Abstract
Sensory nerve autografting is the standard of care for injuries resulting in a nerve gap. Recent work demonstrates superior regeneration with motor nerve grafts. Improved regeneration with motor grafting may be a result of the nerve’s Schwann cell basal lamina tube size. Motor nerves have larger SC basal lamina tubes, which may allow more nerve fibers to cross a nerve graft repair. Architecture may partially explain the suboptimal clinical results seen with sensory nerve grafting techniques. To define the role of nerve architecture, we evaluated regeneration through acellular motor and sensory nerve grafts. Thirty-six Lewis rats underwent tibial nerve repairs with 5 mm double-cable motor or triple-cable sensory nerve isografts. Grafts were harvested and acellularized in University of Wisconsin solution. Control animals received fresh motor or sensory cable isografts. Nerves were harvested after 4 weeks and histomorphometry was performed. In 6 animals per group from the fresh motor and sensory cable graft groups, weekly walking tracks and wet muscle mass ratios were performed at 7 weeks. Histomorphometry revealed more robust nerve regeneration in both acellular and cellular motor grafts. Sensory groups showed poor regeneration with significantly decreased percent nerve, fiber count, and density (p < 0.05). Walking tracks revealed a trend toward improved functional recovery in the motor group. Gastrocnemius wet muscle mass ratios show a significantly greater muscle mass recovery in the motor group (p < 0.05). Nerve architecture (size of SC basal lamina tubes) plays an important role in nerve regeneration in a mixed nerve gap model.
Keywords: nerve regeneration, peripheral nerve, motor nerve, preferential motor reinnervation, nerve architecture, acellularized nerve, nerve graft, sensory nerve, nerve transfer
INTRODUCTION
Sensory nerve autografting is the accepted technique for reconstruction of peripheral nerve defects despite suboptimal outcomes (Kline, 1990, Mackinnon and Dellon, 1988, Mackinnon, et al., 2001, Sunderland, 1978, Terzis and Smith, 1990). Recent studies suggest that modality specific regeneration (MSR) may influence nerve regrowth. Brushart and colleagues demonstrated that regenerating motor axons preferentially choose the motor pathway (Preferential Motor Reinnervation, or PMR) following injury (Brushart, 1988, Madison, et al., 1996). Motor nerve grafts result in a four fold increase in nerve total fiber counts compared to sensory nerve grafts (Brenner, et al., 2006, Nichols, et al., 2004). Possible governing mechanisms for this phenomenon include differences in biochemical markers, Schwann cell phenotype, neurotrophic support, and/or nerve architecture (Brenner, et al., 2006, Brushart, 1988, Brushart, 1993, Brushart and Seiler, 1987, Ghalib, et al., 2001, Martini, et al., 1994, Martini, et al., 1992, Nichols, et al., 2004).
Differences in neurotrophic factor presence and expression have been identified in motor and sensory nerves: insulin-like growth factor (Caroni and Grandes, 1990, Hansson, et al., 1986), basic fibroblast growth factor (Danielsen, et al., 1988, Grothe, et al., 1991), and ciliary neurotrophic factor (Arakawa, et al., 1990, Forger, et al., 1993, Manthorpe, et al., 1986, Oppenheim, et al., 1991), are all believed to affect motor nerve phenotypes. Other studies evaluating the sciatic and facial nerves demonstrated that the presence or application of glial cell line-derived neurotrophic factor and neurturin reduced the amount of axonal cell death, and promoted motor nerve regeneration (Fine EG, 2002, Florian MB, 2002, Hoke A, 2002, MC, 2004, Oppenheim RW, 2000).
Repair of a tibial nerve defect with motor, sensory, and mixed nerve grafts demonstrated significantly improved regeneration through motor nerve cable grafts (femoral motor nerve to the quadriceps) compared to sensory nerve cable grafts (femoral cutaneous nerve) (Nichols, et al., 2004). This held true even if a single cable of motor nerve was used (Brenner, et al., 2006). However, when nerve graft architecture was disrupted and nerve cellularity was maintained (i.e., Schwann cells), the benefit seen with motor graft material was lost (LLoyd, 2007). Neurotrophic differences alone do not completely account for the differences in regeneration observed with motor and sensory nerve grafting.
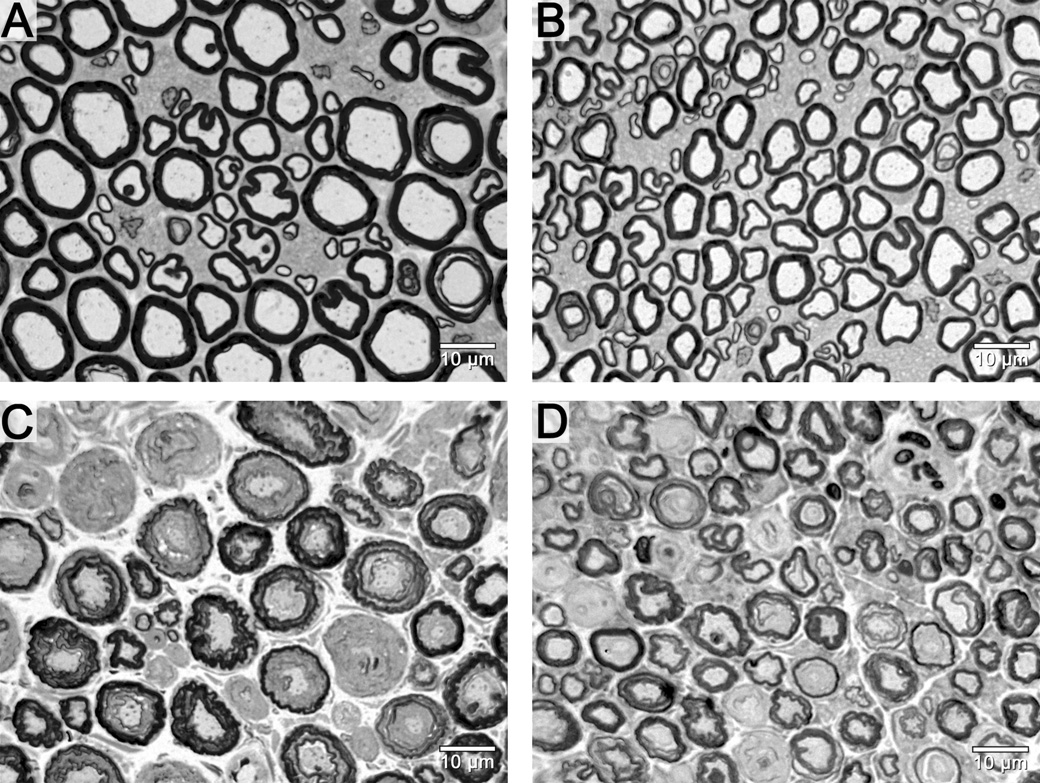
Previous studies have demonstrated that regenerating axons prefer to grow along Schwann cell basal lamina tubes (Ramon y Cajal, 1928), and intact nerve architecture allows the basal lamina tubes to guide regenerating axons to the desired distal target (Brown MC, 1981). The Schwann cell basal lamina tubes than contain motor fibers are larger than those that contain sensory fibers (Figure 1A/B). The present study tests the hypothesis that architectural differences between motor and sensory nerves confer the regeneration advantage seen with motor nerve grafting in a mixed nerve gap model.
