Abstract
We investigated the molecular determinants of Ca2+-activated chloride current (CaCC) expressed in adult sensory neurons after a nerve injury. Dorsal root ganglia express the transcripts of three gene families known to induce CaCCs in heterologous systems: bestrophin, tweety, and TMEM16. We found with quantitative transcriptional analysis and in situ hybridization that nerve injury induced upregulation of solely bestrophin-1 transcripts in sensory neurons. Gene screening with RNA interference in single neurons demonstrated that mouse Best1 is required for the expression of CaCC in injured sensory neurons. Transfecting injured sensory neurons with bestrophin-1 mutants inhibited endogenous CaCC. Exogenous expression of the fusion protein green fluorescent protein–Bestrophin-1 in naive neurons demonstrated a plasma membrane localization of the protein that generates a CaCC with biophysical and pharmacological properties similar to endogenous CaCC. Our data suggest that Best1 belongs to a group of genes upregulated by nerve injury and supports functional CaCC expression in injured sensory neurons.
Introduction
After peripheral nerve injury, sensory neurons switch from a transmitting mode to a regrowth mode, and large scale analyses of transcriptional modifications established that injured sensory neurons express a panel of injury-related and regeneration-associated genes (Costigan et al., 2002; Xiao et al., 2002; Méchaly et al., 2006). Axotomy upregulates Ca2+-activated chloride current (CaCC) expression in sensory, sympathetic, and nodose neurons (Sánchez-Vives and Gallego, 1994; Lancaster et al., 2002; André et al., 2003).
Conditioning lesions increase the ability of the associated primary afferent neurons to regenerate successfully in vivo (Tanaka et al., 1992; Jacob and McQuarrie, 1993) and in vitro (Smith and Skene, 1997; Lankford et al., 1998). Using an in vitro model of regenerative growth, we have shown that a close relationship exists between the growth competence of sensory neurons and CaCC expression (André et al., 2003). In addition, we have revealed that phosphorylation of the Na+–K+–2Cl− cotransporter, NKCC1, is responsible for an increase in intracellular chloride concentration among regenerating sensory neurons and positively controls regenerative growth velocity (Pieraut et al., 2007). Altogether, these data suggest that chloride homeostasis and associate molecules may fulfill a major role in nerve regeneration and survival of neurons of the peripheral nervous system. Thus far, a major pitfall in elucidating the role of CaCC in cellular function has been the difficulty in identifying its molecular counterpart.
To date, research has identified three families of Ca2+-activated Cl− channels: the bestrophin (Sun et al., 2002), the tweety (Suzuki and Mizuno, 2004), and the TMEM16 families (Caputo et al., 2008; Schroeder et al., 2008; Yang et al., 2008). In mice, the bestrophin family consists of three genes, denominated Best1, Best2, and Best3 (Krämer et al., 2004). Bestrophin-1 is expressed in the retinal pigment epithelium (Marmorstein et al., 2000), and BEST1 mutations are responsible for Best vitelliform macular dystrophy in humans, a retinopathy attributable to degeneration of the retinal pigment epithelium (Marquardt et al., 1998; Petrukhin et al., 1998). Bestrophin-2 is expressed in mouse non-pigmented epithelium, colon epithelia, and olfactory epithelia (Bakall et al., 2008). Although changes in aqueous dynamics in Best2−/− mice suggest that Best2 does not function as a CaCC in the eye (Zhang et al., 2009), it is a potential candidate for the Ca2+-activated Cl− channel in mouse olfactory transduction (Pifferi et al., 2006). Bestrophin-3 is essential for the generation of calcium-sensitive cGMP-dependent Cl− channels in rat mesenteric arteries (Matchkov et al., 2008). Heterologous expression of members of the tweety family, TTYH2 and TTYH3, yielded a maxi-Cl− channel sensitive to Ca2+, whereas TTYH1 did not respond to calcium (Suzuki and Mizuno, 2004). Recently, heterologous expression of Tmem16a and Tmem16b yielded a CaCC with biophysical properties more closely related to an endogenous current in mammary and salivary glands, although they lacked specific consensus sites for Ca2+ binding (Caputo et al., 2008; Schroeder et al., 2008; Yang et al., 2008).
To examine the contribution of the six candidate genes to CaCC expressed after nerve injury, we performed a functional gene screening in axotomized sensory neurons.
Materials and Methods
Surgery and cell culture.
Adult Swiss and C57BL/6 mice (6–8 weeks old; CERJ) were housed in cages with a 12 h light/dark cycle and fed food and water ad libitum. Best1−/− mice were generated as described previously (Marmorstein et al., 2006). The care and use of mice conformed to institutional policies and guidelines. Mice were deeply anesthetized by isoflurane inhalation. The left sciatic nerve was exposed at the midthigh, sectioned, and a 3–5 mm fragment removed. Five days after surgery, neuronal cultures were established from left lumbar (L4–L5) dorsal root ganglia as reported previously (André et al., 2003).
Gene knockdown and plasmid transfection experiments.
