0.38, indicating that 38% of the variability in BAC could be accounted for by the variation in alcohol dose consumed.
Figure 2.
Best-fit regression line of the relationship between alcohol intake (in grams per kilogram) during a 30 min alcohol-drinking session and BACs attained (in milligram per milliliter) as derived from a study of 45 B6 mice.
Immunoblotting
Chronic, continuous alcohol consumption elevates NAC levels of Homer2 and associated glutamate receptor proteins (Szumlinski et al., 2008b). To determine whether or not alcohol drinking upregulates the mesocorticolimbic expression of members of the mGluR–Homer–PI3K signaling pathway in the fully extended SHAC drinking model (for discussion, see Finn et al., 2005), B6 mice were subjected to six bouts of SHAC drinking over an 18 d period with 5% alcohol available for 30 min, every third day (see above). Control animals received tap water in an identical 50 ml sipper tube during each of the 30 min sessions. Animals were decapitated 24 h after the sixth alcohol presentation, brains were sectioned (1.0 mm thick) along the coronal plane, and the entire prefrontal cortex (PFC), NAC, dorsal striatum, and hippocampus were dissected out over ice. Because the mGluR5F1128R mutation reduced binge drinking with the SHAC procedure (see Results), a second experiment assessed genotypic differences in basal NAC protein expression in experimentally naive WT and mGluR5F1126R mutant mice. Finally, a third immunoblotting experiment was conducted on NAC tissue from selectively bred SHAC and SLAC mice (see above) to further relate genetic vulnerability in binge alcohol drinking to mGluR/Homer/PI3K expression in the NAC (i.e., was mGluR5/Homer/PI3K expression in the NAC a correlated response to selection for binge drinking?). For this experiment, frozen whole brains from S4 SHAC and SLAC offspring 3 months after alcohol testing were sectioned along the coronal plane (1 mm thick) at the level of the NAC, and the entire NAC and dorsal striatum were dissected out over ice.
As described in recent reports by our group (Ary and Szumlinski, 2007; Ary et al., 2007; Szumlinski et al., 2008b), the tissue from all the experiments outlined above was homogenized in a medium consisting of 0.32 m sucrose, 2 mm EDTA, 1% w/v SDS, 50 μm phenylmethylsulfonyl fluoride, and 1 μg/ml leupeptin, pH 7.2, and 50 mm sodium fluoride, 50 mm sodium pyrophosphate, 20 mm 2-glycerol phosphate, 1 mm p-nitrophenyl phosphate, and 2 μm microcystin-LR were included to inhibit phosphatases. Samples were then subjected to low-speed centrifugation at 10,000 × g for 20 min. Protein determinations were performed using the Bio-Rad DC protein assay according to the instructions of the manufacturer, and homogenates were stored at −80°C until immunoblotting was completed.
For immunoblotting, protein samples (5–20 μg) were subjected to SDS-PAGE. Bis-Tris gradient gels (4–12%) (Invitrogen) were used for separation of Homers, PI3K, and the p(Tyr)p85α PI3K binding motif, the latter of which was used to index PI3K activity (Zhang et al., 2006). Tris-acetate gradient gels (3–8%) (Invitrogen) were used for separation of Homers, as well as the glutamate receptor proteins. Proteins were transferred to PVDF membranes and preblocked with PBS containing 0.1% (v/v) Tween 20 and either 5% (w/v) bovine serum albumin [for p(Tyr)p85α PI3K binding motif] or 5% (w/v) nonfat dried milk powder (for all other proteins) for no less than 1 h before overnight incubation with primary antibodies. The following rabbit polyclonal antibodies were used: anti-Homer 2a/b and anti-Homer 1b/c (1:1000 dilution; Dr. Paul F. Worley, Johns Hopkins University School of Medicine, Baltimore, MD), anti-mGluR5 (1:1000 dilution; Upstate Cell Signaling Solutions), anti-NR2a and anti-NR2b (1:1000 dilution; Calbiochem), anti-PI3K antibody (1:1000 dilution; Upstate Cell Signaling Solutions), and anti-p(Tyr)p85α PI3K binding motif (1:500; Cell Signaling Technology). An anti-mGluR1a mouse polyclonal antibody (1:1000 dilution; Upstate Cell Signaling Solutions) was also used. Membranes were washed, incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:20,000 to 1:40,000 dilution; Upstate) or anti-mouse secondary antibody (1:20,000 to 1:40,000 dilution; Jackson ImmunoResearch) for 90 min, and washed again, and immunoreactive bands were detected by enhanced chemiluminescence using either ECL Plus (GE Healthcare) or Pierce SuperSignal West Femto (Thermo Fisher Scientific). A rabbit anti-calnexin polyclonal primary antibody (Stressgen) was also used to index protein loading and transfer. The levels of immunoreactivity for all proteins were quantified using NIH Image J, and the immunoreactivity for each protein of interest for each animal was first normalized to that of its appropriate calnexin signal to provide a protein/calnexin ratio. These ratios were then normalized to the mean ratios for each protein of the water or genetic control for each individual gel (n = 3–4 per gel).
Surgical procedures
The surgical procedures for implanting bilateral guide cannulae into the NAC of mice (i.e., B6, WT, or TG) were similar to those used in previous studies (Lominac et al., 2006; Szumlinski et al., 2007, 2008b; Kapasova and Szumlinski, 2008). Mice were anesthetized by inhalation of isoflurane with 4% oxygen as the carrier gas. Mice were mounted in a David Kopf Instruments stereotaxic device with tooth and ear bars adapted for mice. The animal's skull was exposed and leveled, and holes were drilled based on a set of coordinates from bregma for the NAC shell [anteroposterior (AP), +1.3; mediolateral (ML), ±0.5 mm; dorsoventral (DV), −2.3 mm] or NAC core (AP, +1.3; ML, ±0.7 mm; DV, −2.0 mm), according to the mouse brain atlas of Paxinos and Franklin (2004). The skull was prepared for polymer resin application, and stainless steel guide cannulae (20-gauge, 10 mm long; Small Parts) were lowered such that the tips of the cannulae were 2 mm above the NAC. A mound of resin was placed around the guide cannulae for stabilization and then light cured. The incision was closed with a tissue adhesive. To prevent continuous externalization, dummy cannulae (24 gauge; length equivalent to guide cannula) were placed inside the guide cannulae and only removed before drug testing. After surgery, the animals are allowed to recover for 4–5 d before the start of the SHAC drinking procedures.
AAV vectors
Our previous studies conducted in B6 and B6-hybrid mice demonstrated a facilitatory role for NAC Homer2 expression in alcohol intake, under both non-operant and operant conditions (Szumlinski et al., 2005b, 2008b). Because repeated bouts of binge drinking were found to elevate NAC expression of Homer2 (see Results), we used a similar adeno-associated viral vector (AAV) gene-transfer approach to examine the functional relevance of increasing and decreasing NAC Homer2 expression for binge-drinking behavior. The procedures for generating AAVs carrying Homer2b cDNA have been described in detail by Klugmann et al. (2005) and Klugmann and Szumlinski (2008). In brief, Homer2b was expressed as an N-terminal fusion protein with the hemagglutinin (HA) tag in a recombinant AAV backbone containing the 1.1 kb cytomegalovirus immediate early enhancer/chicken β-actin (CBA) promoter (AAV–Homer2b). The same backbone encoding Renilla green fluorescent protein (hrGFP) was used as control (AAV–GFP). For generation of Homer2b-specific small hairpin RNAs (shRNAs), we searched the Homer2b mRNA using an shRNA design algorithm (Ambion) and identified the targets sequences H2b#1 (5′-GUGUGAAUAUGUCUCUGAGTT-3′) and H2b#2 (5′-CACAGAGUGCUGCCAAUGUTT-3′). Similarly, a sequence (5′-ACUACCGUUGUUAUAGGTGTT-3′) with no significant homology to any endogenous target (universal negative control) was cloned to serve as a negative control for potential nonspecific effects mediated by delivery of shRNA. We generated sense and antisense oligonucleotides corresponding to these targets, annealed them, and cloned them into an AAV plasmid that simultaneously drives the expression of the shRNAs and hrGFP as described previously (Franich et al., 2008). In this cassette, shRNAs for knockdown of Homer2b were driven by the human U6 promoter inserted upstream of the CBA promoter in the AAV–GFP vector (Franich et al., 2008). This bicistronic strategy allowed for easy identification of cells transduced by the shRNA-expressing vector. Packaging of chimeric AAV1/2 vector was performed as described previously (Klugmann et al., 2005), and genomic titers were determined using a Prism 7700 sequence detector system (Applied Biosystems) with primers designed to woodchuck posttranscriptional regulatory element (During et al., 2003).
