Abstract
Congenital central hypoventilation syndrome (CCHS) children show cognitive and affective deficits, in addition to state-specific loss of respiratory drive. The caudate nuclei serve motor, cognitive, and affective roles, and show structural deficits in CCHS patients, based on gross voxel-based analytic procedures. However, the magnitude and regional sites of caudate injury in CCHS are unclear. We assessed global caudate nuclei volumes with manual volumetric procedures, and regional volume differences with three-dimensional surface morphometry in 14 CCHS (mean age ± SD: 15.1 ± 2.3 years; 8 male) and 31 control children (15.1 ± 2.4 years; 17 male) using brain magnetic resonance imaging (MRI). Two high-resolution T1-weighted image series were collected using a 3.0 Tesla MRI scanner; images were averaged and reoriented (rigid-body transformation) to common space. Both left and right caudate nuclei were outlined in the reoriented images, and global volumes calculated; surface models were derived from manually-outlined caudate structures. Global caudate nuclei volume differences between groups were evaluated using a multivariate analysis of covariance (covariates: age, gender, total intracranial volume). Both left and right caudate nuclei volumes were significantly reduced in CCHS over control subjects (left, 4293.45 ± 549.05 mm3 vs 4626.87 ± 593.41 mm3, p < 0.006; right, 4376.29 ± 565.42 mm3 vs 4747.81 ± 578.13 mm3, p < 0.004). Regional deficits in CCHS caudate volume appeared bilaterally, in the rostral head, ventrolateral mid, and caudal body. Damaged caudate nuclei may contribute to CCHS neuropsychological and motor deficits; hypoxic processes, or maldevelopment in the condition may underlie the injury.
Keywords: Magnetic Resonance Imaging, Brain, Basal ganglia, Striatum, Hypoxia, Cognition
Introduction
Congenital central hypoventilation syndrome (CCHS) is a rare genetic disorder characterized by reduced drive to breathe, primarily during sleep, reduced ventilatory sensitivity to CO2 and O2, various autonomic deficits, developmental delays, and multiple affective and cognitive issues, with a wide range of expression of both physiological and neuropsychological characteristics (Haddad et al., 1978, Paton et al., 1989, American Thoracic Society, 1999, Vanderlaan et al., 2004, Ruof et al., 2008). Mutations in PHOX2B, a transcription protein for cellular development which targets neurons in autonomic and brainstem respiratory ganglia (Dauger et al., 2003, Stornetta et al., 2006), are found in over 90% of CCHS patients (Weese-Mayer et al., 2003, Matera et al., 2004), and those mutations appear to underlie the primary respiratory and autonomic characteristics of the syndrome. The neuropsychologic and cognitive aspects of CCHS would depend on reduced integrity of limbic regions and basal ganglia, including the caudate nuclei, and other rostral brain areas serving emotional and memory functions. Immunoreactive cells to Phox2b are found in selected suprapontine areas of the mouse, particularly the hippocampus and isolated cortical areas (Lein et al., 2007), and may contribute to the cognitive and affective behaviors; such cells do not appear in the caudate nuclei. The caudate nuclei, which are especially susceptible to certain forms of hypoxia (Veasey et al., 2004, Pulsipher et al., 2006), and other suprapontine structures could also be affected by hypoxic exposure resulting from sleep hypoventilation, the principal characteristic of the syndrome, or by secondary consequences of impaired perfusion from damaged autonomic ganglia.
The caudate nuclei play significant roles in learning, mood, memory, and cognition (Mendez et al., 1989, Poldrack et al., 1999, Levitt et al., 2002, Matsuo et al., 2008). The structure shows volume deficits in patients with conditions accompanied by cognitive impairment, including fronto-temporal lobar degeneration (Looi et al., 2008), Down syndrome (Haier et al., 2008), and schizotypal personality disorder (Levitt et al., 2002). The caudate structures comprise elements of circuitry for higher cognitive and limbic functions, including pathways to the dorsolateral prefrontal and lateral orbito-frontal cortices (Lehericy et al., 2004, Postuma and Dagher, 2006). Areas within the caudate nuclei, which serve functions mediated by these different projections, are topographically organized (Alexander et al., 1986, Hontanilla et al., 1994), but specific regions within the caudate nuclei affected in CCHS are unknown.
Caudate injury in CCHS was initially detected by voxel-based analytic procedures that required cross-subject normalization of brain images into a common space (Kumar et al., 2005, Kumar et al., 2006). Such procedures can affect structural assessment, since the presence of excessive cerebrospinal fluid (CSF) in adjacent ventricles, a characteristic of CCHS, can influence such evaluation. Moreover, voxel-based techniques, as well as the particular imaging procedures used provided insufficient spatial resolution to localize regional damage within caudate structures. By contrast, manual volumetric procedures, using high-resolution T1-weighted images, combined with three-dimensional (3D) surface reconstruction and morphometry can provide both volumetric and map-based regional information of the structure.