Pooled non-targeting control siRNA or specific siRNA against mouse bestrophin Best1 and Best3, tweety 2 and 3, Tmem16a and b used in this study were the on-target plus SMART pools from Dharmacon (Perbio Science). The plasmid coding for a C-terminal green fluorescent protein (GFP)-tagged mBest1 fusion protein is described by Bakall et al. (2003). From pEGFP–mBest1, we generated the Best1 mutant, pEGFP–R92C and pEGFP–G299E. For in vitro transfection, neurons were individually electroporated 4 h after plating (Boudes et al., 2008). Electrode tips were filled with 8 μl of 145 mm KCl, 10 mm HEPES containing either dextran-fluorescein (3 mm)/siRNA (1 μm) or plasmid DNA (0.1 μg/ml). For in vivo transfection, siRNA was delivered intrathecally as described previously (Pieraut et al., 2007).
Quantitative reverse transcription-PCR.
Total RNA was extracted from lumbar L4–L5 dorsal root ganglia (DRG) of naive and axotomized adult mice using the RNeasy Mini Kit (Qiagen). All primers used in this study were designed with Primers 3.0 software. Mouse Best1 forward 5′TGGCAGAACAGCTCATCAAC and reverse 5′GCTGCCTCGTTCCAGTACAT. Mouse Best3 forward 5′CCGCTGACTTCTTGTTCAAA and reverse 5′ACTTGCAGAGGCCTGTCTGT. Mouse Tmem16a forward 5′TTCGTCAATCACACGCTCTC and reverse 5′GGGGTTCCCGGTAATCTTTA. Mouse Tmem16b forward 5′CTGATCACACCCCCTTCCTA and reverse 5′CTTCCTTACCACCAGGTCCA. Mouse ATF3 forward 5′ACAACAGACCCCTGGAGATG and reverse 5′CCTTCAGCTCAGCATTCA. Mouse Sprr1a forward 5′CCAGCAGAAGACAAAGCAGA and reverse 5′GGGCAATGTTAAGAGGCTCA. All product lengths were restricted between 100 and 150 pb to allow the highest efficiency of amplification. cDNAs were synthesized with 0.5–2 μg of RNA by using the SuperScript first-strand synthesis system for real-time (RT)-PCR (Invitrogen) in the presence of 50 ng random hexamers μg−1 of RNA. The reaction was incubated at 25°C (10 min), 42°C (50 min), and 70°C (15 min).
Real-time PCR was performed using SYBR Green I dye detection on the LightCycler system (Roche Diagnostics) as described previously (Méchaly et al., 2006), except that the annealing temperature was increased from 54 to 59°C to optimize DNA amplification. After PCR amplification, a melting curve analysis was generated to check the specificity of the PCR. The relative amounts of cDNA were calculated using the comparative delta-Ct method from two independent experiments. The two reference genes were polymerase (RNA) II polypeptide J and DEAD box polypeptide 48.
In situ hybridization. Mouse Best1 and Best3 antisense and sense cRNA probes were synthesized with the DIG (Digoxigenin) labeling system (Roche Diagnostics). In situ hybridization was performed according to Méchaly et al. (2006).
Electrophysiological measurements.
Calcium-activated chloride currents in DRG neurons were recorded after 1 or 3 d in vitro (DIV). To ensure that we were not analyzing a nonspecific cationic current described to have smaller amplitude than CaCC (<500 pA) (Currie and Scott, 1992), we set a minimal value of current amplitude to say that it is a CaCC. This amplitude was 500 pA. Of course this is a bias, and siRNA treatment probably leads to CaCC amplitude inferior to 500 pA. This is expressed in our study as percentage of expression. In neurons having a CaCC superior or equal to 500 pA, we analyzed mean current amplitude. Although this analysis underestimates the blocking effects of siRNA on current amplitude, it has the advantage to eliminate possible artifacts linked to the nonspecific cationic current. Plasmid-transfected sensory neurons were recorded 24 h after electroporation. Whole-cell patch-clamp recordings were made at room temperature under conditions optimized for the isolation of calcium and chloride currents separately from other voltage-activated currents. The bathing solution contained (in mm) the following: 140 tetraethylammonium chloride, 2 CaCl2, 1.5 MgCl2, 10 HEPES, and 10 glucose, and the pH was adjusted to 7.4 with CsOH. Recording pipettes were filled with the following solution (in mm): 145 CsCl, 10 HEPES, 2 Mg-ATP, 0.5 Na2–GTP, and pH 7.35 adjusted with CsOH. All recordings were made using an Axopatch 200B amplifier (Dipsi Industrie).
Imaging of sensory neurons.
Transfected control or axotomized neurons with pEGFP–Best1 or pEGFP were imaged with a Zeiss 5 Live Duo confocal microscope, 63× objectives. CellMask was purchased from Invitrogen. Similar results were obtained for at least six transfected neurons in each condition.
Statistical analysis.
All values are reported as mean ± SEM. Comparisons of the means observed in different groups were performed using a χ2, a Student's t test, or an ANOVA (one-way ANOVA with Newman–Keuls post hoc test) as appropriate (Graphpad Prism 5).