AAV infusion
The procedures for infusing AAVs were identical to those used in previous mouse studies (Szumlinski et al., 2004, 2005b, 2008b; Lominac et al., 2005). One week after surgery, 33 gauge injector cannulae (12 mm long; threaded through a 24 gauge adapter for stability) were lowered bilaterally into the NAC and AAVs infused at a rate of 0.1 μl/min for 5 min (total volume is 0.50 μl/side). A similar infusion procedure produces neuronal transduction that is restricted to <1 mm of the infusion site and is maximal for both AAV–cDNAs and AAV–shRNAs at 3 weeks after infusion (Szumlinski et al., 2004, 2005b, 2006, 2008b; Lominac et al., 2005; Klugmann and Szumlinski, 2008) (see also Fig. 4). In the case of the shRNAs, AAV infusion of 0.5 μl/side produces a 50% reduction in total Homer2 protein expression within the NAC, without affecting Homer1b/c protein expression, as assessed by immunoblotting (Klugmann and Szumlinski, 2008). For all experiments involving intra-NAC AAV infusion, testing began 3 weeks later. As we examined the site specificity of Homer2b manipulations during binge-drinking behavior, after AAV testing, mice were perfused transcardially with saline, followed by a 4% paraformaldehyde solution, and immunocytochemical staining for the HA tag was performed to localize the extent of transduction efficiency within the NAC and to examine for gross signs of neurotoxicity, as conducted previously (Szumlinski et al., 2004, 2005b, 2006, 2008b; Klugmann et al., 2005; Lominac et al., 2005). As described in greater detail below, in all, three distinct AAV studies were conducted in series (respectively, NAC shell cDNA, NAC core cDNA, and NAC shell shRNA) with 3–4 months intervening between studies. For each study, mice were tested in two cohorts of five to six mice/AAV treatment/cohort, and the cohorts were spaced 2 months apart.
Figure 4.
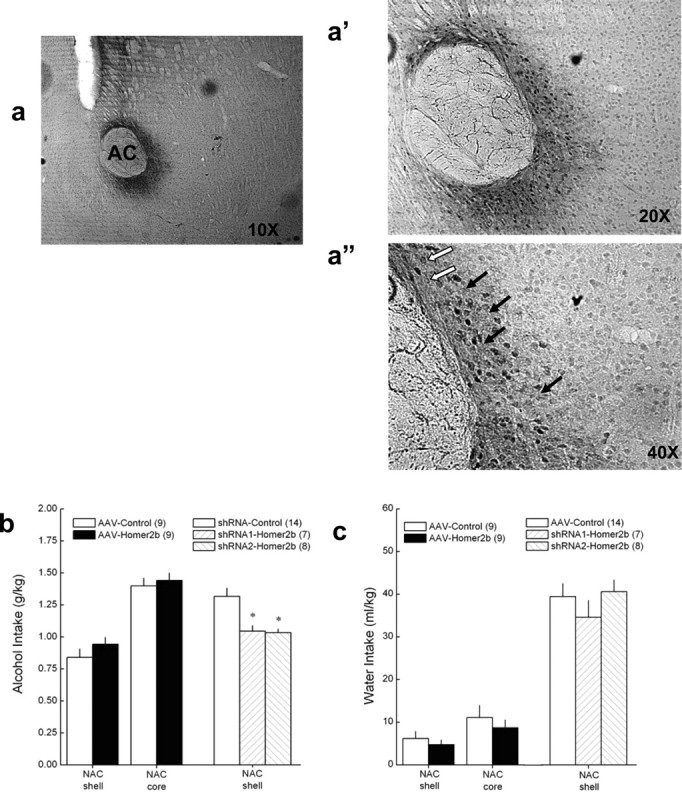
shRNA-mediated knockdown of NAC shell Homer2b levels blunts binge alcohol drinking. a–a″, Representative micrographs of immunostaining for AAV-transfected HA-tagged Homer2b in the NAC core. Filled arrows in subpanel a″ (40× magnification of tissue section) indicate cytoplasmic and process staining, and open arrows indicate staining that is localized more to the plasma membrane. b, Summary of the effects of intra-NAC shell and intra-NAC core infusions of AAVs carrying Homer2 cDNA, Homer2 shRNA, or control shRNA during the average alcohol intake of B6 mice over the course of a week of 30 min limited alcohol access in the SHAC procedure. c, Summary of the effects of intra-NAC shell and core infusions of the AAVs on the average water intake of mice on intervening 30 min limited water access days. The data in b and c represent the means ± SEM of the number of animals indicated in the figure. *p < 0.05 versus control.
Intracranial drug infusion procedures
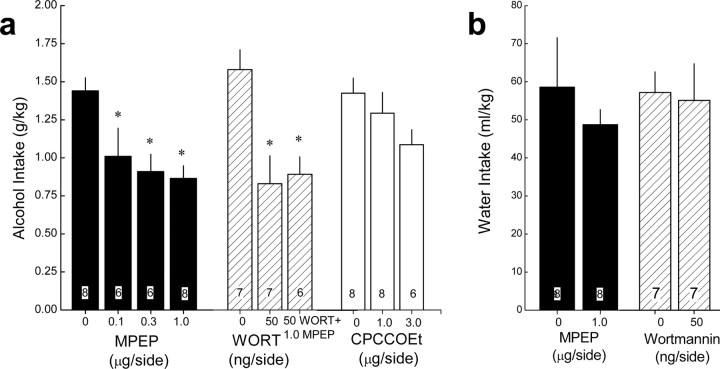
To establish a necessary role for NAC group 1 mGluR and PI3K activation in the maintenance of excessive alcohol drinking, the effects of the local infusion of mGluR5, mGluR1, and PI3K antagonists during binge drinking with the SHAC procedure were determined in three separate experiments (one per compound). In these behavioral pharmacological experiments, mice were presented with 5% alcohol for 30 min, every third day, until stable intake was established (<10% variability across three consecutive presentations; approximately three to four presentations). MPEP [2-methyl-6-(phenylethynyl)pyridine hydrochloride] (0, 0.1, 0.3, and 1.0 μg/side; Sigma-Aldrich), the mGluR1a antagonist CPCCOEt [7-(hydroxyimino)cyclopropa[b] chromen-1a-carboxylate ethyl ester] (0, 1.0, and 3.0 μg/side; Tocris Cookson), and a PI3K-selective dose of the antagonist wortmannin (0 vs 50 ng/side; Sigma-Aldrich) (Rong et al., 2003) were infused through a 33 gauge injector (12 mm long; threaded through a 24 gauge adapter for stability) at a rate of 0.25 μl/min for a total volume of 0.25 μl/side using a Harvard PhD2000 automated syringe pump, and the injectors remained in place for an additional 60 s. MPEP and wortmannin were dissolved in sterile water for infusion, and CPCCOEt was dissolved in water containing cylcodextrin (45% w/v; Sigma-Aldrich), and water or a 45% w/v cyclodextrin solution served as the appropriate control (Schroeder et al., 2005; Hodge et al., 2006; Lominac et al., 2006; Besheer et al., 2008a). In these antagonist studies, an intra-NAC infusion of neither the water nor cyclodextrin vehicle affected alcohol intake relative to that exhibited by these same mice in the absence of any microinjection (e.g., non-infused, 1.31 ± 0.12 g/kg vs water infused, 1.44 ± 0.09 g/kg vs cyclodextrin infused, 1.42 ± 0.09 g/kg), indicating that, if any disruption in the osmotic balance of the NAC was produced by our vehicle pretreatments, it did not influence binge alcohol drinking. Immediately after infusion, mice were returned to their home cage and presented with the 5% alcohol-containing sipper tube for 30 min. Within each experiment, mice received at least two alcohol bottle presentations between intra-NAC drug tests to examine for potential carryover effects of pretreatment and to ensure that alcohol drinking had stabilized. In none of the experiments were carryover effects observed during either alcohol or water intake on the intervening days (i.e., there were no significant differences between the average pretest intake and intake on the intervening nontest days; data not shown). The order of the dosing was randomized across test days, and, to reduce the amount of tissue damage caused by repeated testing and to reduce the risk of infection, mice were tested with a maximum of three doses (i.e., not all mice in the MPEP study received all antagonist doses). To avoid disruption of drinking behavior on subsequent test days, BACs were not assessed but rather were predicted based on the mean alcohol intake according to the results of the regression analysis provided in Figure 2. To assess for nonspecific effects of intra-NAC antagonist infusion, the maximally effective dose for reducing alcohol intake was then examined during water intake during a 30 min session. Standard cresyl violet histochemical procedures were used to verify injector cannulae localization in the NAC shell (Szumlinski et al., 2005b, 2008b; Lominac et al., 2006; Kapasova and Szumlinski, 2008).
Continuous alcohol access procedures
NAC Homer2 overexpression and intra-NAC infusion of a glutamate reuptake inhibitor increase, whereas Homer2 or mGluR5 deletion and systemic pretreatment with mGluR5 antagonists reduce, alcohol intake of B6 mice under continuous alcohol access procedures (Bäckström et al., 2004; Olive et al., 2005; Szumlinski et al., 2005b, 2008b; Lominac et al., 2006; Besheer et al., 2008a; Blednov and Adron Harris, 2008). Thus, we next determined whether or not the effects of the mGluR5F1128R mutation during drinking within the SHAC procedure (see Results) generalized to voluntary alcohol preference drinking. For this, an independent study examined the mGluR5F1128R TG and WT mice for genotypic differences in alcohol intake and preference using a four-bottle-choice procedure as described previously (Szumlinski et al., 2005b, 2008b; Lominac et al., 2006; Bäckström and Hyytiä, 2007). In brief, WT and TG mice were presented simultaneously with four 50 ml sipper tubes containing 0, 3, 6, and 12% alcohol (v/v) and allowed to drink undisturbed in the home cage over a 24 h period. For 3 weeks, bottles were weighed daily at 10:00 A.M., and the total alcohol intake was calculated on a gram per kilogram weight basis. The preference for each alcohol concentration was calculated as the percentage of the total fluid consumed from the four bottles. The average alcohol and water intake, as well as alcohol preference, during the last week of testing were used in the analysis of genotypic differences. In both the SHAC and continuous alcohol access procedures, spillage attributable to bottle manipulation was monitored by weighing the appropriate number of bottles on empty cages, and the average spillage for a given day (0.05–0.1 ml) was subtracted from the experimental data for that day.