Here, we aimed to assess caudate nuclei volumes in CCHS and similar age- and gender-distributed control subjects with manual volumetric procedures, and to determine regional differences with 3D surface morphometry procedures, using high-resolution T1-weighted imaging.
Experimental Procedures
Subjects
Fourteen CCHS children (mean age ± SD: 15.1 ± 2.3 years; range: 12-18 years; 8 male) and 31 control subjects (15.1 ± 2.4 years; 10-19 years; 17 male) were studied. The CCHS diagnosis was based on standard criteria indicated by the American Thoracic Society (American Thoracic Society, 1999), and subjects were recruited through the CCHS family network (http://www.cchsnetwork.org). Of the 14 CCHS subjects, six showed evidence of PHOX2B mutations, two were inconclusive, and the status of the remaining six CCHS patients was unknown. Although CCHS patients may require ventilatory support during sleep and waking, we included only those who required ventilatory support during sleep. CCHS patients with other conditions, including cardiovascular or neurological issues, or with diagnosed Hirschsprung's disease that could induce additional neural injury through malnutrition, were excluded. Brain imaging in all CCHS and control subjects was performed without anesthesia or sedatives, and subjects were provided rest from the scanner if required. Control subjects were healthy, without any diagnosed neurological disorder or other issues that could alter brain tissue integrity, and were recruited through advertisements at the university campus targeting parents to bring their children to participate in this study.
All control and CCHS subjects or their parents/caretakers provided informed written consent prior to the study, and the protocol was approved by the Institutional Review Board of the University of California at Los Angeles.
Magnetic resonance imaging
Brain imaging studies were performed with a 3.0 Tesla magnetic resonance imaging scanner (Magnetom Trio; Siemens, Erlangen, Germany). Subjects lay supine, and foam pads were placed on both sides of the head to reduce head motion during data collection. Two high-resolution T1-weighted image series were collected using a magnetization prepared rapid acquisition gradient-echo (MPRAGE) pulse sequence [repetition-time (TR) = 2200 ms; echo-time (TE) = 3.05 ms; inversion-time = 1100 ms; flip-angle = 10°; matrix size = 256 × 256; field-of-view (FOV) = 220 × 220 mm; slice thickness = 1.0 mm]. Proton-density (PD) and T2-weighted images were also collected in the axial plane, using a dual-echo turbo spin-echo pulse sequence (TR = 8000 ms; TE1, 2 = 17, 133 ms; flip-angle = 150°; matrix size = 256 × 256; FOV = 240 × 240 mm; slice thickness = 5.0 mm), for visual assessment.
Data analysis
T1, T2, and PD-weighted images were evaluated for the presence of any neural pathology, such as major infarcts, cystic lesions, or any other mass lesions. Both high-resolution T1-weighted image series were evaluated for any head motion-related or other artifacts.
We used the statistical parametric mapping package SPM5 (Wellcome Department of Cognitive Neurology, UK; http://www.fil.ion.ucl.ac.uk/spm/), MRIcron (Rorden et al., 2007), and Matlab-based (The MathWorks Inc, Natick, MA) custom software to process the brain images, delineate caudate nuclei structures, and evaluate caudate nuclei volumes.
Both high-resolution T1-weighted image series were realigned to remove variation from potential motion, and averaged to increase signal-to-noise ratio. The averaged images were bias-corrected for image signal intensity variation, and reoriented into a common space, using a 6-parameter rigid-body (non-distorting) affine transformation, and sampled to 0.9×0.9×0.9 mm.
Intracranial volume calculation
The bias-corrected and reoriented images were used to partition gray matter, white matter, and CSF, resulting in probability maps, using a unified segmentation approach (Ashburner and Friston, 2005). The gray, white, and CSF probability maps were summed for each voxel, and each voxel classified as “intracranial” if the voxel probability was greater than 0.5; intracranial voxels were counted in each subject, and total intracranial volume calculated.
Caudate tracing and volume calculation
A single investigator, unaware of subject group assignment, delineated the left and right caudate nuclei with MRIcron using the reoriented and resampled brain images. Both left and right heads and bodies of the caudate nuclei, caudal to the level of the posterior thalamus, were outlined using axial views; anatomic landmarks were followed in all other views. Coronal and sagittal views were used to verify the structure boundaries. Delineated voxels in each caudate nucleus were counted, and volumes of the structure on each side were calculated by multiplying the number of counted voxels by the volume of one voxel.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS, V 15.0, Chicago, IL) was used for statistical evaluation. Both biophysical and demographic numerical variables were assessed with independent-samples t-tests and the categorical variable (gender) was assessed with a Chi-square test. Left and right caudate nuclei volumes were assessed between groups using a multivariate analysis of covariance (MANCOVA), with age, gender, and total intracranial volume included as covariates.
Correlations between caudate nuclei volume and age in CCHS and control subjects were evaluated with Pearson's correlation. Intra- and inter-tracer reliabilities were established with intraclass correlation coefficient (ICC) procedures.