Results
Nerve injury increases Best1 expression in dorsal root ganglia
Among the potential candidate genes reported to code for a CaCC, we have previously shown that L4–L5 axotomized DRG in mice contain Best1, Best3, Ttyh2, and Ttyh3 (Al-Jumaily et al., 2007). In addition to these genes, we report expression of the recently identified Tmem16a and Tmem16b (Fig. 1A). To examine whether specific changes in transcript levels accompanied the increased CaCC expression in injured sensory neurons, we conducted quantitative RT-PCR on control and axotomized L4–L5 DRG. Although we observed no change in the level of Tmem16a, Tmem16b, and Best3, nerve injury promoted a fourfold increase in Best1 transcripts (Fig. 1B). We previously reported no quantitative changes in Ttyh2 and Ttyh3 transcripts after nerve injury (Al-Jumaily et al., 2007). For positive control of axotomy, we analyzed transcript levels of ATF3 and Sprr1a, two known markers of injury (Méchaly et al., 2006; Seijffers et al., 2007). After axotomy, normalized expression levels were increased from 0.003 ± 0.001 to 2.10 ± 0.17, n = 3, for ATF3 and from 0.005 ± 0.001 to 2.11 ± 0.25, n = 3, for Sprr1a (data not shown). As Western blots with the available antibodies, anti-Best1, failed to give reliable results, we performed in situ hybridization to detect Best1 transcript localization in DRG. In situ hybridization confirmed the increase in Best1 transcript in sensory neurons from axotomized DRG (Fig. 1C). In addition, Best1 expression appeared stronger in large and medium sensory neurons. Collectively, these data indicate that among the six candidate genes, nerve injury leads exclusively to Best1 upregulation in sensory neurons.
Figure 1.
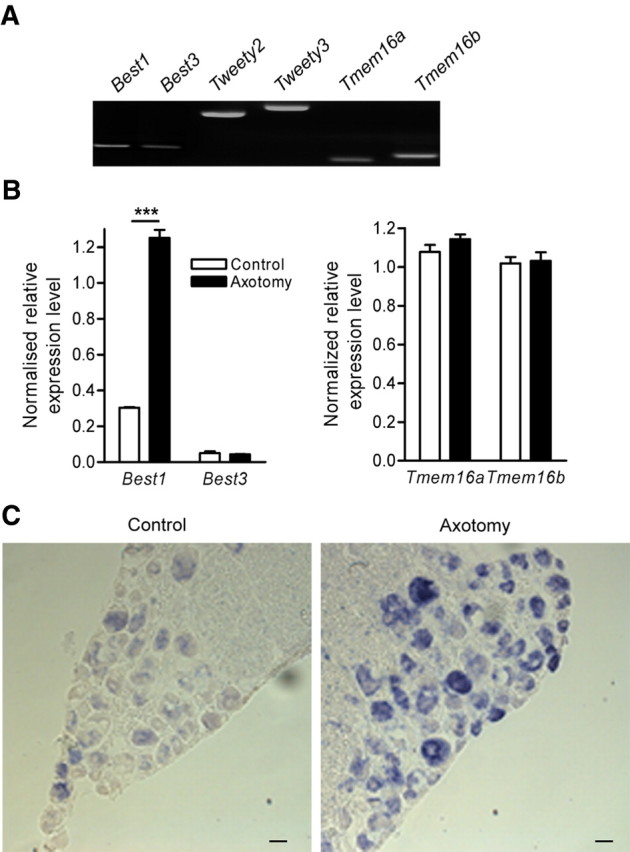
Nerve injury upregulates Best1 expression. A, RT-PCR analysis of candidate genes for Ca+-activated Cl− current in L4–L5 dorsal root ganglia 5 d after sciatic nerve section. B, qRT-PCR analysis of bestrophin-1, -3, Tmem16a and Tmem16b transcripts in lumbar L4–L5 DRG show a significant increase in Best1 transcripts after peripheral nerve injury (n = 4; ***p < 0.001, ANOVA with Bonferroni post hoc test). C, In situ hybridization of Best1 on normal and axotomized adult DRG (scale bar, 50 μm).
Screening candidate genes reveals that bestrophin-1 is involved in injury-induced CaCC expression in sensory neurons
To evaluate the functional relevance of the six genes on CaCC amplitude and expression in axotomized neurons, we applied the RNA interference using the single-cell electroporation method (Boudes et al., 2008). To optimize the proportion of transfected neurons expressing endogenous CaCC, we selected axotomized “conditioned” neurons according to their early neurite initiation 4 h after cell plating (André et al., 2003; Pieraut et al., 2007). Following a ramp protocol from −80 to +40 mV to activate voltage-gated Ca2+ current, we clearly observed CaCC activation as an inward tail current at −80 mV (Fig. 2A). Three days after siRNA electroporation, electrophysiological recordings indicated that only siRNA–Best1 decreased the amplitude of the inward tail current (−1.68 ± 0.23 nA, n = 22 and −0.89 ± 0.09 nA, n = 16 under siCONTROL and siRNA–Best1, respectively; p < 0.05). In addition to its effects on CaCC amplitude, siRNA–Best1 also decreased by half the percentage of neurons expressing CaCC (Fig. 2B,C). siRNA–Best3, -Ttyh2, -Ttyh3, -Tmem16a, and -Tmem16b changed neither CaCC amplitude nor expression (Fig. 2B,C). To validate the silencing efficiency of RNA interference, we performed quantitative RT-PCR after in vivo siRNA–Best1 or siCONTROL transfection of L4–L5 ganglia through intrathecal delivery, once a day for 5 d. Sciatic nerve injury was performed 2 d after starting siRNA treatment. Under siRNA treatment, Best1 transcripts expressed in DRG were reduced to 27.7 ± 6.1%, n = 3 compared with siCONTROL treated DRG (p < 0.01, t test). Specificity of siRNA Best1 treatment was confirmed by showing no significant effects on Best3 transcripts level (supplemental data, available at www.jneurosci.org as supplemental material). These data indicate that among the six candidate genes, only Best1 may be required for CaCC expression in axotomized sensory neurons.