Figure 1.
Photomicrographs of femoral motor and sensory nerve cross-sections. The fresh motor nerve (A) has larger diameter, but, fewer fibers in comparison to the fresh sensory nerve (B). These larger endoneurial tubes may mechanically provide a more favorable size conduit for nerve regeneration than the smaller sensory endoneurial tube. Comparisons between fresh motor (A) and cold motor (C) or fresh sensory (B) and cold sensory (D) nerves demonstrate the small amount of shrinkage that takes place during the cold preservation procedure. Scale bars in the bottom right hand corner.
MATERIALS & METHODS
Nerves were decellularized in University of Wisconsin organ preservation solution for 7 weeks, leaving intact, the basal lamina tube architecture and laminin in the extracellular membrane (Evans, et al., 1998). After 7 weeks of preservation, nerves lack immunogenicity and can support regeneration in a short nerve gap model (Fox, et al., 2005).
Experimental Design
We assigned 12 animals randomly to each of 2 graft recipient groups: fresh motor and fresh sensory and 6 animals to each of 2 graft recipient groups: cold motor and cold sensory (Table I). An additional 18 animals were used as nerve donors.
Animals in group I received 5 mm motor (femoral motor branch to quadriceps) isografts, animals in group II received 5 mm sensory (femoral cutaneous branch) isografts, animals in group III received 5 mm cold preserved (decellularized) motor isografts, and animals in group IV received 5 mm cold preserved sensory isografts. The choice of 5 mm motor and sensory grafts stems from the availability of donor nerve. Specifically, the rat femoral motor nerve can reliably provide 5 mm of length without significant neurolysis. A longer graft such as 1 cm grafts used in other studies requires significant neurolysis and may include sensory fibers in the motor donor graft.
Due to the differences in nerve diameters and cross-sectional area (Brushart and Seiler, 1987), the size disparity between sensory and motor nerves was normalized by constructing a triple cable sensory graft and a double cable motor graft. This arrangement provides grafts of comparable diameter and area (Nichols, et al., 2004).
In the rodent model, timing of evaluation is critical because of the superlative regenerative capacity of these animals. Early evaluation prevents “blow-through”, where true differences in histological recovery are masked by robust regeneration not seen in humans (Brenner MJ, 2008). Because of these considerations, a four-week endpoint was used to evaluate histological recovery. A seven-week endpoint was then chosen to provide information on functional recovery, which lags histological findings since muscle reinnervation and function is required.
Methods
Adult male Lewis rats (Harlan Sprague–Dawley, Indianapolis, IN) weighing 310 to 410 grams were housed in a central animal facility, given a rodent diet (PicoLab Rodent Diet 20 #5053, PMI Nutrition International) and water ad libitum. All surgical procedures, experimental manipulations, and peri-operative care measures were carried out in strict accordance with National Institutes of Health guidelines and were approved by the university institutional Animal Studies Committee. Animals were returned to the animal facility following surgical procedures and monitored for infection, weight loss, or other disability.
Cold-Preservation
Fifteen milliliters of University of Wisconsin (UW) organ preservation solution supplemented with penicillin G (200,000 U/L), regular insulin (40 U/L), and dexamethasone (16 mg/L) was aliquoted into sterile Petri dishes. Nerves were placed into solution at the time of harvest and maintained at 4°C for 7 weeks. Cold preservation solution was changed weekly until the time of implantation.
Surgical Procedures
General anesthesia was achieved by subcutaneous injection of 75 mg/kg ketamine hydrochloride (Fort Dodge Animal Health, Fort Dodge, IA) and 0.5 mg/kg medetomidine hydrochloride (Orion Corporation, Espoo, Finland). Animals were then shaved, prepped, and surgeries were performed under aseptic conditions. Donor nerves were harvested through a longitudinal groin incision to expose the femoral neurovascular bundle. The saphenous nerve was traced back to the proximal femoral trunk where the motor branch of the femoral nerve was identified branching to the quadriceps muscles using a Wild M651 operating microscope (Leica Microsystems, Deerfield, IL) under 16X magnification. Both branches were neurolysed proximally and harvested by sharp transection with microscissors. Under 25X magnification the nerves were measured and cut into 5 mm cables. To facilitate handling, cables were joined together at each end with one epineurial 11–0 nylon rosette stitch.
In the recipient animals, the right tibial nerve was exposed through a routine gluteal muscle splitting incision. The tibial nerve was divided sharply 1 cm distal to the sciatic trifurcation, and 5 mm sensory or motor cable grafts were coapted using an 11-0 nylon microsuture epineurial repair at 25X magnification. One to two sutures were placed per cable to optimally align nerve fascicles. Incisions were closed in two layers with 6–0 vicryl muscular sutures and 4–0 nylon skin sutures. Donor animals were sacrificed with intracardiac injection of Euthasol (Delmarva Laboratories, Des Moines, IA) while still under general anesthesia. Experimental animals were reversed with antipamezole HCl (Pfizer Animal Health, Exton, PA), allowed to recover in a heated area and then returned to the animal housing facility. At the 4-week endpoint, the animals were re-anesthetized, and nerve harvests were performed by reopening the prior muscle splitting incision. The nerve graft and a 1 cm portion of native nerve both proximal and distally were harvested for animals designated for histomorphometric analysis.
Functional Outcome Measures
The 6 additional animals in group I (fresh motor) and group II (fresh sensory) underwent weekly walking track analysis for 7 weeks. At the 7 week endpoint gastrocnemius muscles were harvested for wet muscle mass ratios. The later endpoint was selected for functional outcome measures because functional recovery lags behind histologic recovery.
Walking Track Analysis
Walking tracks were conducted as previously described (Bain, et al., 1989, Brown, et al., 1989). The walking track consisted of an 8 × 40 cm track with exposed X-ray film placed on the bottom. The hind feet of the rat were dipped in X-ray film developer and then the rat was allowed to walk down the track; the hind footprints appeared as the developer reacted with the film. Measurements of the footprints from the walking tracks were used to calculate the print length factor as a measure of the tibial function index (Bain, et al., 1989, de Medinaceli, et al., 1982).