Intra- and inter-tracer reliabilities
We established intra- and inter-tracer reliabilities for delineating the caudate nuclei. A single investigator, who performed all the structural tracings, repeated the same procedure in 6 randomly-selected CCHS and control subjects. Of these 6 subjects, two were CCHS, and four were control subjects. In the same subject subgroup, another investigator also outlined the structures. Both intra-tracing (ICC = 0.97, p < 0.001) and inter-tracing (ICC = 0.95, p < 0.001) reliabilities were high.
3D caudate surface morphometry
We used an anatomical surface modeling approach, together with surface-based statistics (Butters et al., 2009) to compare regional caudate nuclei volumes between CCHS and control groups. Surface models were derived from outlined left and right caudate nuclei, and equivalent surface points of the caudate nuclei were derived with anatomical mesh modeling procedures applied to each subject (Thompson et al., 2004). We derived the medial 3D curve, the centerline of the structure in the anterior-posterior direction, for each caudate nucleus model of individual CCHS and control subjects, and at each point we computed the “radial distance” of the caudate, based on the distance of each surface point from the 3D medial curve (Lin et al., 2005). Two sample t-tests were performed at each surface point to map associations between group and radial distance, and statistical maps indicating regional group differences in caudate nuclei were generated. We controlled for multiple comparisons (across the mesh points) by performing permutation testing, resulting in an overall p-value for the statistical map on each side. This p-value expresses the probability of observing the overall pattern of group effects by chance. Regional p-values corresponding to group differences were displayed using a color code, on averaged 3D surface models derived from all subjects.
Results
Biophysical and demographic variables of CCHS and control subjects are summarized in Table 1. Age, gender, and body-mass-index showed no significant differences between CCHS and control groups.
Table 1.
Biophysical and demographic variables of CCHS and control subjects.
| Variables | CCHS [n =14] | Control [n = 31] | p-values |
|---|---|---|---|
| Age (mean ± SD, years) | 15.14 ± 2.27 | 15.14 ± 2.36 | 0.993 |
| Gender (Male: Female) | 8:6 | 17:14 | 0.885 |
| BMI (mean ± SD, kg/m2) | 20.67 ± 3.80 | 21.36 ± 4.30 | 0.609 |
Table legend: SD = Standard deviation; BMI = Body mass index.
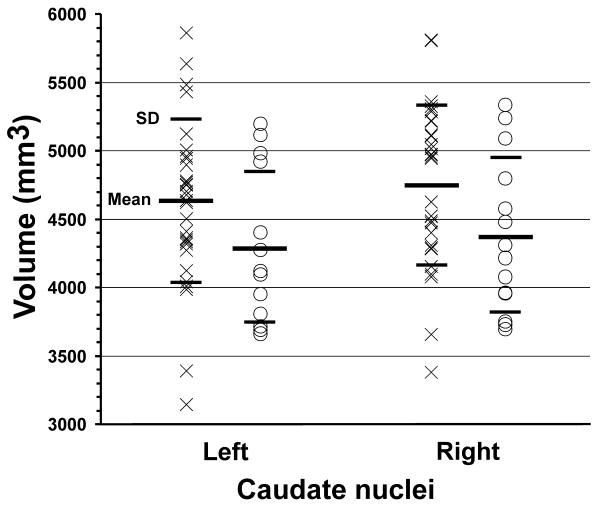
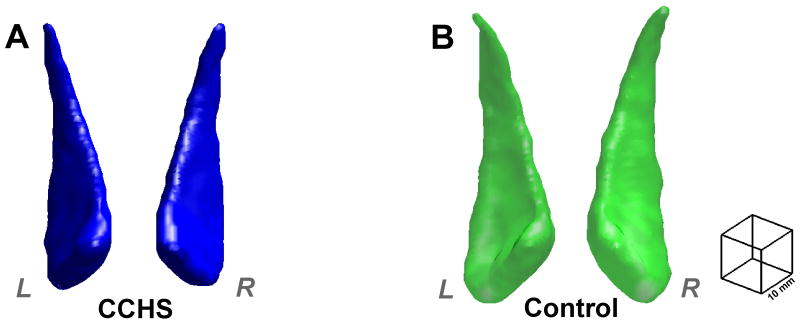
Left and right mean caudate nuclei volumes of CCHS and control subjects are summarized in Table 2, and values from individual subjects are displayed in scatter plots (Fig. 1). The 3D rendering shows bilateral caudate nuclei volume deficits in a CCHS compared to a comparable control subject (Fig. 2). Left and right mean caudate volumes were significantly reduced in CCHS over control groups, controlling for age, gender, and total intracranial volume (MANCOVA; left, p < 0.006; right, p < 0.004). No significant correlations were detected between caudate nuclei volumes and age in CCHS or control groups.
Table 2.
Caudate nuclei volumes from CCHS and control subjects.
| Brain site | CCHS [n = 14] (mean ± SD, mm3) |
Control [n = 31] (mean ± SD, mm3) |
* p values |
|---|---|---|---|
| Left caudate | 4293.45 ± 549.05 | 4626.87 ± 593.41 | < 0.006 |
| Right caudate | 4376.29 ± 565.42 | 4747.81 ± 578.13 | < 0.004 |
Table legend: SD = Standard deviation,
p-values, corrected for total intracranial volume, age, and gender derived from MANCOVA.