Figure 2.
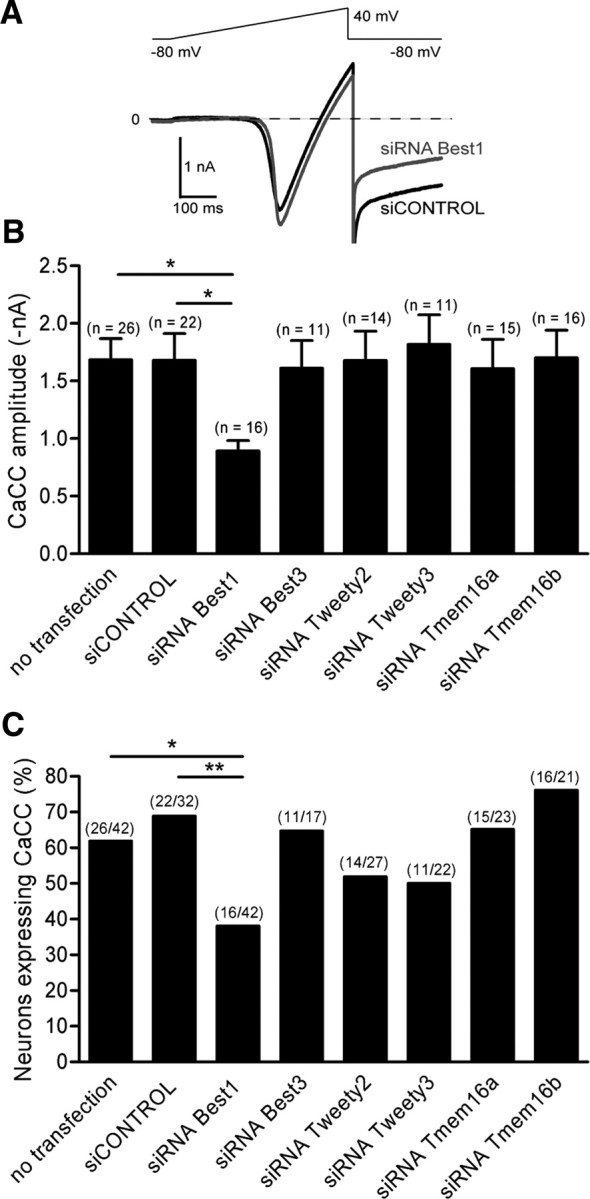
Bestrophin1 transcript inhibition decreases Ca2+-activated Cl− current amplitude and expression in axotomized sensory neurons. A, Representative traces of voltage-gated Ca2+ current and CaCC recorded in axotomized neurons 3 d after 1 μm siCONTROL or siRNA Best1 electroporation. A ramp protocol from −80 to +40 mV activated the voltage-gated Ca2+ current, followed by an inward tail current at −80 mV, previously identified as CaCC. B, After candidate gene screening with siRNA interference, CaCC amplitude decreased exclusively in siRNA Best1-treated neurons. C, Analysis of the percentage of neurons expressing CaCC after gene screening with siRNA interference. *p < 0.05; **p < 0.01 (χ2 test for expression and ANOVA; ANOVA for amplitude).
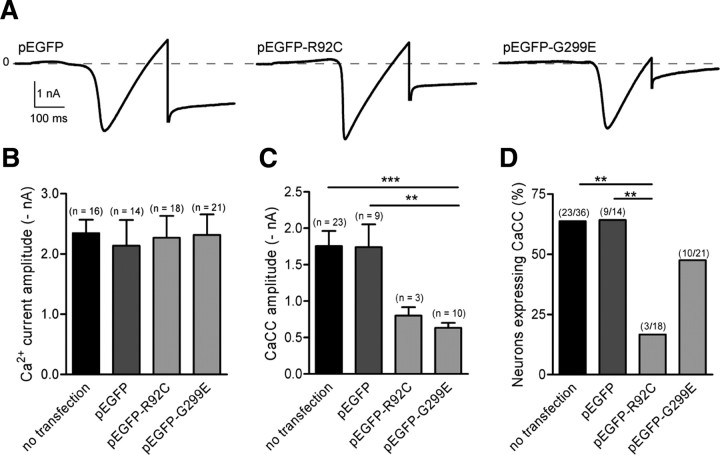
Best1 mutants, R92C and G299E, inhibit CaCC expression in axotomized neurons
To further demonstrate that bestrophin-1 mediated the CaCC expressed in axotomized neurons, we transfected EGFP-tagged Best1 mutants R92C and G299E. According to the human bestrophin-1 topology models, R92 is a conserved amino acid located in the second putative transmembrane domain 2, and G299 is located in the cytosolic C-terminal (Tsunenari et al., 2003; Milenkovic et al., 2007). These mutations are associated with Best disease and generate considerably smaller CaCC than wild-type BEST1 in HEK 293 cells (Sun et al., 2002). Analysis of neurons expressing either pEGFP or mutants did not modify the amplitudes of voltage-gated Ca2+ currents (Fig. 3A,B). EGFP and G299E did not modify the percentage of axotomized neurons expressing CaCC, 65 and 48%, respectively (p > 0.05 relative to the 65% of non-transfected neurons expressing CaCC). In contrast, R92C decreased the percentage of axotomized neurons expressing CaCC, from 65 to 17% (Fig. 3C). Attributable to the low number of neurons expressing CaCC in the presence of R92C, the decrease in CaCC amplitude did not reach statistical significance (p > 0.05, ANOVA) (Fig. 3A,D). Axotomized neurons expressing G299E produced significantly lower CaCC amplitudes than control neurons transfected with pEGFP (Fig. 3A,D). Collectively, these data show that Best1 mutants act in a dominant negative manner on CaCC in native cells.