Wet Muscle Mass Ratios
At the 7 week end-point animals were anesthetized and the bilateral gastrocnemii approached via a posterior tibial incision. Using 10X magnification, these muscles were isolated, removed, and then weighed on an analytical balance. The wet muscle mass ratio between the experimental (right) and control (left) was measured. Animals were then sacrificed as previously described. The ratio was then used as an indicator of muscle mass recovery.
Histomorphometry & Electron Microscopy
Tibial nerves grafts were harvested and fixed in EM grade cold 3% glutaraldehyde solution, post-fixed with 1% osmium tetroxide, dehydrated in ethanol, and then embedded in Araldite 502 (Polysciences Inc., Warrington, PA). Sections were cut 1-micrometer thin and stained with 1% toluidine blue for light microscopy. Microscopic images were examined using a digital image-analysis system linked to Leco Instruments morphometric software described previously (Nichols, et al., 2004). The system displayed the microscope image on a video monitor calibrated to 0.125 mm/pixel. Total fiber number and fascicular area were measured through computer analysis of digitized information based on black and white image scales. A minimum of 500 myelinated fibers were measured to determine the myelin width, axon width, and fiber diameter. From these primary measurements myelin area, nerve fiber area, nerve fiber density (fiber number/mm2), percent neural tissue, percent neural debris, and axon: myelin and axon: fiber area ratios were calculated. For electron microscopy, ultrathin sections were cut using a LKB III ultramicrotome, stained with uranyl acetate-lead acetate and evaluated with a Zeiss 902 electron microscope. Quality of myelination and the relative prevalence of unmyelinated fibers were qualitatively evaluated.
Statistical Analysis
All results are reported as mean ± standard deviation. Statistical analysis was performed using Statistica version 6. Means for histomorphometry, wet muscle mass ratios, and walking track data were compared using analysis of variance (ANOVA). For the histomorphometry data, a post hoc Newman-Keuls test was used for comparison of differences between groups with significance set at p < 0.05.
RESULTS
Histomorphometry and Electron Microscopy
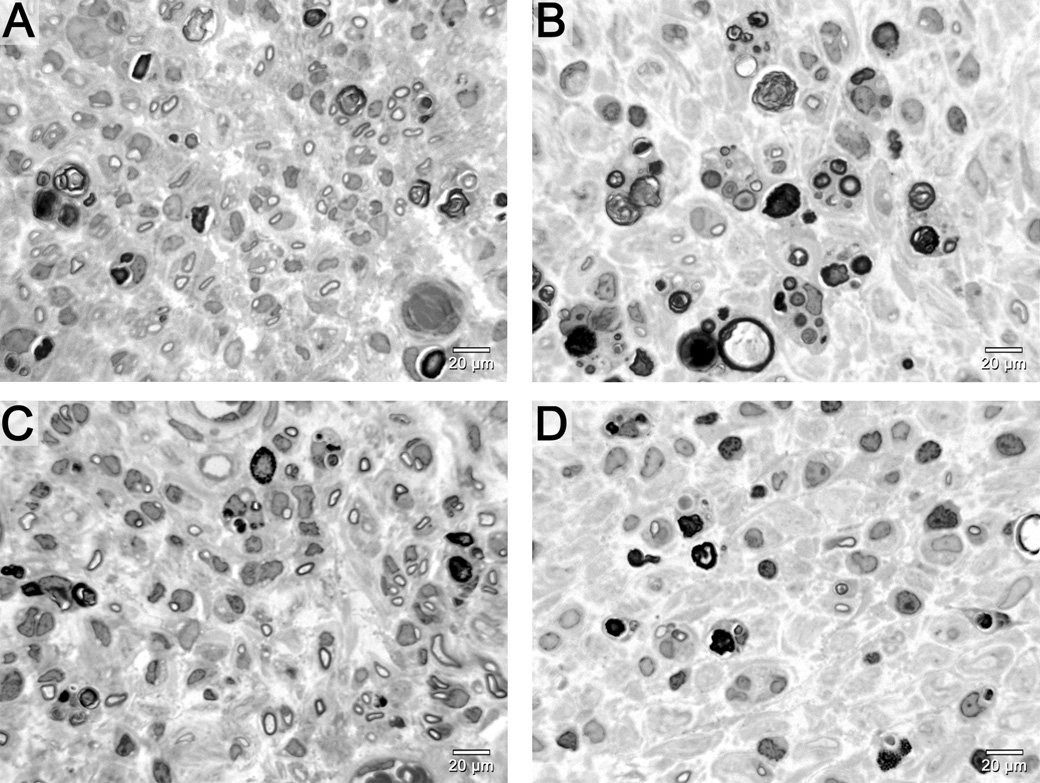
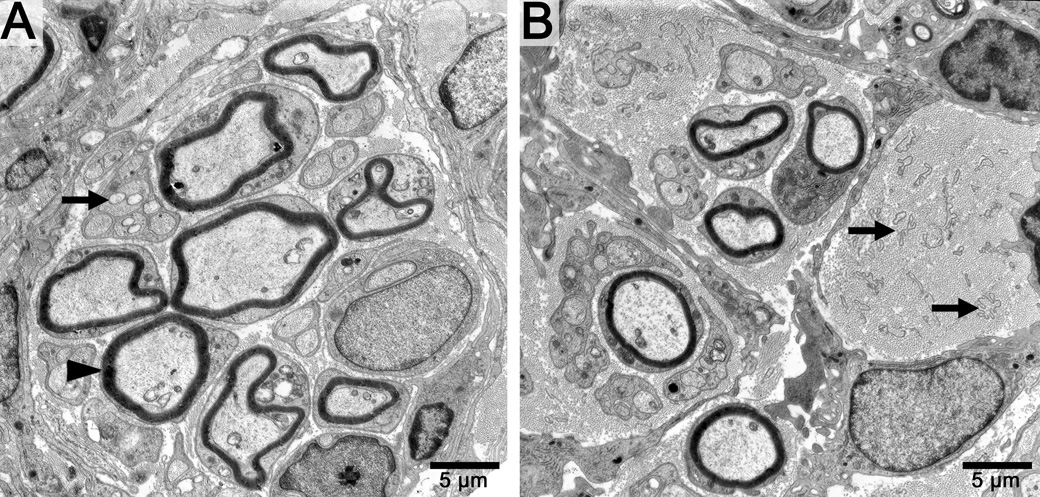
Qualitative examination of distal tibial nerve sections showed greater regeneration in motor nerve graft groups in contrast to the sensory nerve graft groups, which had fewer regenerating fibers. Histology of the nerve segments distal to the grafts revealed improved regeneration in motor groups whether the graft was fresh or decellularized (Figure 2). Electron micrographs of the cold preserved groups demonstrated multiple large robust myelinated neurites in the motor group as well as multiple unmyelinated fibers (Figure 3 A). In contrast, cold sensory nerves had fewer myelinated and unmyelinated fibers (Figure 3B).