Figure 1.
Caudate nuclei volumes from individual control (×) and CCHS (○) subjects. Both left and right caudate volumes are reduced significantly in CCHS compared to control subjects, controlling for age, gender, and total intracranial volume.
Figure 2.
3D rendering (MATLAB, box filter, 3 voxel kernel) of caudate nuclei in a CCHS (A, blue; age 18.3 yrs; male) and control (B, green; age 17.6 yrs; male) subject; both plotted in the same scale (scale, right side). Both left and right caudate nuclei of the CCHS subject show smaller volumes over those of the control subject.
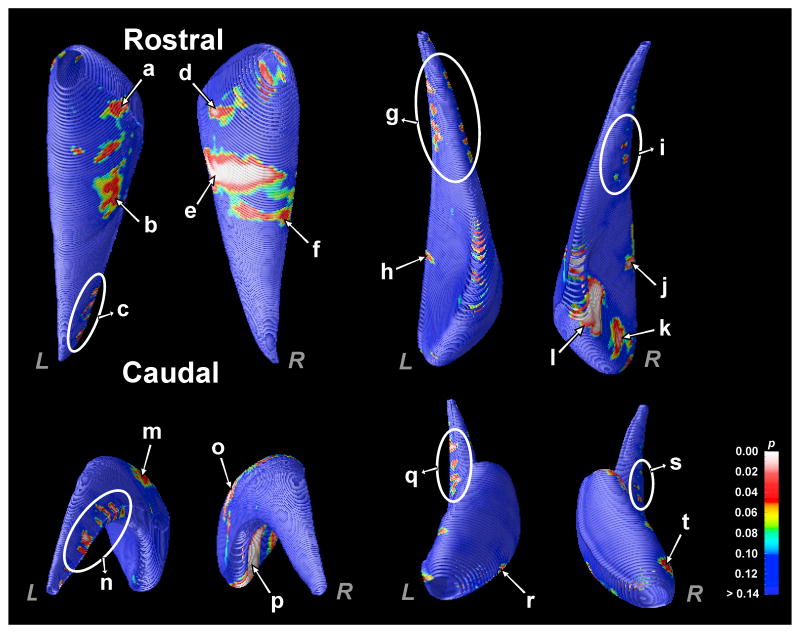
Three-dimensional surface morphometry showed reduced volumes in CCHS patients at specific sites within both left and right caudate structures (Fig. 3), corrected for multiple comparisons using permutation testing (left, p < 0.02; right, p < 0.03). The left caudate in CCHS showed loss in the rostral head (Fig. 3a, r), caudal (Fig. 3c, g, n, q), and ventrolateral mid-body portions (Fig. 3b, h, m) over control subjects. Similarly, the right caudate in CCHS showed loss in the head (Fig. 3d, k, l, p, t), caudal (Fig. 3i, s), and ventrolateral mid-body portions (Fig. 3e, f, j, o), compared to control subjects.
Figure 3.
Probability maps show the significance of the regional reduction of volumes within the caudate in CCHS compared to control subjects. Both caudate nuclei show reduced volumes in the rostral head, ventrolateral mid-body, and caudal body portions. Significance levels are color-coded and displayed on averaged 3D surface models of the caudate nucleus.
Discussion
We found that CCHS subjects showed both global volume reduction and localized sites of volume loss within the caudate nuclei. The caudate nuclei interact with multiple brain sites, including the midbrain, thalamus and prefrontal cortices, and are implicated in a wide range of motor (Gerardin et al., 2004), and higher cognitive and emotional processes (Levitt et al., 2002, Matsuo et al., 2008). Caudate sites earlier showed abnormal functional responses to autonomic and ventilatory challenges in CCHS, and structural injury, based on gross evaluation procedures (Harper et al., 2005, Kumar et al., 2005, Macey et al., 2005a, Macey et al., 2005b, Kumar et al., 2006, 2008). The current study evaluates the extent of damage and localizes the injury to specific sites within the nuclei; these sites may influence specific aspects of cognition, affect, and motor regulation in the syndrome.
Global caudate volume loss in CCHS
Global volume analyses showed an approximate 8% mean volume difference between CCHS and control groups; the extent of deficit should be viewed in the context of other conditions, such as patients with schizotypal personality disorder, who exhibit a 13% reduction in absolute volumes compared to control subjects (Levitt et al., 2002). Thus, the overall volume deficits in CCHS are significant, but not as large as found with severe personality disorders. The volume loss appears appropriate for behavior characteristics in CCHS; although some children are affected in a major fashion, others are not. The particular deficits are more easily understood by examining the regional losses in CCHS caudate nuclei.