Figure 3.
Best1 mutants, R92C and G299E, inhibit CaCC in axotomized neurons. A, Representative traces of CaCC measured at −80 mV following a ramp current to activate voltage-gated Ca2+ current in axotomized neurons transfected with pEGFP, pEGFP–R92C, and pEGFP–G299C. B, Expression of EGFP, EGFP–R92C, or EGFP–G299E does not change voltage-gated Ca2+ amplitudes, whose peak amplitudes elicited during the ramp protocol averaged −2.1 ± 0.4 nA, n = 14; −2.3 ± 0.4 nA, n = 18 and −2.3 ± 0.4 nA, n = 21, respectively. C, Mutation R92C at the transmembrane domain strongly decreases CaCC expression in axotomized sensory neurons, whereas mutation at the cytoplasmic C-terminal domain G299E does not change CaCC expression (χ2 test). D, G299E decreases endogenous CaCC amplitude. Because of the low level of CaCC expression in the presence of R92C, the decreased amplitude of CaCC is not statistically different (ANOVA). **p < 0.01, ***p < 0.001.
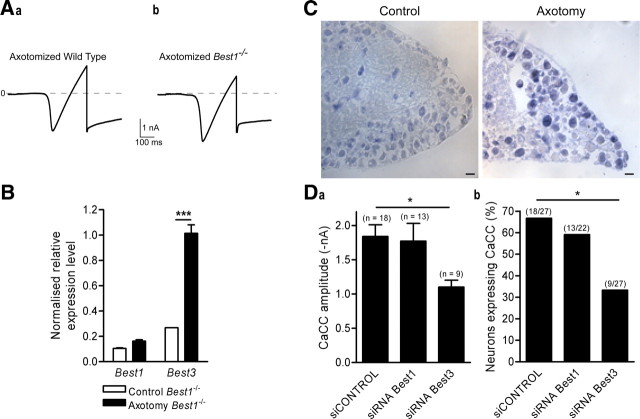
Best3 compensates for Best1 in Best1−/− mice
Our results implied a role for bestrophin-1 in generating neuronal CaCC, which led us to analyze the pattern of CaCC expression in the Best1−/− mice (Fig. 4). Unexpectedly, electrophysiological recordings in sensory neurons from Best1−/− mice showed no significant difference to wild-type mice. Seventy percent of axotomized neurons continued to express CaCC with an amplitude, at −80 mV, averaging −1.6 ± 0.2 nA, n = 15, and the amplitudes of Ca2+ currents were also not significantly different (Fig. 4A). To explain the contradictory results obtained from transfection of Best1 mutant plasmids, Best1 siRNA knockdown and those from the Best1−/− knock-out, we asked if loss of Best1 might be compensated by other members of the bestrophin family. By qRT-PCR, we showed that bestrophin-3 expression is upregulated in naive Best1−/− mice DRG. Indeed, whereas Best1 transcript levels in naive Best1−/− DRG were negligible compared with wild-type DRG (normalized expression level of Best1 was 0.11 ± 0.01, n = 4, and 0.32 ± 0.02, n = 4 respectively; p < 0.01), we measured a significant increase in Best3 mRNA in naive DRG from mutants (normalized expression level of Best3 was 0.06 ± 0.02, n = 4 and 0.26 ± 0.02, n = 4, in naive wild-type and Best1−/− mice, respectively; p < 0.01) (Figs. 1B, 4B). In addition, nerve injury induced a significant fourfold increase in the Best3 transcript in the DRG of Best1−/− mice (Fig. 4B). In situ hybridization confirmed the increase in Best3 transcripts in sensory neurons from axotomized DRG (Fig. 4C). To see if Best3 compensates functionally for Best1 in the null mouse, we applied the RNA interference knockdown strategy using siRNA–Best3 in sensory neurons from Best1−/− mice. Electrophysiological recordings indicated that siRNA–Best3 led to a significant decrease in the amplitude and the percentage of neurons expressing CaCC (Fig. 4D). As expected from the absence of Best1 expression in knock-out mice, siRNA–Best1 had no significant effects on the amplitude or the percentage of axotomized neurons expressing CaCC (Fig. 4D). These data indicate that the expression of CaCC in axotomized neurons from Best1−/− mice results from Best3 upregulation.
Figure 4.