Figure 2.
Distal tibial nerve photomicrographs demonstrate significantly better regeneration in fresh (A) and cold motor (C) nerve graft groups in comparison to fresh (B) and cold sensory (D) graft groups. Fresh and cold motor grafting is similar, while fresh sensory grafting shows more fibers than cold sensory grafting. Scale bar in bottom right hand corner.
Figure 3.
Electron micrographs of distal tibial nerve sections in cold motor and cold sensory nerve groups. A. Note the multiple myelinated (black arrow head) and unmyelinated fibers (black arrow) in the motor group. B. The sensory nerve groups show fewer fibers and multiple empty bands of Bungner which contain Schwann cell lamina tubes (black arrows). Scale bar in bottom right hand corner.
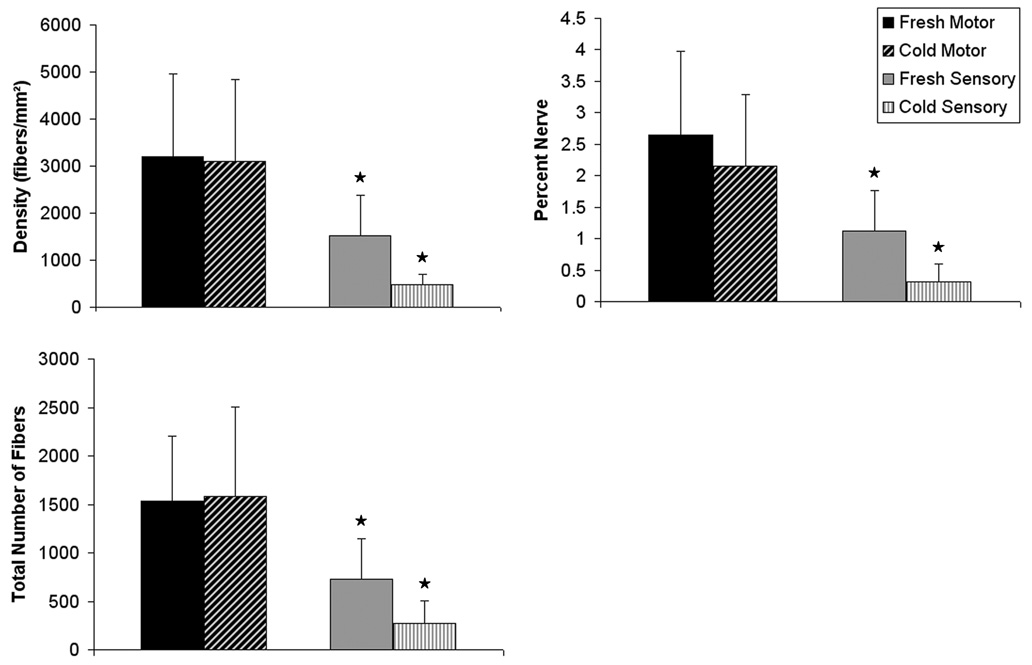
Analysis of the distal tibial nerve tissue harvested from each group revealed significant differences summarized in Table II and Figure 4. The most remarkable differences were seen in the total number of fibers, nerve density, and percent nerve, which all showed much greater regeneration in the motor graft groups (p < 0.05). Total fiber number, density, and percent nerve across fresh and decellularized motor grafts were similar. In contrast, the fresh sensory nerve grafts trended toward better regeneration than the cold preserved sensory nerve grafts although this difference was not statistically significant.
Figure 4.
Histomorphometric comparisons of nerve regeneration across fresh and cold preserved sensory and motor graft groups. Comparable nerve regeneration is noted in fresh and cold motor groups. The fresh sensory and cold nerve graft groups exhibit significantly reduced nerve fiber counts, nerve density, and percent nerve when compared to motor groups (p < 0.05), marked with an asterisk.
Functional Outcome Measures
Walking track analysis
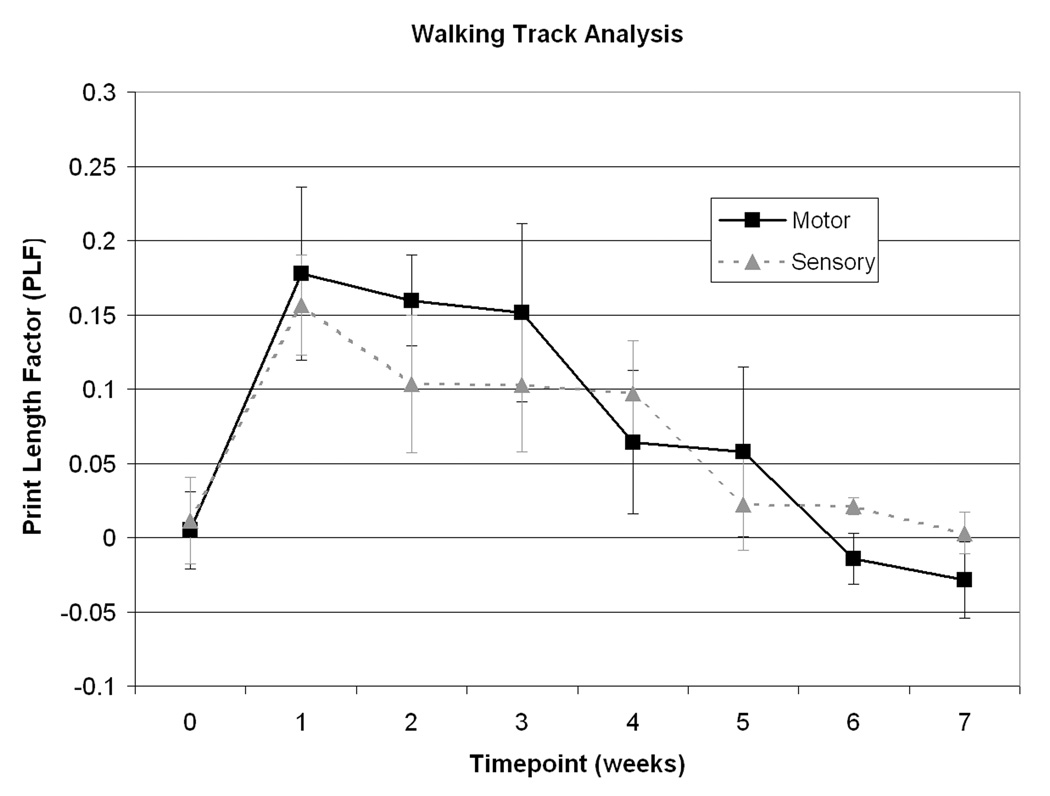
Walking track analysis at 1 week demonstrated maximal lengthening of the print length consistent with a tibial nerve injury. At the 6th and 7th week there was a trend toward greater recovery of function in the motor group compared to the sensory group (Figure 5).