Regional site differences and their significance
Variability in characteristics
CCHS patients show common physiological characteristics, including diminished central chemosensitivity and impaired parasympathetic and sympathetic regulation, as well as the principal life-threatening deficit of loss of drive to the breathing muscles during sleep, a state-specific motor behavior. However, physiological deficits can vary substantially across affected individuals, with a reduced drive to breathe even during waking in some cases, and a range of severity in incidence of cardiovascular abnormalities (Weese-Mayer et al., 2001). Similarly, emotional and cognitive deficits, as well as extent of developmental delays, vary substantially among patients (Vanderlaan et al., 2004, Ruof et al., 2008). The variability in expression of both motor and neuropsychological characteristics in CCHS complicates evaluation of influences from localized caudate injury.
Motor deficits
While recognizing the variability in certain deficits, both impaired developmental motor behaviors and sleep-related suppression of respiratory motor action are characteristic of CCHS. Localized caudate injury may play a role in both motor aspects. Ventrolateral regions of the body showed the most extensive regional injury. Ventrolateral regions, with pronounced projections to the pedunculopontine tegmentum (PPT), help regulate motor behaviors, especially oral motor activity (Allen and Winn, 1995); the PPT is a significant component of the mesencephalic locomotor region (Garcia-Rill and Skinner, 1987). The oscillatory motor patterning influenced by the PPT may extend to the respiratory issues in CCHS, since repetitive locomotion, such as cyclic foot movement, enhances breathing in CCHS patients during sleep (Gozal and Simakajornboon, 2000). Impaired projections from the ventrolateral caudate to the PPT may degrade caudate influences on the PPT, modifying state-related cyclic breathing patterns in sleep.
Damage also occurred in the head of both the left and right caudate nuclei. The extensive caudate roles in other motor regulatory behavior through projections from the caudate head to the supplementary motor and frontal regions, including ventral, medial, and dorsolateral prefrontal cortices (Lehericy et al., 2004), should influence developmental motor patterning significantly, a major concern in CCHS (Vanderlaan et al., 2004).
A characteristic found in many CCHS patients is inappropriate facial expression to emotional stimuli, e.g., unilateral inability to smile to a joke, but appropriate voluntary-initiated smiling. The deficit may represent caudate injury forming a component of the “emotional motor system” (Holstege, 1992). That motor system may provide a component of other emotional responses; dopaminergic processes within the caudate may underlie that structure's response to orgasm in women (Georgiadis et al., 2006). The abundance of serotonergic receptors in the head of the caudate may modulate other neurotransmitter actions, including those of dopamine, gamma-aminobutyric acid and glutamate, and thus a range of motoric actions mediated by those neurotransmitter systems.
Mood and cognitive issues
The midline pontine raphé, the source of rostrally-projecting serotonergic fibers, shows pronounced injury in CCHS (Kumar et al., 2008). A reduced number of serotonergic terminals within the caudate head secondary to the raphé damage may contribute to the tissue loss found here, and relate to mood deficits mediated by that neurotransmitter (Drevets et al., 1999). Altered mood appears consistently across a high proportion of CCHS patients (Vanderlaan et al., 2004). The deficits can appear as anxiety, but a common characteristic is apathy toward self care related to their need for oxygen. Thus, CCHS children are often inattentive to high CO2 or low O2 conditions, and will participate in dangerous behaviors, such as challenges for underwater endurance (a challenge in which they obviously excel). Loss of tissue appeared in the caudate nuclei heads in CCHS; these areas project and receive input from prefrontal, frontal, and limbic sites (Lehericy et al., 2004), and normally are rich in serotonergic fibers (Mori et al., 1985).
Neuropsychological deficits in CCHS also include cognitive disturbances and learning disabilities (Vanderlaan et al., 2004, Ruof et al., 2008), and speech impairments. The cognitive deficits appear as problems in working memory, attention, and social interaction (Ruof et al., 2008). Caudate nuclei injury is classically expressed with several cognitive impairments, including learning and attention deficits (Mendez et al., 1989, Poldrack et al., 1999), short- and long-term memory and retrieval problems (Mendez et al., 1989, Fuh and Wang, 1995), mental flexibility (Lombardi et al., 1999), motivation, and verbal fluency (Fuh and Wang, 1995). The extensive projections from the caudate head to prefrontal and frontal cortical sites may interrupt input involved in mediation of executive function (Newman et al., 2007). Both rostral and caudal portions of the caudate are implicated in different types of working memory functions, as revealed by functional brain imaging studies in both human and animal models (Levy et al., 1997, Chang et al., 2007); the caudal portions injured here may be contributing to the cognitive deficits.
Pathological mechanisms
Although the precise mechanisms of caudate nuclei volume loss in CCHS are unclear, several possibilities emerge. A portion of the injury may result from developmental consequences of PHOX2B mutations characteristic of CCHS. Volume losses in the caudate nuclei also occur in autosomal dominant and other neurodegenerative diseases, including Huntington's disease (Wolf et al., 2009). Animal models of Phox2b mutations show injury localized to visceral ganglia and brainstem autonomic and retrofacial nuclei, and immunoreactivity to Phox2b appears in more rostral areas, such as hippocampus (Dauger et al., 2003, Stornetta et al., 2006, Lein et al., 2007, Dubreuil et al., 2008).