Statistical analysis
Immunoblotting data were statistically evaluated using Student's t tests. The behavioral data for the AAV–cDNA studies were analyzed using unpaired Student's t tests (control vs cDNA) and that for the AAV–shRNA study were analyzed using a between-subjects ANOVA with three levels on the AAV factor (control, shRNA1, and shRNA2). The attrition rate in the AAV studies was one to two animals per group as a result of caudally misplaced microinjectors (i.e., in ventral pallidum). Because not all mice received all intracranial treatments as a result of experimental design (maximum of three microinjections) or loss of guide cannulae patency (maximum of two mice per experiment), to include all of the animals with viable data, the alcohol intake data for the MPEP, wortmannin, and CPCCOEt studies were analyzed using a between-subjects ANOVA with four levels on the MPEP dose factor, three levels on the wortmannin dose factor, and three levels on the CPCCOEt dose factor, respectively. Because there was no attrition in the study examining the effects of either antagonist infusion during water intake, these data were analyzed using paired t tests (control infused vs drug infused). The data for mGluR5F1128R mutant studies were analyzed using between-subjects (WT vs TG) univariate ANOVAs, with the exception of the data for alcohol preference, which was analyzed using a mixed-design ANOVA (genotype × alcohol), with four levels on the within-subjects factor of alcohol (0, 3, 6, and 12% alcohol). There was no attrition as a result of loss of guide cannulae patency or misplaced microinjector placements in the studies examining the combined effects of intra-NAC drug infusion and the mGluR5F1128R mutation during behavior; thus, the data were analyzed using a mixed-design ANOVA (genotype × drug), with three levels on the within-subjects factor of drug (vehicle, MPEP, or wortmannin). When appropriate, post hoc comparisons were made using Fisher's least significant difference test.
Results
Binge alcohol drinking coregulates NAC Homer2 and NR2 expression, as well as PI3K activation in B6 mice
To extend our previous immunoblotting data from an animal model of moderate alcohol intake (Szumlinski et al., 2008b) to one of excessive, binge alcohol drinking (i.e., BACs >0.8 mg/ml) (National Institute on Alcohol Abuse and Alcoholism, 2007), we first examined the effects of six bouts of SHAC during the total protein expression of Homer2a/b, Homer1b/c, their associated glutamate receptors (mGluR1, mGluR5, NR2a, and NR2b), PI3K, as well as the p(Tyr)p85α PI3K binding motif, within several brain regions implicated in the neurobiology of alcoholism, including the NAC, PFC, striatum, and hippocampus (Sullivan and Pfefferbaum, 2005; Bell et al., 2006; Koob and Le Moal, 2008). The B6 mice exposed to the SHAC procedure in this experiment exhibited a mean alcohol intake of 1.6 ± 0.2 g/kg over the six 30-min alcohol bottle presentations, which (based on the results of our regression analysis) (Fig. 2) was predicted to yield a BAC of 1.03 mg/ml and demonstrated previously by our group to result in an actual BAC of 1.09 ± 3.73 mg/ml (Szumlinski et al., 2007). These data indicate (1) the feasibility of using the results of our regression analysis to predict BACs from observations of alcohol intake in our studies, and (2) by National Institute on Alcohol Abuse and Alcoholism standards (National Institute on Alcohol Abuse and Alcoholism, 2007), the experimental mice in the immunoblotting study exhibited binge alcohol consumption under our SHAC procedures.
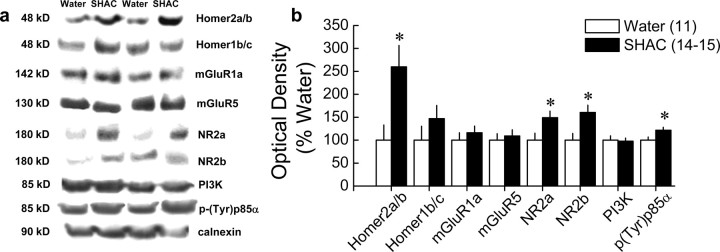
As summarized in Figure 3 and Table 1, binge alcohol drinking coregulated Homer2a/b, NR2a/b, and p(Tyr)p85α PI3K binding motif levels selectively within the NAC. Consistent with the effects of continuous alcohol drinking (Szumlinski et al., 2008b), binge drinking under the SHAC procedure more than doubled NAC Homer2a/b expression (t(23) = 2.22; p = 0.04), without affecting significantly Homer1b/c levels (Fig. 3). Moreover, the SHAC procedure-induced rise in NAC Homer2a/b was accompanied by a smaller, albeit significant, increase in NAC levels of NR2a (t(23) = 2.35; p = 0.03) and NR2b (t(23) = 2.75; p = 0.01), but binge drinking under the SHAC procedure did not increase the total protein expression of either group 1 mGluR subtype (Fig. 3). Consistent with the hypothesis that excessive alcohol drinking induces the activation of PI3K within the NAC, B6 mice exposed to the SHAC procedure exhibited increased p(Tyr)p85α PI3K binding motif levels (t(24) = 2.34; p = 0.03), without any change in total PI3K expression.
Figure 3.
Repeated bouts of binge alcohol intake with the SHAC procedure elevate NAC protein expression of Homer2, NR2 subunits, and PI3K activation in B6 mice. a, Representative immunoblots for the total protein levels of Homer2a/b, Homer1b/c, mGluR1, mGluR5, NR2a, NR2b, PI3K, p(Tyr)p85α PI3K binding motif [p(Try)p85α], and calnexin (loading control) in the NAC of groups of mice killed at 24 h after six bouts of 30 min access to 5% alcohol (SHAC) or water. b, Summary of the change in protein expression after withdrawal from six bouts of limited access to alcohol, expressed as a percentage of the average protein expression of water-drinking controls. Compared with water controls, binge alcohol intake with the SHAC procedure significantly increased Homer2a/b, NR2a/b, and p(Tyr)p85α expression (t tests, *p < 0.05). Data in b represent the mean ± SEM of the number of animals indicated in the figure.
Table 1.
Summary of the means ± SEM of the effects of six bouts of 30 min access to 5% alcohol on Homer1b/c, Homer2a/b, mGluR1, mGluR5, NR2a, NR2b, and PI3K within the PFC, dorsal striatum, and hippocampus of mice
| PFC |
Striatum |
Hippocampus |
||||
|---|---|---|---|---|---|---|
| Water | Ethanol | Water | Ethanol | Water | Ethanol | |
| Homer1b/c | 100 ± 6.4 | 108.9 ± 11.9 | 100 ± 11.6 | 103 ± 6.4 | 100 ± 9.29 | 97.87 ± 6.92 |
| Homer2a/b | 100 ± 11.5 | 89.9 ± 11.4 | 100 ± 7.1 | 88.3 ± 10.5 | 100 ± 13.91 | 112.98 ± 11.04 |
| mGluR1a | 100 ± 7.3 | 108.8 ± 9.6 | 100 ± 11.8 | 92.5 ± 9.4 | 100 ± 16.92 | 308.12 ± 54.95* |
| mGluR5 | 100 ± 20.46 | 108.43 ± 25.38 | 100 ± 12.2 | 104.1 ± 12.0 | 100 ± 13.62 | 152.93 ± 15.53* |
| NR2a | 100 ± 6.8 | 101.8 ± 10.9 | 100 ± 8.1 | 88.4 ± 11.3 | 100 ± 15.22 | 164.86 ± 20.5 |
| NR2b | 100 ± 7.7 | 146.0 ± 18.4* | 100 ± 11.6 | 97.5 ± 10.2 | 100 ± 7.09 | 166.58 ± 15.12* |
| PI3K | 100 ± 9.6 | 75.7 ± 8.9 | 100 ± 10.5 | 111.7 ± 11 | 100 ± 9.75 | 98.37 ± 9.42 |
The data are expressed as a percentage of the mean optical density values of water-drinking controls. Water, n = 11; binge ethanol, n = 14–15;
*p < 0.05 (t test).
In contrast to the data for the NAC (Fig. 3), binge alcohol drinking under the SHAC procedure failed to significantly affect Homer/mGluR/NR2/PI3K protein expression or the levels of p(Tyr)p85α PI3K binding motif within the dorsal striatum. Of all the proteins examined, only expression of NR2b increased within the PFC (Table 1). However, as summarized in Table 1, binge alcohol drinking under the SHAC procedure increased the hippocampal protein expression of mGluR1a (t(25) = 2.62; p = 0.02) and mGluR5 (t(27) = 2.24; p = 0.03), as well as NR2b (t(25) = 3.01; p = 0.01), but did not affect the expression of the other proteins examined. These data demonstrate for the first time that a history of binge alcohol drinking promotes PI3K signaling selectively within the NAC, which appears to be associated with a rise in Homer2a/b, but not group 1 mGluR, expression.