Figure 5.
Walking track analysis of functional recovery. No significant differences were seen between groups. However, a trend toward improved functional recovery was seen in the motor group.
Wet Muscle Mass Ratios
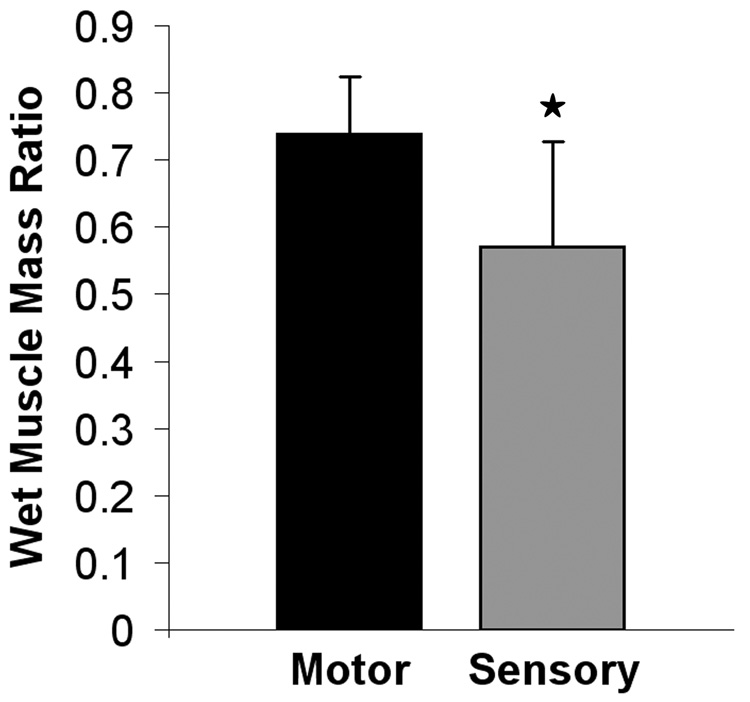
Analysis of the gastrocnemius wet muscle mass ratios demonstrated a significantly greater recovery of muscle mass in the motor group compared to the sensory group, p < 0.05 (Figure 6).
Figure 6.
Wet muscle mass ratios. Significance marked with asterisk, p<0.05.
DISCUSSION
Until recently, little interest surrounded the use of motor nerves to repair predominantly motor nerve defects. However, emerging research regarding preferential motor reinnervation (PMR), in addition to studies investigating phenotypic differences between sensory and motor Schwann cells, modality specific regeneration (MSR) and now nerve graft architecture collectively bring into question the widespread use of sensory grafts for motor nerve gap repair.
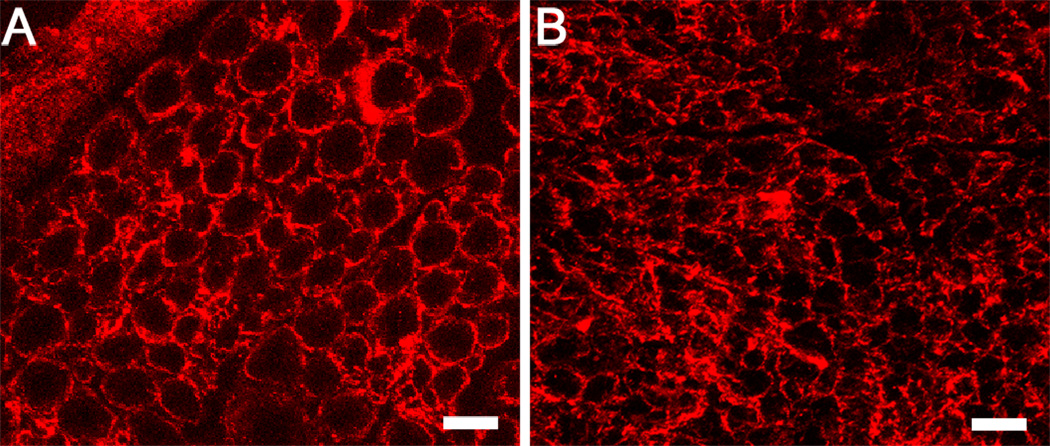
Our previous work demonstrated that addition of either minced fresh motor or sensory nerve, with cells and biochemical factors intact but architecture disrupted, does not differentially impact regeneration through a silicone conduit in the rat model (LLoyd, 2007). Chemical and cellular differences between motor and sensory nerves are not sufficient in isolation to explain observed differences in performance. In this study we find that motor grafts used to repair a mixed nerve defects have a 4 fold increase in the number of regenerating fibers through the grafts as compared to sensory grafts, and that this phenomenon appears to be related in part to the architecture of motor nerves; motor nerve grafts have larger diameter endoneurial tubes as compared to sensory nerve grafts (Figure 7).
Figure 7.
Comparison of sensory and motor nerve laminin architecture. Nerve cross-sections were stained with polyclonal anti-laminin antibodies conjugated to Cy3 flurophore. Motor nerve laminin tubes (A) have a greater diameter than sensory (B). Scale bar = 10µm.
Regardless of cellularity, more fibers cross a motor nerve graft than a sensory graft (Table II). It is known that regenerating axons prefer to grow along SC basal lamina tubes and that sensory nerves have a higher fiber count, which means that more Schwann cell basal lamina tubes are available. However, the size of Schwann cell basal lamina tubes appears to be an important factor when nerve fibers are selecting an endoneurial tube. Close inspection of electron micrographs from sensory grafts revealed multiple empty small-caliber SC basal lamina tubes which were not seen in the motor group (Figure 3B). These SC basal lamina tubes were available but not utilized by the regenerating fibers. One possible explanation is that these sensory SC basal lamina tubes were so small that they posed a size barrier to regenerating axons.