Best3 compensates for Best1 in Best1 knock-out mice. A, Typical current traces recorded with a ramp protocol from −80 to +40 mV for 500 ms to activate voltage-gated Ca2+ current. Activation of CaCC is measured at −80 mV as an inward tail current in wild-type (a) and Best1−/− (b) mice. B, qRT-PCR for bestrophin transcript expression in Best1−/− mice shows an increase in Best3 transcript levels in axotomized L4–L5 DRG compared with controls (n = 4; ***p < 0.001, ANOVA with Bonferroni post hoc test). C, In situ hybridization of Best3 on normal and axotomized adult DRG (scale bar, 50 μm). D, siRNA electroporation of axotomized neurons from Best1−/− mice shows a significant inhibitory effect of siRNA–Best3 on tail current amplitude (a) (ANOVA) and expression (b) (χ2 test) compared with siCONTROL and siRNA–Best1; *p < 0.05.
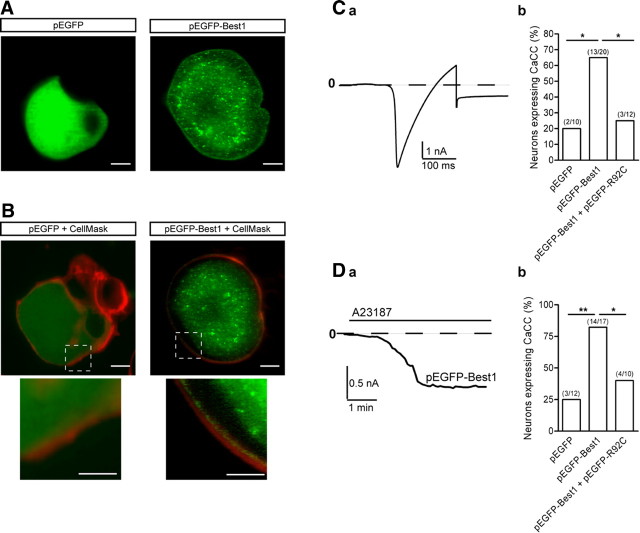
Exogenous Best1 localizes close to the plasma membrane and induces Ca2+-activated current in naive sensory neurons
To localize Best1 in neurons, we used single-cell electroporation to transfect sensory neurons with pEGFP–Best1. We prepared confocal images of live neurons 1 d after transfection. Control experiments with pEGFP showed a strong uniform fluorescent signal in the cytoplasm and nucleus, with no fluorescence associated with the plasma membrane (Fig. 5A). Confocal images of exogenous EGFP-tagged Best1 in live adult sensory neurons showed no nuclear staining but a granular cytoplasmic fluorescence and an intense expression pattern consistent with localizing close to the plasma membrane (Fig. 5A). We next labeled the plasma membrane with a red fluorescent stain, CellMask. Superimposing the EGFP and red fluorescent images confirmed the uniform cytoplasmic localization of GFP (Fig. 5B). Using the same protocol, when we superimposed images of EGFP-tagged Best1 with red CellMask staining, it revealed that Best1 protein localizes to just beneath the plasma membrane (Fig. 5B). These data indicate that the fusion protein EGFP–Best1 shows a specific subcellular pattern of expression consistent with a membrane protein.
Figure 5.
Exogenously expressed Best1 associates with the plasma membrane and induces a Ca2+-activated current in naive sensory neurons. A, Confocal images of live naive sensory neurons at 1 DIV transfected with pEGFP or pEGFP–Best1 (scale bar, 10 μm). B, Same neurons after staining with CellMask (1/5000) for 5 min, a plasma membrane stain (red fluorescence) to delimit the plasma membrane and confirm the GFP-tagged Best1 protein membrane localization (scale bars: 10 μm; inset, 5 μm). C, Typical current traces recorded from a pEGFP–Best1-transfected naive neurons following activation of Ca2+ current with a ramp protocol from −80 to +40 mV (a). pEGFP–Best1 transfection significantly increased the probability of recording an inward tail current at −80 mV compared with transfection with pEGFP. Cotransfection of Best1 with the mutant R92C significantly decreased current expression (b). Amplitude of Best1-induced current at −80 mV is significantly decreased in the presence of R92C (c). D, In the presence of 100 μm Cd2+ and Ni2+ to inhibit voltage-gated Ca2+ currents, the Ca2+ ionophore A23187 at 100 μm activates, at −80 mV, an inward current that needed several minutes to reach a steady amplitude (a). pEGFP–Best1 transfection induces current in 80% of sensory neurons. Cotransfection of Best1 with the mutant R92C decreases the percentage of neurons expressing Ca2+-activated current (b). The amplitude of Best1 current was significantly decreased in the presence of R92C (c). **p < 0.01, *p < 0.05 (χ2 test for expression and t test for amplitude). Number of experiments are in parentheses.