Reduction in binge alcohol drinking by NAC Homer2 knockdown
Homer2 expression within the NAC shell actively regulates alcohol intake under continuous-access and operant self-administration conditions (Szumlinski et al., 2005b, 2008b). Because the SHAC procedure elicited a large rise in the NAC total protein expression of Homer2a/b (Fig. 3), the first series of behavioral experiments used an AAV-mediated neuronal transfection strategy (for review, see Klugmann and Szumlinski, 2008) to examine the functional relevance of the SHAC-induced rise in NAC Homer2 protein expression for excessive, binge alcohol-drinking behavior. As reported previously (Szumlinski et al., 2004, 2005b, 2006, 2008b; Klugmann et al., 2005; Lominac et al., 2005), immunostaining for the HA tag revealed neuronal transfection for all three AAVs used (cDNA–Homer2b, shRNA1–Homer2b, and shRNA2–Homer2b) with no overt signs of neurotoxicity, regardless of vector or the localization of the transfection. Consistent also with our previous reports and as exemplified by Figure 4a and its subpanels (target is NAC core), transfection was observed within both cell bodies and processes within 1 mm of the infusion site (Fig. 4a″).
In contrast to the data obtained using other animal models of alcohol reward/intake (Szumlinski et al., 2005b, 2008b), AAV-mediated Homer2b overexpression within the NAC shell failed to enhance significantly the alcohol intake exhibited by B6 mice with the SHAC procedure, despite the average intakes of both AAV treatment groups being ∼0.75 g/kg over the course of six alcohol bottle presentations (Fig. 4b). As illustrated in Figure 4c, NAC shell Homer2b overexpression also did not affect water intake under SHAC procedures, a finding consistent with our previous reports that NAC Homer2 expression does affect the motivational valence of natural rewards (Szumlinski et al., 2004, 2005a,b, 2008b). To examine the possibility that the effects of NAC Homer2b overexpression during drinking with the SHAC procedure might be subregion specific, the experiment was replicated infusing the AAVs into the NAC core. Again, we failed to detect any significant effect of NAC core Homer2b overexpression during either alcohol intake (Fig. 4b) or water intake (Fig. 4c). These data indicate that elevating NAC Homer2b expression in an animal with a history of binge alcohol drinking is not sufficient to enhance further their alcohol consumption, at least within the confines of the murine SHAC model.
Studies of Homer2 knock-out (KO) mice indicated that NAC Homer2 expression is necessary for alcohol consumption as assessed under both ad libitum access and operant self-administration paradigms (Szumlinski et al., 2005b). To avoid developmental confounds associated with the use of constitutive gene knock-out animals (Gerlai, 2001) yet extend our previous Homer2 KO data to an animal model of binge alcohol drinking, we used an AAV–shRNA infusion strategy, demonstrated previously to reduce NAC shell Homer2b expression by 50% (Klugmann and Szumlinski, 2008). The infusion of two different shRNA–Homer2b constructs into the NAC shell significantly reduced the average amount of alcohol consumed by the B6 mice during the SHAC procedure by ∼20% (Fig. 4b) (F(2,28) = 3.57; p = 0.04), and post hoc analysis indicated no differences between the two shRNA constructs in this regard. Although BACs were not determined in this study, alcohol dose is highly predictive of BACs in the SHAC model (Cronise et al., 2005; Finn et al., 2005). Based on the results of our regression analysis (Fig. 2), the ∼1 g/kg dose of alcohol consumed by shRNA-treated mice is predicted to yield a BAC of ∼0.87 mg/ml, indicating that NAC shell Homer2b knockdown significantly attenuated, but did not prevent, the expression of binge drinking. Consistent with our previous data for Homer2 KO mice (Szumlinski et al., 2004, 2005a,b), NAC shell Homer2b knockdown did not affect the average water intake of the mice exhibited during testing (Fig. 4c), indicating that this manipulation did not influence the motivation for, or physical capacity to, consume fluids. Taken altogether, these data indicate that the large rise in NAC Homer2b expression produced by repeated bouts of binge alcohol drinking, although not sufficient, may be necessary for the full expression of excessive drinking behavior.
Reduction in binge alcohol drinking by NAC mGluR5 and PI3K blockade
Systemic pretreatment with group 1 mGluR antagonists, particularly against the mGluR5 subtype, reduces alcohol intake under both limited and continuous-access conditions (Bäckström et al., 2004; Olive et al., 2005; Schroeder et al., 2005; Hodge et al., 2006; Lominac et al., 2006; Bäckström and Hyytiä, 2007; Besheer et al., 2008a,b; Blednov and Adron Harris, 2008). Although NAC mGluR5 activity is required for cue-induced cocaine-seeking behavior in rats (Bäckström and Hyytiä, 2007), the brain regions involved in mGluR1/5 regulation of alcohol intake remain uncharacterized. Homers interact directly with the C terminus of group 1 mGluRs (Tu et al., 1998; Xiao et al., 1998) and form a complex with PIKE-L, which can regulate both constitutive and stimulated PI3K activity (Rong et al., 2003). The observed SHAC procedure-induced increases in the amount of p(Tyr)p85α PI3K binding motif expression (Fig. 3) suggested an important role for mGluR-mediated stimulation of PI3K activity within the NAC in maintaining binge alcohol-drinking behavior. To test this hypothesis, groups of B6 mice were trained to binge drink alcohol (1.5–1.6 g/kg in 30 min; predicted BACs, ∼1.0 mg/ml) (Fig. 5a) and then pretreated intra-NAC shell with several doses of the selective mGluR5 antagonist MPEP or a PI3K-selective dose of wortmannin (Rong et al., 2003) before a 30 min alcohol-drinking test session. For comparison, the effects of intra-NAC pretreatment with the selective mGluR1 antagonist CPCCOEt were also assessed.
Figure 5.
Blockade of NAC mGluR5 and PI3K, but not mGluR1, reduces binge alcohol drinking in B6 mice. a, Summary of the effects of an intra-NAC shell infusion of various doses of the mGluR5 antagonist MPEP (filled bars), 50 ng/side wortmannin, and the combination of MPEP plus wortmannin (hatched bars), as well as various doses of the mGluR1 antagonist CPCCOEt (open bars) on 5% alcohol intake during a 30 min period with the SHAC procedure. b, Summary of the effects of intra-NAC MPEP and wortmannin on water intake during a 30 min period. The data represent the mean ± SEM of the number of animals indicated in each bar in the figure. *p < 0.05 versus respective vehicle pretreatment (i.e., 0 dose) (least significant difference post hoc tests).
As illustrated in Figure 5a, alcohol intake was reduced dose dependently by intra-NAC MPEP (0, 0.1, 0.3, and 1.0 μg/side; F(3,27) = 5.75; p = 0.004), with estimated BACs of 0.99, 0.87, 0.84, and 0.83 mg/ml, respectively. An attenuation of alcohol intake was observed also after the local infusion of 50 ng/side wortmannin (estimated BACs: 0 ng of wortmannin, 1.03 mg/ml; 50 ng of wortmannin, 0.83 mg/ml), and the coinfusion of 50 ng/side wortmannin with 1.0 μg/side MPEP failed to further reduce alcohol intake (F(2,10) = 5.77; p = 0.02) (Fig. 5a). Thus, NAC mGluR5 signaling through PI3K appears to be involved in the expression of binge alcohol-drinking behavior under the SHAC procedure.
In contrast to the effect of mGluR5 and PI3K blockade, mGluR1 antagonism by intra-NAC CPCCOEt (0, 1.0, and 3.0 μg/side) produced only a moderate reduction in alcohol intake with the SHAC procedure, which failed to reach statistical significance (Fig. 5a). Unfortunately, because of solubility issues, the effects of higher CPCCOEt doses could not be assessed. Thus, it remains to be determined whether or not the inhibitory effect of systemic pretreatment of mGluR1-selective antagonists during alcohol intake (Lominac et al., 2006; Besheer et al., 2008b) involves inhibition of mGluR1 receptors within the NAC shell.
As was observed for NAC Homer2b knockdown (Fig. 4c) and consistent with published studies indicating that systemic mGluR5 blockade or mGluR5 deletion does not reduce the motivation for, or consumption of, natural reinforcers such as water and sucrose nor do these pretreatments alter spontaneous locomotor activity (Bäckström et al., 2004; Olive et al., 2005; Schroeder et al., 2005; Hodge et al., 2006; Lominac et al., 2006; Bäckström and Hyytiä, 2007; Besheer et al., 2008a; Blednov and Adron Harris, 2008), intra-NAC pretreatment with 1.0 μg/side MPEP or 50 ng/side wortmannin did not affect water intake during a 30 min test session (Fig. 5b). These results indicate that neither pretreatment produced an effect on general motivational or motor processes that could have negatively affected the ability to consume fluids under these limited-access conditions.