The size of the endoneurial conduit also likely explains the difference seen in regeneration between cellular and acellular sensory nerve grafts. Although regeneration in the sensory grafts is poor by comparison, the cellular sensory graft supported a greater degree of regeneration than the acellular sensory graft group while both motor groups were similar. We noted that cold preservation reduced the size of the endoneurial conduits, and this may explain in part the decreasing degree of axonal regeneration in a cold sensory graft compared to a fresh sensory graft. Cold preserved sensory nerves undergo shrinkage of their Schwann cell basal lamina tubes creating an even greater size barrier to reinnervation (Figure 1B/D). It is possible that a different method of decellularization resulting in increased conduit size may support superior axonal regeneration.
Our study demonstrates a functional advantage of the motor nerve SC basal lamina tube architecture. Motor nerve grafting results in a statistically significant increase in muscle mass recovery compared to sensory nerve grafting (Figure 6). The walking track data revealed a trend toward improved recovery with motor grafting at the 7 week endpoint; however this difference was not statistically significant (Figure 5). A later timepoint may have revealed a statistically significant difference because functional recovery lags behind histologic recovery.
It is unlikely that there is a single reason for improved axonal regeneration in motor compared with sensory grafts. The phenomenon of PMR highlights the differences between sensory and motor nerves. Beginning in 1987, Brushart and Seiler investigated the behavior of regenerating motor neurons in motor and sensory pathways (Brushart and Seiler, 1987). The authors concluded that end-organ interactions influenced regeneration, a phenomenon called neurotrophism. This body of work elicits the tendency of motor nerves to reinnervate motor pathways when given equal access to both sensory and motor nerve pathways, and clearly demonstrates that motor nerves prefer to innervate muscle when given the opportunity to do so (Brushart, 1993, Brushart, et al., 1998).
Another line of investigation suggests that motor nerve specific growth factors exist, and are largely responsible for improved axonal regeneration in motor nerve defects repaired with motor grafts vs. sensory grafts. Motor-specific growth factors, including pleiotrophin and glial cell line-derived neurotrophic factor (GDNF), are thought to be produced in increased quantity by Schwann cells from motor nerve grafts compared with sensory grafts (Hoke A, 2006 ) as demonstrated by RT-PCR. Functional mimics of the motor-specific carbohydrate HNK-1 have been shown to independently improve return to motor function in a mouse femoral nerve conduit model (Simova O, 2006).
It is a possibility that motor-specific growth factors remained in our cold-preserved grafts, producing a potential confounding factor. However, our previous work has shown that when fresh motor or sensory graft architecture is disrupted by mincing and the resultant material is used to fill a silicone conduit, there is no difference in recovery through that conduit between motor and sensory groups. The mincing procedure leaves cells and protein components, including growth factors, intact – implying that, surprisingly, those constituents alone are not sufficient to produce the difference seen with sensory vs. motor grafting (LLoyd, 2007). The experiment described above provides a compliment to our earlier minced nerve study: cellular and protein components were not sufficient to produce a difference, while sensory vs. motor architecture appears to be.
Conversely, Robinson and Madison’s (2004) more recent work in the rat femoral model demonstrated a hierarchy of trophic support in which sensory end-organ support was more beneficial than the remnant architecture of the silicone capped motor nerve stump. We feel that their conclusion is not mutually exclusive with our own; experiments have repeatedly shown that motor axons are capable of traversing sensory basal lamina. However, their study did not investigate the converse model; namely, a blind-ended sensory branch and a motor branch connected to the end-organ. We would hypothesize, that that experiment would show considerable regeneration of motor axons down the motor branch, to the relative exclusion of the sensory branch. Due to the neurorepulsive effects of silicone, and the trophic support provided by the sensory end-organ, it is not surprising that motor axons predominantly traveled down the sensory branch. While trophic support is undoubtedly important, we think that architecture is also an important contributor to axonal regeneration.
Understanding the contribution of nerve architecture to regeneration opens the door to investigate various methods of reconstituting the motor nerve environment. Short sensory nerve defects can be effectively treated with biodegradable conduits. However, this approach has been unsuccessful for motor nerve gaps. Only autologous nerve is currently used clinically to bridge defects in motor nerves.
The clinical use of musculocutaneous flaps requiring sacrifice of the transferred nerves illustrates that these accompanying motor nerves are functionally expendable (Bostwick J, 1979, Mathes SJ, 1980, Mathes SJ, 1974, Mathes SJ, 1978, McCraw JB, 1980, Nahai F, 1978). A growing number of available selective motor nerve transfers has led peripheral nerve surgeons to reappraise current techniques (Brandt and Mackinnon, 1993, Weber RV, 2004). Multiple expendable motor nerves exist and can be harvested and used with limited morbidity as grafts, and anecdotal evidence supports improved functional recovery when motor nerve grafts are used for motor nerve defects.
For example, in our practice the motor nerve to the gracilis is being used to repair defects in the spinal accessory nerve with excellent return of motor function. However, we must consider the benefit of motor nerve grafting with the potential morbidity of nerve harvest in each individual case. We may be able to generate an effectively unlimited supply of banked donor motor nerve allografts for use in grafting of motor nerve defects. One possibility is that motor nerves will be harvested from cadavers and treated with a number of available and emerging techniques to decellularize the nerve (Evans, et al., 1998, Hudson TW, 2004, Rovak JM, 2005), and eliminate antigenicity (Fox, et al., 2005). Currently the use of nerve allografts requires immunosuppression until host nerve fibers and Schwann cells repopulate the nerve scaffold. Studies in nerve allotransplantation have identified mechanisms to seed acellular nerve grafts with autologous cultured Schwann cells (Ogden, et al., 2000), supporting regenerating nerve fibers across long nerve gaps (Brenner, et al., 2005, Hess JR, 2007).
On the other end of the spectrum, we can use our understanding of the importance of nerve architecture and apply it to research on conduits that reconstitute a motor nerve environment. Biodegradable conduits are now being seeded with Schwann cells and neurotrophic factors (Hadlock T, 1998, Hadlock T, 1999). With the additional ability to construct a conduit based on ideal Schwann cell basal lamina tube size, depending on the desired type of regeneration, peripheral nerve surgery will push past the current microsurgical restraints.
Conclusion
Advances over the last twenty five years are leading to a paradigm shift away from the use of sensory nerve grafts to the use of expendable motor nerve grafts, especially for the recovery of critical motor function. Our work has shown that nerve regeneration across motor grafts results in a 4 fold increase in the number of fibers crossing a mixed nerve gap in comparison to sensory grafts, and that this appears to be related in part to the architecture of motor nerves. Motor nerve grafts have larger diameter endoneurial tubes which allow for more nerve fibers to cross a nerve defect. This finding has implications for our immediate clinical management of nerve gaps, and can guide the future development of tissue engineered nerve conduits constructed with SC basal lamina tubes at an “optimal diameter” for motor nerve regeneration.