Our results show that, under control conditions, DRG express a low level of Best1 transcript, together with a low probability of recording an endogenous CaCC. This led us to study the effects of exogenous Best1 on CaCC expression in naive sensory neurons. The Best1 current was activated with either voltage-gated Ca2+ current (Fig. 5Ca) or with a Ca2+ ionophore, A23187, in the presence of Ca2+ current inhibitors (Fig. 5Da). When recorded using voltage-gated Ca2+ current, transfection with pEGFP did not modify the percentage of naive neurons expressing an inward tail current at −80 mV that amounted 20% of medium and large somatic size neurons (>30 μm) as previously shown. Expression of recombinant EGFP-tagged Best1 significantly increased this percentage to ∼70% of transfected naive neurons with a tail current average amplitude of −0.7 ± 0.2 nA, n = 13 (Fig. 5Cb). Recordings of small soma size neurons (<30 μm) confirm the low level of CaCC expression in these neurons, whether naive or axotomized, that we previously reported (2 of 22 and 4 of 43, respectively) (André et al., 2003). Electroporation of small sensory neurons with pEGFP–Best1 did not induce additional expression of an inward tail current compared with endogenous level (1 of 19). We thus focused the study on sensory neurons having a somatic diameter larger than 30 μm. To examine the effects of the R92C mutation on Best1-induced current, naive neurons were cotransfected with Best1 and R92C plasmids. R92C significantly decreased the expression Best1 current (25% of neurons instead of 70%) (Fig. 5Cb). Best1 current amplitude was −0.34 ± 0.04 nA, n = 3. In another series of experiments, puff application of A23187 induced an inward current at −80 mV that needed between 2 and 5 min to reach steady state (Fig. 5D). In pEGFP transfected neurons, A23187-induced current was observed in 20% of neurons, and this value was increased to 80% in Best1 transfected neurons (Fig. 5Db), whose amplitude amounted to −0.8 ± 0.1 nA, n = 14. In the presence of R92C, A23187 activated a current with significantly smaller amplitude than the Best1-induced current, −0.3 ± 0.1 nA, n = 4, p < 0.05, and the percentage of neurons expressing an inward current at −80 mV was reduced to 40% (Fig. 5Db).
These data demonstrate that exogenous Best1 localizes to plasma membrane and promotes the expression of a Ca2+-activated current in naive sensory neurons.
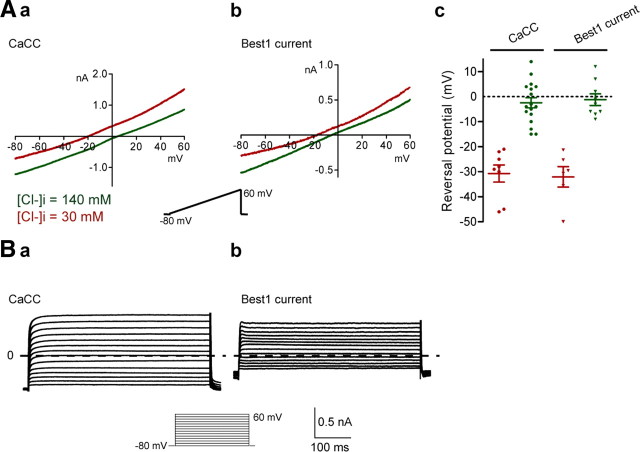
Best1-induced current in naive sensory neurons has similar biophysical and pharmacological properties to endogenous CaCC
To compare the biophysical properties of Best1 current in naive neurons with those of CaCC expressed in axotomized neurons, we applied ramp protocols from −80 to +60 mV in the presence of Ca2+ currents inhibitors. Figure 6A illustrates the current–voltage relationships of maximal current induced under A23187 in axotomized neurons (a) and in Best1-transfected neurons (c). Both conditions produced similarly linear current–voltage relationships. However, in axotomized neurons, amplitude of A23187-induced current at −80 mV holding potential was −1.2 ± 0.1 nA, n = 14: a value significantly smaller than the amplitude of Best1 current recorded in naive neurons (−0.8 ± 0.1 nA, n = 14; p < 0.01, t test).
Figure 6.
Best1 current expressed in naive sensory neurons displays similar biophysical properties than CaCC recorded in axotomized neurons. A, The voltage relationships of Ca2+-activated current recorded in axotomized neurons (a) or in Best1-transfected naive neurons (b) were obtained by subtracting control ramp current from A23187-activated current. Both voltage relationships measured at steady amplitude are linear. By partial substitution with methane sulfonate (30 mm CsCl/115 mm Cs-methane sulfonate) for intracellular chloride ions, reversal potentials were shifted from −2 mV to approximately −30 mV, which indicates Cl− conductance (c). B, Current traces of CaCC expressed in axotomized neurons (a) and of Best1 current expressed in naive neurons (b), recorded with 500 ms depolarizing pulses from −80 to +60 mV in 10 mV increments, show no time dependence.
The reversal potential of ionophore-evoked current in axotomized neurons and in Best1-transfected neurons was close to 0 mV, as expected from equal intracellular and extracellular Cl− concentrations. Under partial substitution of methane sulfonate (30 mm CsCl/115 mm Cs-methane sulfonate) for intracellular chloride ions, the reversal potential of CaCC in axotomized neurons shifted from −2.5 ± 2.0, n = 17, to −30.7 ± 3.4 mV, n = 9 (p < 0.001, t test). The reversal potential of Best1-induced current also shifted to hyperpolarized potentials (from −1.2 ± 2.3 mV, n = 9, to −32.1 ± 4.1 mV, n = 8; p < 0.001, t test) indicating a chloride conductance (Fig. 6Ac). We did not observe time dependence when we constructed current–voltage relationships with 500 ms depolarizing pulses (Fig. 6B). In agreement with the pharmacological properties of CaCC expressed in axotomized neurons, applying 100 μm niflumic acid inhibited 75 ± 10%, n = 4, of Best1 current measured at −80 mV (data not shown). The biophysical properties, pharmacological profile, and chloride permeability of the Best1-induced current matches those of the CaCC expressed in axotomized neurons.