Reduction in binge alcohol drinking by disrupting mGluR5–Homer interaction
The capacity of mGluR5 to regulate PI3K activity in vitro requires Homer interactions with the C terminus of the receptor (Rong et al., 2003). Thus, we next tested the hypothesis that the physical interaction between mGluR5 and Homer is critical for the maintenance of binge alcohol-drinking behavior. For this, the SHAC phenotype of TG mice with an F1128R point mutation in mGluR5 (mGluR5F1128R) that reduces Homer binding to the receptor (Tu et al., 1998) was assessed. As observed in inbred B6 mice (Figs. 4, 5) (Szumlinski et al., 2007), our SHAC procedures elicited high levels of alcohol intake also in WT B6–129 hybrid mice (∼1.5 g/kg per 30 min; estimated BAC of 1.0 mg/ml), which was reduced by ∼75% in littermate mGluR5F1128R mutants (WT, 1.46 ± 0.24 g/kg vs TG, 0.37 ± 0.08 g/kg; F(1,20) = 14.80; p = 0.001) and predicted to result in an estimated BAC of 0.69 mg/ml. Because this estimate is well below the National Institute on Alcohol Abuse and Alcoholism criterion for binge drinking (National Institute on Alcohol Abuse and Alcoholism, 2007), these genotypic differences indicate that the physical interaction between mGluR5 and Homers is necessary for maintaining binge alcohol drinking.
In contrast to the pronounced effect of the mGluR5F1128R mutation during alcohol intake, genotypic differences were not observed for the average water intake (WT, 36.1 ± 6.87 ml/kg vs TG, 30.1 ± 6.65 ml/kg; p > 0.05) or average intake of a palatable 3% sucrose solution (WT, 1.84 ± 0.42 g/kg vs TG, 1.87 ± 0.38 g/kg; p > 0.05) exhibited by the mice during SHAC procedures.
To determine whether or not the blunted SHAC phenotype of mGluR5F1128R mutants extended to a model of voluntary alcohol preference drinking, a separate group of WT and mutant littermates were allowed ad libitum access to four sipper tubes containing 0, 3, 6, and 12% alcohol (v/v) simultaneously in the home cage, and drinking was monitored over a 24 h period for a total of 3 weeks. In stark contrast to the findings with the SHAC procedure, no genotypic differences were observed for total daily alcohol intake (WT, 10.84 ± 2.26 g/kg vs TG, 14.00 ± 1.80 g/kg; p > 0.05), total water intake (WT, 97.07 ± 22.29 ml/kg vs TG, 87.49 ± 15.36 ml/kg; p > 0.05), or alcohol preference (genotype × alcohol concentration; ANOVA, p > 0.05) in this four-bottle-choice, continuous-access procedure. Thus, it does not appear that the reduction in binge alcohol drinking with the SHAC procedure observed in mGluRF1128R mutants reflects a general disruption in the capacity to drink moderate amounts of alcohol. Because both genotypes showed concentration-dependent patterns of voluntary alcohol intake (alcohol effect, F(2,36) = 20.01; p < 0.0001) and preference (alcohol effect, F(3,54) = 3.28; p = 0.03), the mGluR5F1128R mutants clearly can distinguish between the taste of the various ethanol solutions as well as WT animals. These data for (1) water and sucrose consumption under SHAC procedures and (2) alcohol/water preference and consumption under continuous-access procedures, combined with unpublished behavioral phenotyping data indicating no consistent effects of the mGluR5 mutation on various measures of emotionality, motor function, and cognitive processing, all indicate that the reduction in binge alcohol drinking produced by the disruption of mGluR5–Homer interactions does not reflect nonspecific effects of the mutation on general reward, motivational, emotional, or motor processes.
mGluR5–Homer binding is necessary for the anti-binge-drinking effect of NAC mGluR5 and PI3K blockade
To test the hypothesis that signaling through an mGluR5–Homer2–PI3K pathway is critical for excessive, binge alcohol drinking, we next assessed the capacity of intra-NAC infusions of 1.0 μg/side MPEP and 50 ng/side wortmannin to reduce binge drinking with the SHAC procedure in WT and mGluR5F1128R littermates. Replicating the data presented above, marked genotypic differences in binge drinking were observed in vehicle-infused mice, with the intake of the mutants estimated to yield a BAC of 0.8 versus 1.0 mg/ml in WT animals. In contrast to vehicle-infused animals, genotypic differences in binge drinking were not observed in mice pretreated intra-NAC with either MPEP or wortmannin (Fig. 6) (genotype effect: F(1,11) = 4.32, p = 0.05; drug effect: F(2,22) = 4.50, p = 0.02). The data in Figure 6 suggested that the effect of intra-NAC antagonist infusion was selective for WT animals, which was confirmed by the results of one-way ANOVAs conducted separately for each genotype (WT: F(2,12) = 3.97, p = 0.04, post hoc tests; KO, p = 0.29). Together, these data strongly support the notion that intact signaling through an mGluR5–Homer2–PI3K complex within the NAC shell is important for a binge alcohol-drinking phenotype.
Figure 6.
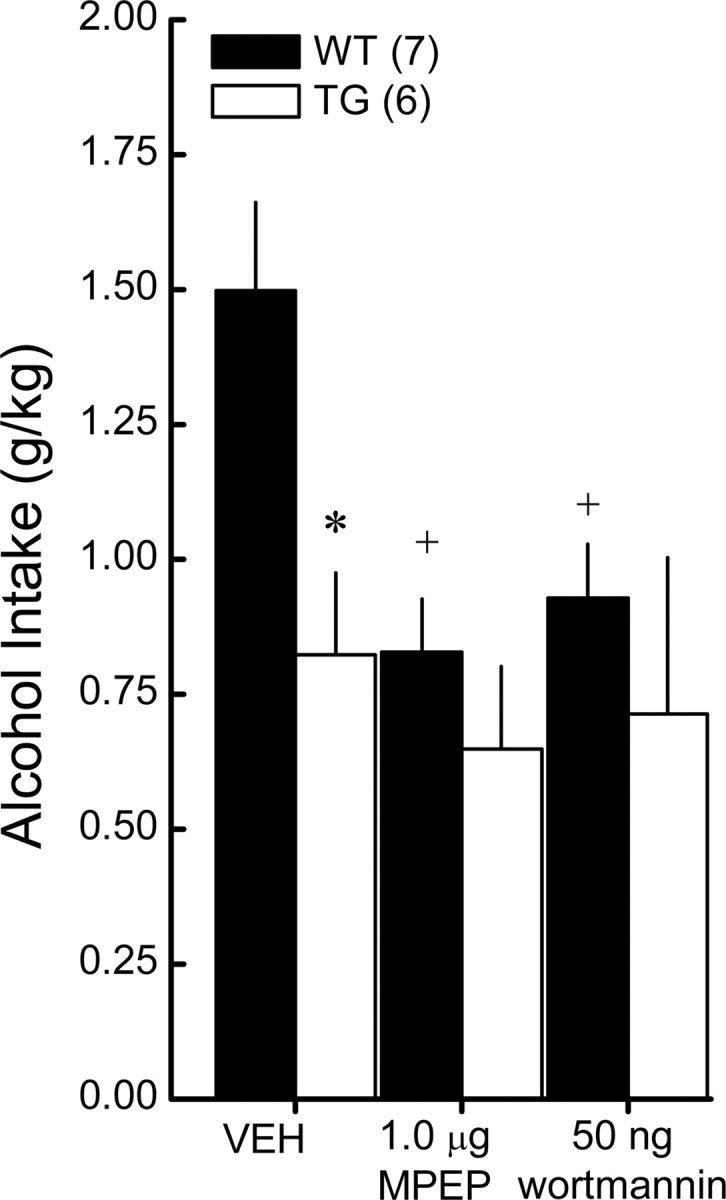
mGluR5–Homer binding is necessary for the anti-binge effects of NAC mGluR5 and PI3K blockade. Summary of the effects of an intra-NAC shell infusion of effective doses of MPEP and wortmannin on the 5% alcohol intake in the SHAC procedure exhibited by WT and mGluR5F1128R TG mice. The data represent the mean ± SEM of the number of animals indicated in the figure. *p < 0.05, WT versus TG; +p < 0.05 versus vehicle (VEH; 0 dose).
Because blunted alcohol intake in Homer2 KO mice is associated with perturbations in glutamate receptor expression within the NAC (Szumlinski et al., 2004, 2005), we next related the genotypic differences in binge drinking within the SHAC procedure to the basal expression of Homers, glutamate receptors, and PI3K, as well as PI3K activity within the NAC. Consistent with the data for whole brain (Fig. 1d,e), naive mGluR5F1128R mutants failed to exhibit significant differences from WT littermates in the total NAC protein expression of any of the glutamate receptor subtypes/subunits examined, nor did they differ significantly in their total Homer1/2 protein expression (Fig. 7). Although a moderate, but nonsignificant genotypic difference was observed regarding total PI3K expression, mGluR5F1128R mutants exhibited an ∼35% reduction in basal PI3K activity, as assessed by NAC levels of p(Tyr)p85α PI3K binding motif (t(17) = 2.56; p = 0.02). These data confirm that the physical interaction between mGluR5 and Homers do not regulate their total protein expression (Tu et al., 1998) (Fig. 1d,e) but that this interaction is required for normal constitutive PI3K activity in vivo. Moreover, these data provide our first evidence that a relationship exists between a genetic propensity for binge alcohol drinking under limited-access conditions and the basal activational state of PI3K within the NAC.