However, the absence of Phox2b immunoreactive neurons in the caudate nuclei of mice suggests other mechanisms of damage. Secondary developmental damage arising from impaired perfusion resulting from initial mutations of PHOX2B targeting autonomic ganglia may lead to injury. Damage to autonomic regulatory areas, including the nucleus of the solitary tract, could significantly alter development or function of the microvasculature, and modify perfusion of perhaps distant brain areas, impairing development of those sites. An indication of the vascular effects can be found in large arteries supplying the brainstem; the basilar artery is significantly dilated, relative to other major vessels (Kumar et al., 2009b), perhaps from excessive constriction of the brainstem microvasculature.
The caudate nuclei are classic targets for hypoxic injury, especially carbon monoxide poisoning (Pulsipher et al., 2006). The sensitivity of the caudate nuclei to hypoxia suggests that hypoventilation accompanying CCHS may play a significant role in the damage found here. Increased levels of inflammatory markers and oxidative damage appear in other animal hypoxia models, such as intermittent hypoxic exposure simulating obstructive sleep apnea (Ohga et al., 2003, Veasey et al., 2004, Zhan et al., 2005). Injury in those models appears in several brain sites, including the fornix, cerebellum, thalamus, hippocampus, and frontal cortices, as well as the caudate nuclei (Gozal et al., 2001, Veasey et al., 2004, Pae et al., 2005).
Loss of afferent and efferent projections from damaged cortical and subcortical regions may impact the caudate nuclei. The caudate nuclei are not alone in structural damage in CCHS. Multiple other areas are severely affected, including the mammillary bodies, hippocampus, cerebellum, medial midbrain, cingulate cortex, and various frontal and parietal cortical areas (Kumar et al., 2005, Kumar et al., 2006, 2008, Kumar et al., 2009a, Macey et al., 2009). The caudate nuclei receive projections from many of these sites, including the ventral medial prefrontal and anterior cingulate cortices (Alexander et al., 1986); the localized caudate injury here may partially result from damage to projections from other distant regions.
Conclusions
Both left and right caudate nuclei volumes are significantly reduced in CCHS over similar age- and gender-distributed control subjects, with the regional volume loss more pronounced in the ventrolateral mid-body; portions of the head and caudal regions are also affected. The projections to mesencephalic locomotor areas, as well as to frontal cortical sites and the thalamus may contribute to the breathing, cognitive, and behavioral deficits found in CCHS. The caudate injury may result from hypoxic processes, impaired perfusion, or maldevelopment associated with mutations of PHOX2B.
Acknowledgments
The authors thank Ms. Rebecca Harper and Ms. Annaise Magliore for assistance with data collection, Dr. Jennifer Ogren for editorial assistance, and parents and children for participating in this study. This research work was supported by the National Institute of Child Health and Human Development R01 HD-22695. P.T. and C.A. were also supported by R01 EB008281 and EB007813 from the National Institute for Biomedical Imaging and Bioengineering (NIBIB).
Abbreviations
- CCHS
Congenital central hypoventilation syndrome
- CSF
Cerebrospinal fluid
- 3D
Three-dimensional
- TR
Repetition-time
- TE
Echo-time
- FOV
Field-of-view
- ICC
Intraclass correlation coefficient
- PD
Proton-density
- MANCOVA
Multivariate analysis of covariance
- PPT
Pedunculopontine tegmentum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Allen LF, Winn P. Excitotoxic lesions of the pedunculopontine tegmental nucleus disinhibit orofacial behaviours stimulated by microinjections of d-amphetamine into rat ventrolateral caudate-putamen. Exp Brain Res. 1995;104:262–274. doi: 10.1007/BF00242012. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Idiopathic congenital central hypoventilation syndrome: diagnosis and management. Am J Respir Crit Care Med. 1999;160:368–373. doi: 10.1164/ajrccm.160.1.16010. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Butters MA, Aizenstein HJ, Hayashi KM, Meltzer CC, Seaman J, Reynolds CF, 3rd, Toga AW, Thompson PM, Becker JT. Three-dimensional surface mapping of the caudate nucleus in late-life depression. Am J Geriatr Psychiatry. 2009;17:4–12. doi: 10.1097/JGP.0b013e31816ff72b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage. 2007;34:1253–1269. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci USA. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuh JL, Wang SJ. Caudate hemorrhage: clinical features, neuropsychological assessments and radiological findings. Clin Neurol Neurosurg. 1995;97:296–299. doi: 10.1016/0303-8467(95)00059-s. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res. 1987;411:1–12. doi: 10.1016/0006-8993(87)90675-5. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR, Kortekaas R, Kuipers R, Nieuwenburg A, Pruim J, Reinders AA, Holstege G. Regional cerebral blood flow changes associated with clitorally induced orgasm in healthy women. Eur J Neurosci. 2006;24:3305–3316. doi: 10.1111/j.1460-9568.2006.05206.x. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Pochon JB, Poline JB, Tremblay L, Van de Moortele PF, Levy R, Dubois B, Le Bihan D, Lehericy S. Distinct striatal regions support movement selection, preparation and execution. Neuroreport. 2004;15:2327–2331. doi: 10.1097/00001756-200410250-00005. [DOI] [PubMed] [Google Scholar]
- Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Simakajornboon N. Passive motion of the extremities modifies alveolar ventilation during sleep in patients with congenital central hypoventilation syndrome. Am J Respir Crit Care Med. 2000;162:1747–1751. doi: 10.1164/ajrccm.162.5.2005012. [DOI] [PubMed] [Google Scholar]
- Haddad GG, Mazza NM, Defendini R, Blanc WA, Driscoll JM, Epstein MA, Epstein RA, Mellins RB. Congenital failure of automatic control of ventilation, gastrointestinal motility and heart rate. Medicine (Baltimore) 1978;57:517–526. doi: 10.1097/00005792-197811000-00003. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Head K, Head E, Lott IT. Neuroimaging of individuals with Down's syndrome at-risk for dementia: evidence for possible compensatory events. Neuroimage. 2008;39:1324–1332. doi: 10.1016/j.neuroimage.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RM, Macey PM, Woo MA, Macey KE, Keens TG, Gozal D, Alger JR. Hypercapnic exposure in congenital central hypoventilation syndrome reveals CNS respiratory control mechanisms. J Neurophysiol. 2005;93:1647–1658. doi: 10.1152/jn.00863.2004. [DOI] [PubMed] [Google Scholar]
- Holstege G. The emotional motor system. Eur J Morphol. 1992;30:67–79. [PubMed] [Google Scholar]
- Hontanilla B, de las Heras S, Gimenez-Amaya JM. Organization of the striatal projections from the rostral caudate nucleus to the globus pallidus, the entopeduncular nucleus, and the pars reticulata of the substantia nigra in the cat. Anat Rec. 1994;238:114–124. doi: 10.1002/ar.1092380113. [DOI] [PubMed] [Google Scholar]
- Kumar R, Lee K, Macey PM, Woo MA, Harper RM. Mammillary body and fornix injury in congenital central hypoventilation syndrome. Pediatr Res. 2009a doi: 10.1203/PDR.0b013e3181b3b363. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Elevated mean diffusivity in widespread brain regions in congenital central hypoventilation syndrome. J Magn Reson Imaging. 2006;24:1252–1258. doi: 10.1002/jmri.20759. [DOI] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Diffusion tensor imaging demonstrates brainstem and cerebellar abnormalities in congenital central hypoventilation syndrome. Pediatr Res. 2008;64:275–280. doi: 10.1203/PDR.0b013e31817da10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Alger JR, Keens TG, Harper RM. Neuroanatomic deficits in congenital central hypoventilation syndrome. J Comp Neurol. 2005;487:361–371. doi: 10.1002/cne.20565. [DOI] [PubMed] [Google Scholar]
- Kumar R, Nguyen H, Macey PM, Woo MA, Harper RM. Dilated basilar arteries in patients with congenital central hypoventilation syndrome. Soc Neurosci Abs. 2009b;89.10 doi: 10.1016/j.neulet.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, McCarley RW, Dickey CC, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Ciszewski AA, Kikinis R, Jolesz FA, Shenton ME. MRI study of caudate nucleus volume and its cognitive correlates in neuroleptic-naive patients with schizotypal personality disorder. Am J Psychiatry. 2002;159:1190–1197. doi: 10.1176/appi.ajp.159.7.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Friedman HR, Davachi L, Goldman-Rakic PS. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J Neurosci. 1997;17:3870–3882. doi: 10.1523/JNEUROSCI.17-10-03870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ, Salamon N, Dutton RA, Lee AD, Geaga JA, Hayashi KM, Toga AW, Engel J, Jr, Thompson PM. Three-dimensional preoperative maps of hippocampal atrophy predict surgical outcomes in temporal lobe epilepsy. Neurology. 2005;65:1094–1097. doi: 10.1212/01.wnl.0000179003.95838.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi WJ, Andreason PJ, Sirocco KY, Rio DE, Gross RE, Umhau JC, Hommer DW. Wisconsin Card Sorting Test performance following head injury: dorsolateral fronto-striatal circuit activity predicts perseveration. J Clin Exp Neuropsychol. 1999;21:2–16. doi: 10.1076/jcen.21.1.2.940. [DOI] [PubMed] [Google Scholar]
- Looi JC, Lindberg O, Zandbelt BB, Ostberg P, Andersen C, Botes L, Svensson L, Wahlund LO. Caudate nucleus volumes in frontotemporal lobar degeneration: differential atrophy in subtypes. Am J Neuroradiol. 2008;29:1537–1543. doi: 10.3174/ajnr.A1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Woo MA, Keens TG, Harper RM. Aberrant neural responses to cold pressor challenges in congenital central hypoventilation syndrome. Pediatr Res. 2005a;57:500–509. doi: 10.1203/01.PDR.0000155757.98389.53. [DOI] [PubMed] [Google Scholar]