Acknowledgement
This study was funded, in part, by Moshe and Jacqueline Tal, Leslie F. Loewe, NIH grant 5R01NS051706-02 (S.E.M.), and NIH T32DC000022-20 resident training grant (A.M.). The authors would like to thank Dr. Chris Nichols, Jason Koob, and Dr. Michael Brenner for their assistance with the pilot work for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Arakawa Y, Sendtner M, Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. J Neurosci. 1990;10:3507–3515. doi: 10.1523/JNEUROSCI.10-11-03507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plastic & Reconstructive Surgery. 1989 Jan;83(1):129–138. doi: 10.1097/00006534-198901000-00024. [see comment]. [DOI] [PubMed] [Google Scholar]
- 3.Bostwick JNF, Wallace JG, Vasconez LO. Sixty latissimus dorsi flaps. Plast Reconstr Surg. 1979;63:31–41. doi: 10.1097/00006534-197901000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Brandt KE, Mackinnon SE. A technique for maximizing biceps recovery in brachial plexus reconstruction. J Hand Surg [Am] 1993;18:726–733. doi: 10.1016/0363-5023(93)90328-Z. [DOI] [PubMed] [Google Scholar]
- 5.Brenner MJ, Hess JR, Myckatyn TM, Hayashi A, Hunter DA, Mackinnon SE. Repair of motor nerve gaps with sensory nerve inhibits regeneration in rats. Laryngoscope. 2006;116:1685–1692. doi: 10.1097/01.mlg.0000229469.31749.91. [DOI] [PubMed] [Google Scholar]
- 6.Brenner MJ, Lowe JB, 3rd, Fox IK, Mackinnon SE, Hunter DA, Darcy MD, Duncan JR, Wood P, Mohanakumar T. Effects of Schwann cells and donor antigen on long-nerve allograft regeneration. Microsurgery. 2005;25:61–70. doi: 10.1002/micr.20083. [DOI] [PubMed] [Google Scholar]
- 7.Brenner MJ, M A, Myckatyn TM, Tung TH, Mendez AB, Hunter DA, Mackinnon SE. Role of Timing in Assessment of Nerve Regeneration. Microsurgery in press. 2008 doi: 10.1002/micr.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown CJ, Mackinnon SE, Evans PJ, Bain JR, Makino AP, Hunter DA, Hare GM. Self-evaluation of walking-track measurement using a Sciatic Function Index. Microsurgery. 1989;10(3):226–235. doi: 10.1002/micr.1920100317. [DOI] [PubMed] [Google Scholar]
- 9.Brown MC, H W. Role of degenerating axon pathways in regeneration of mouse soleus motor axons. J Physiol (Lond) 1981;318:365–373. doi: 10.1113/jphysiol.1981.sp013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brushart TM. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brushart TM. Motor axons preferentially reinnervate motor pathways. J Neurosci. 1993;13:2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brushart TM, Gerber J, Kessens P, Chen YG, Royall RM. Contributions of pathway and neuron to preferential motor reinnervation. J Neurosci. 1998;18:8674–8681. doi: 10.1523/JNEUROSCI.18-21-08674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brushart TM, Seiler WD. Selective reinnervation of distal motor stumps by peripheral motor axons. Exp Neurol. 1987;97:289–300. doi: 10.1016/0014-4886(87)90090-2. [DOI] [PubMed] [Google Scholar]
- 14.Caroni P, Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol. 1990;110:1307–1317. doi: 10.1083/jcb.110.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danielsen N, Pettmann B, Vahlsing HL, Manthorpe M, Varon S. Fibroblast growth factor effects on peripheral nerve regeneration in a silicone chamber model. J Neurosci Res. 1988;20:320–330. doi: 10.1002/jnr.490200306. [DOI] [PubMed] [Google Scholar]
- 16.de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Experimental Neurology. 1982 Sep;77(3):634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- 17.Evans PJ, Mackinnon SE, Levi AD, Wade JA, Hunter DA, Nakao Y, Midha R. Cold preserved nerve allografts: changes in basement membrane, viability, immunogenicity, and regeneration. Muscle Nerve. 1998;21:1507–1522. doi: 10.1002/(sici)1097-4598(199811)21:11<1507::aid-mus21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Fine EG, D I, Papaloizos M, Zurn AD, Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15:589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 19.Florian MB, P P, Bouche N, Aebischer P, Zurn AD. Glian cell line-derived neurotrophic factor released by synthetic guidance channels promotes facial nerve regeneration in the rat. J of Neuroscience Research. 2002;70:746–755. doi: 10.1002/jnr.10434. [DOI] [PubMed] [Google Scholar]
- 20.Forger NG, Roberts SL, Wong V, Breedlove SM. Ciliary neurotrophic factor maintains motoneurons and their target muscles in developing rats. J Neurosci. 1993;13:4720–4726. doi: 10.1523/JNEUROSCI.13-11-04720.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox IK, Jaramillo A, Hunter DA, Rickman SR, Mohanakumar T, Mackinnon SE. Prolonged cold-preservation of nerve allografts. Muscle Nerve. 2005;31:59–69. doi: 10.1002/mus.20231. [DOI] [PubMed] [Google Scholar]
- 22.Ghalib N, Houst'ava L, Haninec P, Dubovy P. Morphometric analysis of early regeneration of motor axons through motor and cutaneous nerve grafts. Ann Anat. 2001;183:363–368. doi: 10.1016/S0940-9602(01)80183-7. [DOI] [PubMed] [Google Scholar]
- 23.Grothe C, Wewetzer K, Lagrange A, Unsicker K. Effects of basic fibroblast growth factor on survival and choline acetyltransferase development of spinal cord neurons. Brain Res Dev Brain Res. 1991;62:257–261. doi: 10.1016/0165-3806(91)90173-g. [DOI] [PubMed] [Google Scholar]
- 24.Hadlock TEJ, Langer R, Vacanti J, Cheney M. A tissue-engineered conduit for peripheral nerve repair. Archives of Otolaryngology-Head and Neck Surgery. 1998;124:1081–1086. doi: 10.1001/archotol.124.10.1081. [DOI] [PubMed] [Google Scholar]
- 25.Hadlock TSC, Koka R, Hunter D, Cheney M, Vacanti J. A novel, biodegradable ppolymer conduit delivers neurotrophins and promotes nerve regeneration. Laryngoscope. 1999;109:1412–1416. doi: 10.1097/00005537-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Hansson HA, Dahlin LB, Danielsen N, Fryklund L, Nachemson AK, Polleryd P, Rozell B, Skottner A, Stemme S, Lundborg G. Evidence indicating trophic importance of IGF-I in regenerating peripheral nerves. Acta Physiol Scand. 1986;126:609–614. doi: 10.1111/j.1748-1716.1986.tb07862.x. [DOI] [PubMed] [Google Scholar]