Figure 7.
mGluR5F1128R mutation reduces NAC basal PI3K activity. a, Representative immunoblots for the total protein levels of Homer2a/b, Homer1b/c, mGluR1, mGluR5, NR2a, NR2b, PI3K, p(Tyr)p85α PI3K binding motif [p(Try)p85α], and calnexin (loading control) in the NAC of experimentally naive WT and mGluR5F1128R TG mice. b, Summary of the genotypic differences in protein expression, expressed as a percentage of average levels of WT controls. Data in b represent the mean ± SEM of the number of mice indicated in the figure. *p < 0.05 versus WT (t tests).
Variance in NAC mGluR/Homer/PI3K expression between selectively bred SHAC and SLAC mice
To determine whether constitutive NAC PI3K activation represented a correlated response to selection, we next conducted immunoblotting on NAC tissue derived from fourth generation (S4) offspring of selectively bred male and female SHAC and SLAC mice. As is evident from an examination of the selection patterns for both alcohol intake and BACs (the selection phenotype), these responses vacillated across these early generations (Fig. 8), as would be expected of responses to selective breeding from an eight-way cross (for discussion, see Crabbe et al., 2009). The observation that the divergence in selection phenotype (BAC), but also alcohol intake, was asymmetrical and that parallel shifts in the absolute responses occurred between SHAC and SLAC lines across generations (Fig. 8a,b) suggests an environmental influence on these measures (e.g., seasonal effects). Of relevance to this report, the selection procedures used (for details, see Materials and Methods) produced a significant divergence in limited-access alcohol drinking across generations, as indexed by the alcohol intake of the mice (Fig. 8a) (genotype effect: F(1,745) = 67.63, p < 0.001; generation effect: F(3,745) = 19.44, p < 0.001; interaction: F(3,745) = 2.61, p = 0.05) and the selection criterion of BACs attained after the second 30 min alcohol-drinking session in the SHAC paradigm (Fig. 8b) (genotype effect: F(1,748) = 68.46, p < 0.001; generation effect: F(3,748) = 21.72, p < 0.001; interaction: F(3,748) = 5.44, p = 0.001; n = 80–104 per line per generation). The significant divergence in alcohol intake, as well as the selection phenotype of BAC, was evident by the second generation of selection, and this divergence remained stable for the subsequent generations.
There was no significant line difference in water intake in offspring from generations S1, S3, and S4, although there was a slight but significant increase in water consumption in the S2 SHAC versus SLAC lines on days 2, 4, and 5 of testing (data not shown). Importantly, there was no correlation between the water and alcohol intake exhibited by either group of mice during testing, nor was there a line difference in total fluid intake on day 3 (alcohol plus water) for any generation (i.e., SHAC mice increased their alcohol intake relative to water, whereas SLAC mice increased their water intake relative to alcohol). Based on such data, in conjunction with the divergence in the lines in ethanol intake, it is unlikely that we were selecting simply on the basis of thirst. Unfortunately, because the feeding patterns of the mice were not measured during the first 3 h of the dark cycle, the possibility exists that our SHAC procedures may have selected for prandial food intake. However, our observation that body weight (and water intake) did not differ across days in the SHAC and SLAC lines suggests also that we are not selecting for simple prandial factors. From the data depicted in Figure 8b, we estimated the heritability of the SHAC/SLAC trait from the multiple r2 from the ANOVA of the line difference in S4 BAC. Heritability was estimated as h2 = 0.16, indicating that 16% of individual differences in BAC after drinking were attributable to genetic influences. These data support the notion that binge alcohol drinking as assessed by the murine SHAC model is a genetically transmissible trait. Notably, the selection pressure and concomitant divergence of the selection phenotype alters the allele frequencies of genes that are relevant to the trait of interest, making selected lines a powerful tool to explore mechanisms underlying the selected trait.
As illustrated in Figure 8, c and d, the S4 offspring of the SHAC and SLAC selected lines differed regarding NAC levels of Homer2a/b (F(1,12) = 6.05; p = 0.03), mGluR1 (F(1,12) = 6.36; p = 0.03), and p(Tyr)p85α (F(1,12) = 10.43; p = 0.008), with the SHAC line exhibiting greater protein expression in all cases when compared with the SLAC line. Although SHAC mice also exhibited elevated mGluR5 expression, this line difference was shy of statistical significance (p = 0.09). These data are consistent with the data from the mGluR5F1128R mutant study (Fig. 7), as well as unpublished immunoblotting data from our laboratory derived from studies of inbred mice (S. P. Goulding, I. Obara, K. D. Lominac, M. Klugmann, and K. K. Szumlinski, unpublished observations) and further the notion that genetic vulnerability to an excessive, binge alcohol-drinking phenotype might relate to the basal functional status of the mGluR–Homer2–PI3K signaling pathway within the NAC.
Discussion
The present report provides in vivo validation of the involvement of an mGluR5–Homer2–PI3K signaling pathway within the NAC shell in binge alcohol-drinking behavior by showing that the site-directed pharmacological and transgenic interruption of this pathway reduces the extent to which mice drink alcohol within the context of the SHAC model of alcoholism. This finding extends previous indications of important roles for Homer2 and mGluR5 in regulating alcohol reward in various rodent models of alcohol intake (Bäckström et al., 2004; Olive et al., 2005; Schroeder et al., 2005; Szumlinski et al., 2005b, 2008b), as well as an association between polymorphisms in genes encoding glutamate receptors and SNPs in the p85 regulatory subunit of PI3K with risky alcohol-drinking behavior in human adolescents (Desrivières et al., 2008; Schumann et al., 2008). As observed after chronic, moderate alcohol intake (Szumlinski et al., 2008b), repeated bouts of binge drinking elevate NAC Homer2 levels, and, consistent with in vitro evidence that Homer proteins complex with the PI3K-enhancer molecule PIKE-L to regulate constitutive PI3K activity (Rong et al., 2003), the binge-drinking-induced rise in NAC Homer2 was concomitant with increased basal PI3K activation. Moreover, an examination of both mutant and selectively bred mouse lines revealed a relationship between genotypic differences in binge alcohol drinking and basal NAC expression of Homer2, group 1 mGluRs, and/or PI3K activity. Together, these results implicate idiopathic and alcohol-drinking-induced increases in the functional status of the mGluR5–Homer2–PI3K signaling pathway within the NAC shell subregion in regulating the propensity to binge drink alcohol under limited-access conditions.
Accumbens glutamate, mGluR5, and binge alcohol-drinking behavior
Clinical and preclinical data support a relationship between the propensity to consume/prefer large amounts of alcohol, a reduction in behavioral sensitivity to alcohol, and alcohol-induced increases in metabolic activity or glutamate release within the NAC or within its major glutamatergic afferent structures (McBride et al., 1986; Selim and Bradberry, 1996; Dahchour et al., 2000; Moselhy et al., 2001; Piepponen et al., 2002; Meyerhoff et al., 2004; Kapasova and Szumlinski, 2008). Repeated bouts of binge alcohol drinking under SHAC procedures sensitizes the capacity of ingested alcohol to elevate NAC levels of extracellular glutamate (Szumlinski et al., 2007), and pharmacological manipulations of NAC glutamate actively regulate alcohol ingestion in mice (Kapasova and Szumlinski, 2008).
Alcohol inhibits mGluR5 function (Minami et al., 1998), and the therapeutic efficacy of acamprosate for treating alcoholism is hypothesized to relate to a reduction in alcohol-induced glutamate hyperexcitability via mGluR5 blockade (Harris et al., 2002; Littleton and Zieglgänsberger, 2003; Lominac et al., 2006). The present data provide the first evidence that glutamate activation of mGluR5 receptors located specifically within the NAC shell is necessary for high levels of binge alcohol-drinking behavior under limited-access conditions and thereby point to NAC shell mGluR5 receptors as important for the anti-alcohol effects of systemic mGluR5 antagonist treatment, a finding consistent with the results of a recent intravenous alcohol self-administration study by Gass and Olive (2009). Because the total protein expression of mGluR5 is unaltered within the NAC after either chronic, continuous alcohol drinking (Szumlinski et al., 2008b) or repeated bouts of binge alcohol drinking (Fig. 3), it is possible that idiopathic or drug-induced anomalies in mGluR5 intracellular signaling might modulate the propensity to consume excessive amounts of alcohol in a manner independent of changes in receptor protein expression.
