Abstract
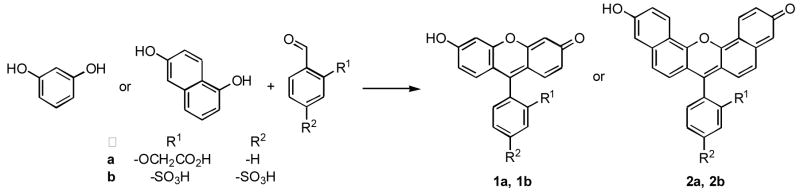
Symmetric and asymmetric xanthene dyes have been prepared by a convenient one-step procedure from aldehyde and diol or m-aminophenol precursors using concentrated phosphoric acid as a solvent. This protocol provides access to water-soluble dyes with large Stoke’s shifts and far-red fluorescence emission. These compounds are envisioned as components of fluorescence-based sensors for a variety of imaging applications.
As part of an effort to prepare activateable far-red and near infrared fluorescence-based probes for interrogation of pathological processes at a molecular level, we are interested in development of efficient routes to new long-wavelength dye platforms. Fluorescein and other xanthene dyes are used commonly for a variety of fluorescence-based diagnostic and imaging applications. In recent years a number of analyte sensors have been designed using these scaffolds either via synthesis of new xanthene based dyes1,2 or by chemical modification at the 3′ or 6′ positions of the dye.3–5 Often these probes are prepared via multi-step synthetic procedures involving the reaction of a benzophenone with a resorcinol derivative.1 Many of the current sensors based on xanthene fluorophores have relatively poor water solubility, requiring organic co-solvents for aqueous work and typically have fluorescence emission less than 550 nm. The short wavelength emission from these probes makes them susceptible to interference from tissue autofluorescence in biological sensing applications. Therefore, there is a need for further development of new routes for the preparation of xanthene dyes that are water-soluble, have long wavelength absorption and emission, and are amenable to modification into fluorescence-based analyte sensors. Furthermore, due to the enhanced optical transparency of tissue from approximately 650 to 900 nm,6 probes based on these far-red emitting dyes may find utility in a variety of in vivo imaging applications.
We have found that aldehydes react with resorcinol or 1,6-dihydroxynaphthalene in the presence of 85% phosphoric acid in a one step procedure to generate a variety of fluorescein and naphthofluorescein analogs (Scheme 1).7 Most previous routes to xanthene dyes that use aldehyde precursors involve isolation of a triphenylmethane or dihydroxyxanthene intermediate that is subsequently oxidized to generate the final dye.8–10 For the synthesis of naphthofluorescein derivatives 2a and 2b, the use of more common catalysts such as methanesulfonic acid or zinc chloride resulted in lower yields and formation of multiple side products. However, use of concentrated phosphoric acid as the solvent results in clean formation of the desired naphthofluoresceins, which are then isolated by column chromatography in up to 52% yield. This procedure represents a significant improvement over a previous route to sulfonaphthofluoresceins, which has a reported overall yield of less than 2%.11
Scheme 1.
Conditions: 85% H3PO4, 125°C, 2h.
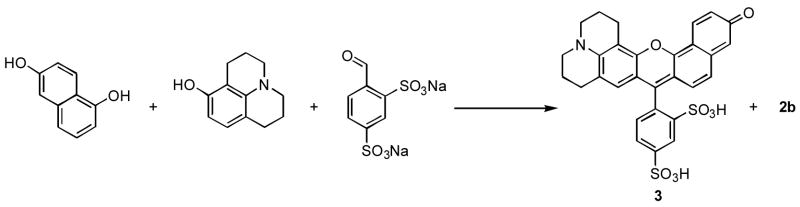
Interestingly, it is not possible to prepare the analogous rhodamine derivatives employing concentrated phosphoric acid as a solvent. Although, microwave irradiation of a mixture containing 1,6-dihydroxynaphthalene, 8-hydroxyjulolidine, and benzaldehyde 2,4-disulfonic acid disodium salt at 200 °C yielded the asymmetric rhodanaphthofluor, 3 (Scheme 2).12 In this reaction, the symmetric naphthofluorescein is also generated in a 35:65 ratio of 2b to 3, respectively, as determined by LCMS.