- Macey PM, Richard CA, Kumar R, Woo MA, Ogren JA, Avedissian C, Thompson PM, Harper RM. Hippocampal volume reduction in congenital central hypoventilation syndrome. PLos One. 2009;4:e6436. doi: 10.1371/journal.pone.0006436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Woo MA, Macey KE, Keens TG, Saeed MM, Alger JR, Harper RM. Hypoxia reveals posterior thalamic, cerebellar, midbrain, and limbic deficits in congenital central hypoventilation syndrome. J Appl Physiol. 2005b;98:958–969. doi: 10.1152/japplphysiol.00969.2004. [DOI] [PubMed] [Google Scholar]
- Matera I, Bachetti T, Puppo F, Di Duca M, Morandi F, Casiraghi GM, Cilio MR, Hennekam R, Hofstra R, Schober JG, Ravazzolo R, Ottonello G, Ceccherini I. PHOX2B mutations and polyalanine expansions correlate with the severity of the respiratory phenotype and associated symptoms in both congenital and late onset central hypoventilation syndrome. J Med Genet. 2004;41:373–380. doi: 10.1136/jmg.2003.015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Rosenberg DR, Easter PC, MacMaster FP, Chen HH, Nicoletti M, Caetano SC, Hatch JP, Soares JC. Striatal volume abnormalities in treatment-naive patients diagnosed with pediatric major depressive disorder. J Child Adolesc Psychopharmacol. 2008;18:121–131. doi: 10.1089/cap.2007.0026. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Adams NL, Lewandowski KS. Neurobehavioral changes associated with caudate lesions. Neurology. 1989;39:349–354. doi: 10.1212/wnl.39.3.349. [DOI] [PubMed] [Google Scholar]
- Mori S, Ueda S, Yamada H, Takino T, Sano Y. Immunohistochemical demonstration of serotonin nerve fibers in the corpus striatum of the rat, cat and monkey. Anat Embryol (Berl) 1985;173:1–5. doi: 10.1007/BF00707298. [DOI] [PubMed] [Google Scholar]
- Newman LM, Trivedi MA, Bendlin BB, Ries ML, Johnson SC. The relationship between gray matter morphometry and neuropsychological performance in a large sample of cognitively healthy adults. Brain Imaging Behav. 2007;1:3–10. doi: 10.1007/s11682-007-9000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol. 2003;94:179–184. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- Pae EK, Chien P, Harper RM. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett. 2005;375:123–128. doi: 10.1016/j.neulet.2004.10.091. [DOI] [PubMed] [Google Scholar]
- Paton JY, Swaminathan S, Sargent CW, Keens TG. Hypoxic and hypercapnic ventilatory responses in awake children with congenital central hypoventilation syndrome. Am Rev Respir Dis. 1989;140:368–372. doi: 10.1164/ajrccm/140.2.368. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Prabhakaran V, Seger CA, Gabrieli JD. Striatal activation during acquisition of a cognitive skill. Neuropsychology. 1999;13:564–574. doi: 10.1037//0894-4105.13.4.564. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Pulsipher DT, Hopkins RO, Weaver LK. Basal ganglia volumes following CO poisoning: A prospective longitudinal study. Undersea Hyperb Med. 2006;33:245–256. [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Ruof H, Hammer J, Tillmann B, Ghelfi D, Weber P. Neuropsychological, behavioral, and adaptive functioning of Swiss children with congenital central hypoventilation syndrome. J Child Neurol. 2008;23:1254–1259. doi: 10.1177/0883073808318048. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Vanderlaan M, Holbrook CR, Wang M, Tuell A, Gozal D. Epidemiologic survey of 196 patients with congenital central hypoventilation syndrome. Pediatr Pulmonol. 2004;37:217–229. doi: 10.1002/ppul.10438. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Zhou L, Maher BS, Silvestri JM, Curran ME, Marazita ML. Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am J Med Genet A. 2003;123A:267–278. doi: 10.1002/ajmg.a.20527. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Silvestri JM, Huffman AD, Smok-Pearsall SM, Kowal MH, Maher BS, Cooper ME, Marazita ML. Case/control family study of autonomic nervous system dysfunction in idiopathic congenital central hypoventilation syndrome. Am J Med Genet. 2001;100:237–245. doi: 10.1002/ajmg.1249. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Vasic N, Schonfeldt-Lecuona C, Ecker D, Landwehrmeyer GB. Cortical dysfunction in patients with Huntington's disease during working memory performance. Hum Brain Mapp. 2009;30:327–339. doi: 10.1002/hbm.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan G, Fenik P, Pratico D, Veasey SC. Inducible nitric oxide synthase in long-term intermittent hypoxia: hypersomnolence and brain injury. Am J Respir Crit Care Med. 2005;171:1414–1420. doi: 10.1164/rccm.200411-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]