Accumbens mGluR–Homer2 interactions and binge alcohol-drinking behavior
Consistent with the notion that mGluR5-mediated signaling contributes to an excessive alcohol-drinking phenotype, the SHAC selected line exhibited a marked rise in the NAC Homer2a/b expression relative to the SLAC selected line. Thus, an increase in this Homer isoform demonstrated previously to play an active role in regulating various aspects of alcohol reward in mice (Szumlinski et al., 2005b, 2008b) is a correlated response to selection. As observed on mGluR5 blockade or mGluR5 deletion (see above), Homer2 deletion produces an alcohol-avoiding behavioral phenotype (Szumlinski et al., 2005b), which can be “rescued” by AAV-mediated restoration of NAC Homer2 expression. Moreover, AAV-mediated Homer2b overexpression within the NAC shell of both inbred B6 mice and B6-hybrid mice augments the rewarding/reinforcing properties of alcohol (Szumlinski et al., 2005b, 2008b). However, mimicking the SHAC procedure-induced rise in NAC Homer2b levels by AAV-mediated Homer2 overexpression failed to augment alcohol intake in this binge alcohol-drinking model. The precise explanation for this negative result might relate to the following: (1) a ceiling effect on alcohol drinking imposed by the limited-access conditions of the SHAC procedure (although the alcohol intake of both AAV-treated groups in the NAC shell study was relatively low compared with that of the NAC core and shRNA study) (see Fig. 4b); (2) competition for proline-rich motif binding between exogenous protein and the very high background of endogenously expressed Homer2 produced by repeated, binge alcohol intake (Fig. 3); or (3) differences in the underlying motivational factors governing alcohol intake under limited-access, forced-choice conditions. Nevertheless, the observation that shRNA-mediated knockdown of NAC Homer2 expression reduced binge alcohol drinking (Fig. 4b) in a manner consistent with the anti-alcohol phenotype of constitutive Homer2 knock-out mice (Szumlinski et al., 2005b) and, akin to that observed in (1) mice treated intra-NAC with an mGluR5 antagonist (Fig. 5) and (2) mice with a transgenic disruption of the Homer binding site on mGluR5 (Fig. 6), supports an important role for mGluR5 signaling through Homer2 in excessive alcohol drinking. This notion is strengthened by the observation that an intra-NAC infusion of MPEP failed to reduce further the relatively low alcohol intake of mGluR5F1128R mutants (Fig. 6). Although not negating a potential role for Homer1 gene products in regulating binge alcohol drinking, this collection of data supports an important role for the physical interaction between mGluR5 and Homer2 in the anti-binge effects of mGluR5 antagonists.
Accumbens PI3K activity and binge alcohol-drinking behavior
Complementing previous in vitro data (Rong et al., 2003) and supporting a potential role for PI3K activity in excessive alcohol intake, the SHAC procedure-induced rise in NAC Homer2a/b expression was accompanied by elevations in p(Tyr)p85α PI3K binding motifs (Fig. 3), an index of PI3K activation (Zhang et al., 2006). Moreover, the basal NAC expression of p(Tyr)p85α PI3K binding motifs was elevated in a selectively bred mouse line exhibiting high binge alcohol intake [i.e., SHAC line (Fig. 8)] and reduced in mGluR5F1128R mutant mice exhibiting blunted binge-drinking behavior (Fig. 7). Pharmacological antagonism of PI3K activity within the NAC shell significantly reduced binge alcohol drinking (estimated BACs, ∼0.83 mg/ml), and this effect was not additive with the reduction in drinking produced by mGluR5 inhibition (Fig. 5) nor by transgenic inference of mGluR5–Homer binding (Fig. 6). Although not negating a potential role for Homer2 effects on the conductance of voltage- and ligand-gated ion channels (e.g., the NMDA receptor) or group 1 mGluR-mediated activation of IP3-dependent calcium release or PKC activation (Tu et al., 1998; Kammermeier et al., 2000; Hwang et al., 2005; Mao et al., 2005; Sala et al., 2005; Szumlinski et al., 2005b; Yamamoto et al., 2005; Kammermeier and Worley, 2007; Kammermeier, 2008), the collection of data presented in this report argues strongly in favor of increased mGluR5-mediated activation of PI3K, presumably via Homer2, as a critical molecular adaptation contributing to the propensity to binge drink excessive amounts of alcohol. Given the recent association of SNPs in PI3K with risky alcohol-drinking behavior in adolescent humans (Desrivières et al., 2008; Schumann et al., 2008), the present data have direct relevance not only for our basic understanding of how repeated bouts of excessive, binge alcohol drinking impact excitatory neurotransmission but also inform as to potential gene candidates mediating vulnerability to this prevalent form of alcoholism.
Footnotes
This work was funded by National Institutes of Health Grants AA016650 [Integrative Neuroscience Initiative on Alcoholism (INIA) West] and AA015351 (K.K.S.), AA013478 (INIA West) (D.A.F.), AA13519 (INIA West) and AA10760 (J.C.C.), and DA 00266 and DA011742 (P.F.W.), grants from the Department of Veterans Affairs (D.A.F., J.C.C.), and an Emerging Research Excellence Award, University of Auckland (M.K.).
References
- Ary and Szumlinski, 2007.Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: a two-species comparison. Brain Res. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Ary et al., 2007.Ary AW, Aguilar VR, Szumlinski KK, Kippin TE. Prenatal stress alters limbo-corticostriatal Homer protein expression. Synapse. 2007;61:938–941. doi: 10.1002/syn.20439. [DOI] [PubMed] [Google Scholar]
- Bäckström and Hyytiä, 2007.Bäckström P, Hyytiä P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Bäckström et al., 2004.Bäckström P, Bachteler D, Koch S, Hyytiä P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Bell et al., 2006.Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Besheer et al., 2008a.Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008a;32:209–221. doi: 10.1111/j.1530-0277.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer et al., 2008b.Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats. Alcohol. 2008b;42:13–20. doi: 10.1016/j.alcohol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov and Adron Harris, 2008.Blednov YA, Adron Harris R. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11:775–793. doi: 10.1017/S1461145708008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe, 1990.Crabbe JC. Animal models in neurobehavioral genetics: methods for estimating genetic correlation. In: Jones BC, Mormede P, editors. Neurobehavioral genetics: methods and applications. New York: CRC; 1990. pp. 121–138. [Google Scholar]
- Crabbe et al., 1990.Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Crabbe et al., 1999.Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK. Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends Neurosci. 1999;22:173–179. doi: 10.1016/s0166-2236(99)01393-4. [DOI] [PubMed] [Google Scholar]
- Crabbe et al., 2009.Crabbe JC, Metten P, Rhodes JS, Yu CH, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiatry. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronise et al., 2005.Cronise K, Finn DA, Metten P, Crabbe JC. Scheduled access to ethanol results in motor impairment and tolerance in female C57BL/6J mice. Pharmacol Biochem Behav. 2005;81:943–953. doi: 10.1016/j.pbb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Dahchour et al., 2000.Dahchour A, Hoffman A, Deitrich R, de Witte P. Effects of ethanol on extracellular amino acid levels in high- and low-alcohol sensitive rats: a microdialysis study. Alcohol Alcohol. 2000;35:548–553. doi: 10.1093/alcalc/35.6.548. [DOI] [PubMed] [Google Scholar]
- Desrivières et al., 2008.Desrivières S, Krause K, Dyer A, Frank J, Blomeyer D, Lathrop M, Mann K, Banaschewski T, Laucht M, Schumann G. Nucleotide sequence variation within the PI3K p85 alpha gene associates with alcohol risk drinking behaviour in adolescents. PLoS ONE. 2008;3:e1769. doi: 10.1371/journal.pone.0001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Witte, 2004.De Witte P. Imbalance between neuroexcitatory and neuroinhibitory amino acids causes craving for ethanol. Addict Behav. 2004;29:1325–1339. doi: 10.1016/j.addbeh.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Dole and Gentry, 1984.Dole VP, Gentry RT. Toward an analogue of alcoholism in mice: scale factors in the model. Proc Natl Acad Sci U S A. 1984;81:3543–3546. doi: 10.1073/pnas.81.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During et al., 2003.During MJ, Young D, Baer K, Lawlor P, Klugmann M. Development and optimization of adeno-associated virus vector transfer into the central nervous system. In: Machida CA, editor. Viral vectors for gene therapy: methods and protocols. Vol 76. Totowa, Canada: Humana; 2003. pp. 221–236. [DOI] [PubMed] [Google Scholar]
- Falconer and Mackay, 1996.Falconer SD, Mackay TFC. Introduction to quantitative genetics. Harlow, UK: Longman; 1996. [Google Scholar]
- Finn et al., 2005.Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC. A procedure to produce high alcohol intake in mice. Psychopharmacology. 2005;178:471–480. doi: 10.1007/s00213-004-2039-8. [DOI] [PubMed] [Google Scholar]
- Finn et al., 2007.Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Franich et al., 2008.Franich NR, Fitzsimons HL, Fong DM, Klugmann M, During MJ, Young D. AAV vector-mediated RNAi of mutant huntingtin expression is neuroprotective in a novel genetic rat model of Huntington's disease. Mol Ther. 2008;16:947–956. doi: 10.1038/mt.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass and Olive, 2008.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass and Olive, 2009.Gass JT, Olive MF. Role of protein kinase C epsilon (PKCvarepsilon) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology (Berl) 2009;204:587–597. doi: 10.1007/s00213-009-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai, 2001.Gerlai R. Gene targeting: technical confounds and potential solutions in behavioral brain research. Behav Brain Res. 2001;125:13–21. doi: 10.1016/s0166-4328(01)00282-0. [DOI] [PubMed] [Google Scholar]