Scheme 2.
Conditions: H3PO4, microwave 200 °C, 15 min.
Dyes 1b, 2b, and 3, which contain two sulfonate moieties, are highly soluble in aqueous solution under acidic or basic conditions. Whereas, compounds 1a and 2a, the phenoxyacetic acid based fluorophores, only have good aqueous solubility under neutral or basic conditions.
As expected, fluorescein-based dyes 1a and 1b (Figure 1) are optically similar to fluorescein. The absorption and emission wavelengths as well as quantum yields and extinction coefficients are reported in Table 1. Sulfonation of the bottom ring at the 3- and 5-positions results in a slight bathochromic shift of the absorption maximum of 1b (Figure 1) when compared to fluorescein. The measured quantum yields for 1a and 1b are slightly lower than the 92% reported for fluorescein. The extinction coefficients of 1a and 1b are 29 and 20% lower than that of fluorescein, respectively. Naphthofluorescein derivatives 2a and 2b have their absorbance and fluorescence emission red-shifted by over 100 nm when compared to their fluorescein analogs in aqueous solution. This coupled with the > 60 nm Stoke’s shifts observed for naphthofluoresceins may prove advantageous for biological imaging applications. The hybrid rhodanaphthofluor, 3, (Figure 2) combines the advantageous properties of the fluoresceins (good quantum yields) and naphthofluoresceins (long wavelength absorption and emission). With good water solubility, long wavelength absorption and emission, a quantum yield of 30%, and an extinction coefficient of 46,000 cm−1M−1, 3 is a promising candidate for development into a long wavelength analyte sensor.
Figure 1.
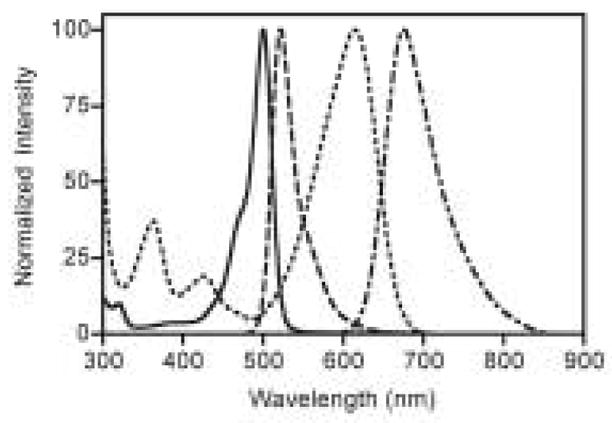
Absorption spectra of 1b and 2b (solid and doted lines, respectively) and fluorescence emission spectra of 1b and 2b (dashed and dot-dashed lines, respectively) in 0.1 M aqueous bicarbonate buffer pH 10.0.
Table 1.
Optical properties of the fluorophores in 0.1 M bicarbonate buffer, pH 10.0.
Figure 2.
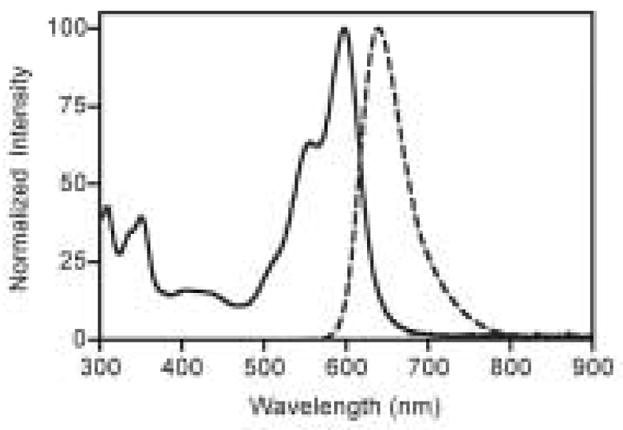
Absorption (solid line) and fluorescence emission (dashed line) spectra of 3 in 0.1 M aqueous bicarbonate buffer pH 10.0.