- 27.Hess JR, B M, Fox IK, Nichols CM, Myckatyn TM, Hunter DA, Rickman SR, Mackinnon SE. Use of cold-preserved allografts seeded with autologous Schwann cells in the treatment of a long-gap peripheral nerve injury. Plast Reconstr Surg. 2007;119:246–259. doi: 10.1097/01.prs.0000245341.71666.97. [DOI] [PubMed] [Google Scholar]
- 28.Hoke AGT, Zochodne DW, Sulaiman OAR. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Experimental Neurology. 2002;173:77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- 29.Hoke ARR, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudson TW, L S, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10:1346–1358. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 31.Kline DG. Surgical repair of peripheral nerve injury. Muscle Nerve. 1990;13:843–852. doi: 10.1002/mus.880130911. [DOI] [PubMed] [Google Scholar]
- 32.LLoyd B, Luginbuhl R, Brenner M, Rocque B, Tung T, Myckatyn T, Hunter D, Mackinnon S. Use of motor nerve material in peripheral nerve repair with conduits. Microsurgery. 2007;27:138–145. doi: 10.1002/micr.20318. [DOI] [PubMed] [Google Scholar]
- 33.Mackinnon SE, Dellon AL. Nerve repair and nerve grafting. In: Mackinnon SE, Dellon AL, editors. Surgery of the Peripheral Nerve. New York: Thieme Medical Publishers; 1988. pp. 89–121. [Google Scholar]
- 34.Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–1429. doi: 10.1097/00006534-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Madison RD, Archibald SJ, Brushart TM. Reinnervation accuracy of the rat femoral nerve by motor and sensory neurons. J Neurosci. 1996;16:5698–5703. doi: 10.1523/JNEUROSCI.16-18-05698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manthorpe M, Skaper SD, Williams LR, Varon S. Purification of adult rat sciatic nerve ciliary neuronotrophic factor. Brain Res. 1986;367:282–286. doi: 10.1016/0006-8993(86)91603-3. [DOI] [PubMed] [Google Scholar]
- 37.Martini R, Schachner M, Brushart TM. The L2/HNK-1 carbohydrate is preferentially expressed by previously motor axon-associated Schwann cells in reinnervated peripheral nerves. J Neurosci. 1994;14:7180–7191. doi: 10.1523/JNEUROSCI.14-11-07180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martini R, Xin Y, Schmitz B, Schachner M. The L2/HNK-1 Carbohydrate Epitope is Involved in the Preferential Outgrowth of Motor Neurons on Ventral Roots and Motor Nerves. Eur J Neurosci. 1992;4:628–639. doi: 10.1111/j.1460-9568.1992.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 39.Mathes SJ, A B. Advances in muscle and musculocutaneous flaps. Clin Plast Surg. 1980;7:15–26. [PubMed] [Google Scholar]
- 40.Mathes SJ, M J, Vasconez LO. Muscle transposition flaps for coverage of lower extremity defects: anatomic considerations. Surg Clin North Am. 1974;54:1337–1354. doi: 10.1016/s0039-6109(16)40489-5. [DOI] [PubMed] [Google Scholar]
- 41.Mathes SJ, V L. Mocutaneous free-flap transfer. Anatomical and experimental considerations. Plast Reconstr Surg. 1978;62:162–166. doi: 10.1097/00006534-197808000-00002. [DOI] [PubMed] [Google Scholar]
- 42.MC B. Motoneurons crave glial cell line-derived neurotrophic factor. Experimental Neurology. 2004;190:263–275. doi: 10.1016/j.expneurol.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 43.McCraw JB, V L. Musculocutaneous flaps: principles. Clin Plast Surg. 1980;7:9–13. [PubMed] [Google Scholar]
- 44.Nahai FSJ, Hill HL, Vasconez LO. The tensor fascia lata musculocutenous flap. Annals of Plastic Surgery. 1978;1:372–379. doi: 10.1097/00000637-197807000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Nichols CM, Brenner MJ, Fox IK, Tung TH, Hunter DA, Rickman SR, Mackinnon SE. Effects of motor versus sensory nerve grafts on peripheral nerve regeneration. Exp Neurol. 2004;190:347–355. doi: 10.1016/j.expneurol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Ogden MA, Feng FY, Myckatyn TM, Jensen JN, Grand AG, Wood PW, Hunter DA, MacKinnon SE. Safe injection of cultured schwann cells into peripheral nerve allografts. Microsurgery. 2000;20:314–323. doi: 10.1002/1098-2752(2000)20:7<314::aid-micr2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 47.Oppenheim RW, P A, Prevette D, Snider WD, Shen L. Glial cell line-derived neurotrophic factor and developing mammalianmotoneurons: regulation of programmed cell death among motoneuron subtypes. J Neurosci. 2000;20:5001–5011. doi: 10.1523/JNEUROSCI.20-13-05001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oppenheim RW, Prevette D, Yin QW, Collins F, MacDonald J. Control of embryonic motoneuron survival in vivo by ciliary neurotrophic factor. Science. 1991;251:1616–1618. doi: 10.1126/science.2011743. [DOI] [PubMed] [Google Scholar]
- 49.Ramon y, Cajal S. Degeneration and regeneration in the nervous system. London: Oxford University Press; 1928. [Google Scholar]
- 50.Robinson GA, Madison RD. Motor neurons preferentially reinnervate cutaneous pathways. Experimental Neurology. 2004;190:407–413. doi: 10.1016/j.expneurol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Rovak JM, B K, Boxer LK, Wood SC, Mungara AK, Cederna PS. Peripheral nerve translpantation: the role of chemical acellularization on eliminating allograft antigenicity. J Reconstructive Microsurgery. 2005;21:207–213. doi: 10.1055/s-2005-869828. [DOI] [PubMed] [Google Scholar]
- 52.Simova O, I A, Mehanna A, Liu J, Dihné M, Bächle D, Sewald N, Loers G, Schachner M. Carbohydrate mimics promote functional recovery after peripheral nerve repair. Ann Neurol. 2006;60:430–437. doi: 10.1002/ana.20948. [DOI] [PubMed] [Google Scholar]
- 53.Sunderland S. Nerves and Nerve injuries. Edinburgh: Livingstone; 1978. [Google Scholar]
- 54.Terzis JK, Smith KL. The peripheral nerve: structure, function, and reconstruction. New York: Raven; 1990. [Google Scholar]
- 55.Weber RV, M S. Nerve transfer in the upper extremity. J of the American Society for Hand Surgery. 2004;4:200–213. [Google Scholar]