- Grahame and Grose, 2003.Grahame NJ, Grose AM. Blood alcohol concentrations after scheduled access in high-alcohol-preferring mice. Alcohol. 2003;31:99–104. doi: 10.1016/j.alcohol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Harris et al., 2002.Harris BR, Prendergast MA, Gibson DA, Rogers DT, Blanchard JA, Holley RC, Fu MC, Hart SR, Pedigo NW, Littleton JM. Acamprosate inhibits the binding and neurotoxic effects of trans-ACPD, suggesting a novel site of action at metabotropic glutamate receptors. Alcohol Clin Exp Res. 2002;26:1779–1793. doi: 10.1097/01.ALC.0000042011.99580.98. [DOI] [PubMed] [Google Scholar]
- Hodge et al., 2006.Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology. 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang et al., 2005.Hwang JI, Kim HS, Lee JR, Kim E, Ryu SH, Suh PG. The interaction of phospholipase C-beta3 with Shank2 regulates mGluR-mediated calcium signal. J Biol Chem. 2005;280:12467–12473. doi: 10.1074/jbc.M410740200. [DOI] [PubMed] [Google Scholar]
- Kammermeier, 2008.Kammermeier PJ. Endogenous homer proteins regulate metabotropic glutamate receptor signaling in neurons. J Neurosci. 2008;28:8560–8567. doi: 10.1523/JNEUROSCI.1830-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier and Worley, 2007.Kammermeier PJ, Worley PF. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc Natl Acad Sci U S A. 2007;104:6055–6060. doi: 10.1073/pnas.0608991104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier et al., 2000.Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J Neurosci. 2000;20:7238–7245. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasova and Szumlinski, 2008.Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Klugmann and Szumlinski, 2008.Klugmann M, Szumlinski KK. Targeting Homer genes using AAV: lessons learned from behavioural and neurochemical studies. Behav Pharmacol. 2008;19:485–500. doi: 10.1097/FBP.0b013e32830c369f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann et al., 2005.Klugmann M, Symes CW, Leichtlein CB, Klaussner BK, Dunning J, Fong D, Young D, During MJ. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci. 2005;28:347–360. doi: 10.1016/j.mcn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Koob and Le Moal, 2008.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Krystal et al., 2003.Krystal JH, Petrakis IL, Mason G, Trevisan L, D'Souza DC. N-methyl-d-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Lê et al., 2001.Lê AD, Israel Y, Juzytsch W, Quan B, Harding S. Genetic selection for high and low alcohol consumption in a limited-access paradigm. Alcohol Clin Exp Res. 2001;25:1613–1620. doi: 10.1111/j.1530-0277.2001.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Littleton and Zieglgänsberger, 2003.Littleton J, Zieglgänsberger W. Pharmacological mechanisms of naltrexone and acamprosate in the prevention of relapse in alcohol dependence. Am J Addict. 2003;12(Suppl 1):S3–S11. doi: 10.1111/j.1521-0391.2003.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Lominac et al., 2005.Lominac KD, Oleson EB, Pava M, Klugmann M, Schwarz MK, Seeburg PH, During MJ, Worley PF, Kalivas PW, Szumlinski KK. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J Neurosci. 2005;25:11586–11594. doi: 10.1523/JNEUROSCI.3764-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac et al., 2006.Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lovinger, 1996.Lovinger DM. Interactions between ethanol and agents that act on the NMDA-type glutamate receptor. Alcohol Clin Exp Res. 1996;20(Suppl):187A–191A. doi: 10.1111/j.1530-0277.1996.tb01773.x. [DOI] [PubMed] [Google Scholar]
- Mao et al., 2005.Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride et al., 1986.McBride WJ, Murphy JM, Lumeng L, Li TK. Effects of ethanol on monoamine and amino acid release from cerebral cortical slices of the alcohol-preferring P line of rats. Alcohol Clin Exp Res. 1986;10:205–208. doi: 10.1111/j.1530-0277.1986.tb05072.x. [DOI] [PubMed] [Google Scholar]
- Meyerhoff et al., 2004.Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middaugh et al., 1999.Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Minami et al., 1998.Minami K, Gereau RW, 4th, Minami M, Heinemann SF, Harris RA. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus oocytes. Mol Pharmacol. 1998;53:148–156. doi: 10.1124/mol.53.1.148. [DOI] [PubMed] [Google Scholar]
- Moselhy et al., 2001.Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, 2004.National Institute on Alcohol Abuse and Alcoholism. NIAAA Newsletter. Vol 3. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2004. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, 2007.National Institute on Alcohol Abuse and Alcoholism. Bethesda, MD: National Institutes of Health; 2007. FAQs for the general public. http://www.niaaa.nih.gov/FAQs/General-English/default.htm. [Google Scholar]
- Olive et al., 2005.Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Paxinos and Franklin, 2004.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coodinates. Oxford: Elsevier; 2004. [Google Scholar]
- Piepponen et al., 2002.Piepponen TP, Kiianmaa K, Ahtee L. Effects of ethanol on the accumbal output of dopamine, GABA, and glutamate in alcohol-tolerant and alcohol-nontolerant rats. Pharmacol Biochem Behav. 2002;74:21–30. doi: 10.1016/s0091-3057(02)00937-1. [DOI] [PubMed] [Google Scholar]
- Rhodes et al., 2005.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rong et al., 2003.Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Sala et al., 2005.Sala C, Roussignol G, Meldolesi J, Fagni L. Key role of the postsynaptic density scaffold proteins Shank and Homer in the functional architecture of Ca2+ homeostasis at dendritic spines in hippocampal neurons. J Neurosci. 2005;25:4587–4592. doi: 10.1523/JNEUROSCI.4822-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder et al., 2005.Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology. 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PubMed] [Google Scholar]
- Schumann et al., 2008.Schumann G, Johann M, Frank J, Preuss U, Dahmen N, Laucht M, Rietschel M, Rujescu D, Lourdusamy A, Clarke TK, Krause K, Dyer A, Depner M, Wellek S, Treutlein J, Szegedi A, Giegling I, Cichon S, Blomeyer D, Heinz A, Heath S, Lathrop M, Wodarz N, Soyka M, Spanagel R, Mann K. Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry. 2008;65:826–838. doi: 10.1001/archpsyc.65.7.826. [DOI] [PubMed] [Google Scholar]
- Selim and Bradberry, 1996.Selim M, Bradberry CW. Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: comparison between the Lewis and Fischer 344 rat strains. Brain Res. 1996;716:157–164. doi: 10.1016/0006-8993(95)01385-7. [DOI] [PubMed] [Google Scholar]
- Siggins et al., 2003.Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, De Lecea L. Glutamatergic transmission in opiate and alcohol dependence. Ann N Y Acad Sci. 2003;1003:196–211. doi: 10.1196/annals.1300.012. [DOI] [PubMed] [Google Scholar]
- Sullivan and Pfefferbaum, 2005.Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Szumlinski et al., 2004.Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, Rohrer J, Griffin W, 3rd, Toda S, Champtiaux NP, Berry T, Tu JC, Shealy SE, During MJ, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate vulnerability to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Szumlinski et al., 2005a.Szumlinski KK, Lominac KD, Kleschen MJ, Oleson EB, Dehoff MH, Schwarz MK, Seeberg PH, Worley PF, Kalivas PW. Behavioural and neurochemical phenotyping of Homer1 mutant mice: possible implications for schizophrenia. Genes Brain Behav. 2005a;4:273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- Szumlinski et al., 2005b.Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005b;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski et al., 2006.Szumlinski KK, Abernathy KE, Oleson EB, Klugmann M, Lominac KD, He DY, Ron D, During M, Kalivas PW. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacology. 2006;31:768–777. doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- Szumlinski et al., 2007.Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology (Berl) 2007;190:415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- Szumlinski et al., 2008a.Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008a;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski et al., 2008b.Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 over-expression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008b;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth and Gardiner, 2000.Toth LA, Gardiner TW. Food and water restriction protocols: physiological and behavioral considerations. Contemp Top Lab Anim Sci. 2000;39:9–17. [PubMed] [Google Scholar]
- Tu et al., 1998.Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Urizar et al., 2007.Urizar NL, Yang Z, Edenberg HJ, Davis RL. Drosophila homer is required in a small set of neurons including the ellipsoid body for normal ethanol sensitivity and tolerance. J Neurosci. 2007;27:4541–4551. doi: 10.1523/JNEUROSCI.0305-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao et al., 1998.Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto et al., 2005.Yamamoto K, Sakagami Y, Sugiura S, Inokuchi K, Shimohama S, Kato N. Homer 1a enhances spike-induced calcium influx via L-type calcium channels in neocortex pyramidal cells. Eur J Neurosci. 2005;22:1338–1348. doi: 10.1111/j.1460-9568.2005.04278.x. [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2006.Zhang X, Mi J, Wetsel WC, Davidson C, Xiong X, Chen Q, Ellinwood EH, Lee TH. PI3 kinase is involved in cocaine behavioral sensitization and its reversal with brain area specificity. Biochem Biophys Res Commun. 2006;340:1144–1150. doi: 10.1016/j.bbrc.2005.12.114. [DOI] [PubMed] [Google Scholar]