In summary, a new synthetic procedure for the synthesis of xanthene dyes utilizing concentrated phosphoric acid as the solvent is detailed. Using this procedure it is possible to prepare fluorescein, naphthofluorescein, and rhodanaphthofluor derivatives. The far-red absorption and emission from the naphthofluorescein and rhodanaphthofluor derivatives may prove useful for development of these dyes into fluorescence-based probes for optical imaging applications.
Supplementary Material
HPLC traces for all compounds, demonstrating product purity.
Acknowledgments
This research was supported in part by NIH grants CA86355 (P50) and HL080731 (U01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nolan EM, Burdette SC, Harvey JH, Hilderbrand SA, Lippard SJ. Inorg Chem. 2004;43:2624–2635. doi: 10.1021/ic035158+. [DOI] [PubMed] [Google Scholar]
- 2.Nolan EM, Racine ME, Lippard SJ. Inorg Chem. 2006;45:2742–2749. doi: 10.1021/ic052083w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotman B, Zderic JA, Edelstein M. Proc Natl Acad Sci USA. 1963;50:1–6. doi: 10.1073/pnas.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Setsukinai KI, Urano Y, Kakinuma K, Majima HJ, Nagano T. J Biol Chem. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- 5.Zaikova TO, Rukavishnikov AV, Birrell GB, Griffith HO, Keana JFW. Bioconjugate Chem. 2001;12:307. doi: 10.1021/bc0001138. [DOI] [PubMed] [Google Scholar]
- 6.Weissleder R, Ntziachristos V. Nat Med. 2003;9:123–8. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 7.General procedure for synthesis of symmetric dyes 1–2. To a solution of the appropriate aldehyde (0.25 mmol) in 85% aqueous phosphoric acid (1 mL) is added resorcinol or 1,6-dihydroxynaphthalene (0.5 mmol). The resulting mixture is then heated in an oil bath with stirring at 125 °C for 2 h. After cooling, the crude product is precipitated by addition of water (15 mL), then filtered and washed with water (2 × 15 mL). For the dyes containing sulfonate groups, EtOAc is used to precipitate and wash the products instead of water. Column chromatography of the product on silica gel (eluting with: CH2Cl2, 0.1% TFA, and a gradient from 2.5 to 10.0% MeOH) or reverse phase C-18 silica (eluting with: water, 0.1% TFA, and a gradient from 20 to 40% MeOH) affords the desired products. Purity of all compounds was confirmed by HPLC. Compound 1a. Yield 21 mg, 23%. 1H NMR (400 MHz, d6-DMSO): 7.59 (1H, t, J = 7.4 Hz), 7.31 (1H, d, J = 6.7 Hz), 7.17–7.23 (4H, m), 6.72–6.75 (4H, m), 4.70 (2H, s). High resolution ESI-MS [M+H]+ calcd. for C21H15O6+: 363.0863, found: 363.0872. Compound 1b. Yield 25 mg, 22%. 1H NMR (400 MHz, d6-DMSO): 8.26 (1H, s, J = 1.3 Hz), 7.78 (1H, dd, J = 7.8, 1.6 Hz), 7.40 (2H, s, J = 9.2 Hz), 7.27 (2H, d, J = 2.1 Hz), 7.24 (1H, d, J = 7.9 Hz), 7.14 (2H, dd, J = 9.2, 2.0 Hz). High resolution ESI-MS [M+H]+ calcd. for C19H13O9S2+: 448.9996, found: 449.0002. Compound 2a. Yield 59 mg, 51%. 1H NMR (400 MHz, d6-DMSO): 9.33 (2H, d, J = 9.1 Hz), 8.03 (2H, d, J = 9.4 Hz), 7.76 (1H, t, J = 8.0 Hz), 7.55–7.59 (4H, m), 7.53 (2H, d, J = 1.7 Hz), 7.35–7.45 (3H, m), 4.73 (2H, s). High resolution ESI-MS [M+H]+ calcd. for C29H19O6+: 463.1176, found: 463.1173. Compound 2b. Yield 71 mg, 52%. 1H NMR (400 MHz, d6-DMSO): 8.95 (2H, d, J = 9.0 Hz), 8.41 (1H, s), 7.89 (1H, d, J=7.9 Hz), 7.81 (2H, d, J=9.2 Hz), 7.35–7.25 (7H, m). High resolution ESI-MS [M+H]+ calcd. for C27H17O9S2+: 549.0309, found: 549.0309.
- 8.Bacci JP, Kearney AM, Van Vranken DL. J Org Chem. 2005;70:9051–3. doi: 10.1021/jo051243i. [DOI] [PubMed] [Google Scholar]
- 9.Gee KR, Zhou ZL, Quian WJ, Kennedy R. J Am Chem Soc. 2002;124:776–777. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- 10.He Q, Miller EW, Wong AP, Chang CJ. J Am Chem Soc. 2006;128:9316–7. doi: 10.1021/ja063029x. [DOI] [PubMed] [Google Scholar]
- 11.Lee LG, Berry GM, Chen CH. Cytometry. 1989;10:151–64. doi: 10.1002/cyto.990100206. [DOI] [PubMed] [Google Scholar]
- 12.Synthesis and characterization of compound 3. To a solution obtained by heating 8-hydroxyjulolidine (95 mg, 0.5 mmol) and benzaldehyde 2,4-disulfonic acid disodium salt (155 mg, 0.5 mmol) in 85% aqueous phosphoric acid (1 mL) was added finely powdered 1,6-dihydroxynaphthalene (40 mg, 0.25 mmol). The resulting mixture was subjected to microwave irradiation in an open vessel at 200 °C using a CEM Discover LabMate reactor for 15 min. Upon cooling, the viscous dark pink mixture was treated with acetone (50 mL), filtered, and washed with acetone (2 × 10 mL). Column chromatography using a 70 g reverse phase Isolute C-18 column from Biotage (eluting with: water, 0.1% TFA, and a gradient from 10 to 40% MeOH in 5% increments) affords 3 (14 mg, 10%) as a dark pink solid. The purity of 3 was confirmed by HPLC. 1H NMR (400 MHz, d6-DMSO): 8.60 (1H, d, J = 9.1 Hz), 8.29 (1H, d, J = 1.7 Hz), 7.77 (1H, dd, J = 7.8, 1.7 Hz), 7.60 (1H, d, J = 9.0 Hz), 7.38 (1H, dd, J = 9.0, 2.2 Hz), 7.30 (1H, d, J = 2.2 Hz), 7.20 (1H, d, J = 7.8 Hz), 6.96 (1H, d, J = 9.0 Hz), 6.88 (1H, s), 3.58–3.67 (4H, m), 3.13–3.18 (2H, m), 2.73–2.78 (2H, m), 2.07–2.10 (2H, m ), 1.88–1.93 (2H, m). High resolution ESI-MS [M+H]+ calcd. for C29H24NO8S2+: 578.0938, found: 578.0934.
- 13.Velapoldi RA, Tønnesen HH. J Fluorescence. 2004;14:465–471. doi: 10.1023/b:jofl.0000031828.96368.c1. [DOI] [PubMed] [Google Scholar]
- 14.Mujumdar RB, Ernst LA, Mujumdar SR, Lewis CJ, Waggoner AS. Bioconjugate Chem. 1993;4:105–111. doi: 10.1021/bc00020a001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HPLC traces for all compounds, demonstrating product purity.