Discussion
These results demonstrate a requirement for bestrophin-1 in CaCC expression in injured sensory neurons. Concomitant upregulation of bestrophin-1 transcripts and CaCC expression in injured DRG, together with the decreased CaCC amplitude and expression in axotomized neurons under siRNA–Best1, Best1 mutants R92C and G299E, and expression of Best1 current in naive neurons, all tend toward the need for bestrophin-1 in functional Cl− current expression in axotomized neurons.
The hypothesis that bestrophins function as Ca2+-activated Cl− channels arose from their association with Best disease. Experimental evidence came predominantly from their expression in heterologous systems (Sun et al., 2002; Hartzell et al., 2005). However, no data are available on the mouse Best1 expression in heterologous systems, and thus our study provides the first description of the mBest1-induced current in a native system. Our results show that Best1 expression in sensory neurons induces a CaCC, with time and voltage dependence, and niflumic acid inhibition similar to endogenous CaCC expressed in injured neurons. However, the amplitude of Best1 current induced in naive neurons is significantly smaller than endogenous CaCC induced by injury, a result that could be related to different intracellular regulatory mechanisms between naive and axotomized neurons (Frings et al., 2000; Hartzell et al., 2005). In situ hybridization experiments demonstrate that Best1 is not expressed in all sensory neurons, which is consistent with the endogenous expression of CaCC in ∼70% of medium and large somatic size axotomized neurons. Surprisingly, forced expression of Best1 in naive neurons does not increase this proportion (65%). Analysis of the small soma size neurons (<30 μm) confirmed that CaCC was much less expressed in this subset of neurons under control and after nerve injury (10%), and forced expression of Best1 does not induce CaCC in these neurons. From these data, it appears that the specificity of Best1 current would not be uniquely related to its transcript expression but also to its protein trafficking or phosphorylation state. Population-specific expression of both endogenous CaCC and exogenous Best1 current could be related to the need for the coexpression with bestrophin-1 of a molecular partner specific to a sensory subpopulation for a functional expression. This proposal is consistent with the differences in current amplitude between exogenous expression of Best1 in naive neurons and endogenous CaCC in axotomized neurons. The other hypothesis would be that bestrophin-1 is an auxiliary subunit necessary for Cl− channel functional expression. Expression of auxiliary subunits for functional Cl− channels has been reported, such as the β-subunit Barttin for ClC-K channels in the inner ear (Estévez et al., 2001). The recent findings that human BEST1 negatively regulates voltage-dependent Ca2+ current amplitude in retinal pigment epithelium (RPE) cells (Rosenthal et al., 2006), most probably through interaction with the Cav1β subunit (Yu et al., 2008), suggest an auxiliary subunit function for BEST1. It should be noted that in the study of Yu et al. (2008), such an interaction was not observed with mouse Best1, and however that may be, protein interactions do not exclude Best1 as being a chloride channel (Levitan, 2006).
Most mutations identified in the Best disease lead to smaller CaCC amplitudes or they do not function when heterologously expressed. Moreover, we show that the mutants R92C and G299E suppress the current produced by coexpression of wild-type channels, which is consistent with a dominant-negative mechanism of action (Hartzell et al., 2008). Our results are thus in agreement with dominant effects of R92C and G299E mutants on endogenous CaCC and Best1 currents, through defective channels composed of both mutant and wild-type subunits. The CaCC inhibition with the mutants also supports the proposal that native bestrophin-1 oligomerizes (Stanton et al., 2006). In addition to the electrophysiological data, the localization of tagged recombinant Best1 at the plasma membrane is consistent with its functions as an ion channel or a close molecular partner. The overexpressed protein also localized to intracellular membranes or compartments, as estimated by the punctiform cytosolic appearance. Although exogenous protein expression does not directly replicate the behavior of its endogenous counterpart, studies recently used this molecular approach to convincingly mimic the localization and function of endogenous Best1 protein in rat retinal pigment epithelium (Marmorstein et al., 2004). Remarkably, in Best1−/− mice, we witnessed a substantial increase in Best3 transcript that contributes to CaCC expression. Interestingly, we also show with qRT-PCR that siRNA–Best1 does not induce an upregulation of Best3 transcript in axotomized DRG from wild-type mice. These results demonstrate that the compensatory effects observed in the Best1−/− mice would not be related to a loss of inhibition of Best1 over Best3. Best1 is located at chromosome 19B and Best3 at chromosome 10D2. Such different gene locations point to the complexity of the regulatory cascades leading to these compensatory effects. In addition, this compensation questions whether Best1 and Best3 expression is regulated by similar transcription factors in sensory neurons and in RPE cells (Esumi et al., 2009). In addition to transcription factors, nerve injury induces post-transcriptional modifications attributable to the synthesis and the secretion of various growth factors, cytokines, cell adhesion molecules (Makwana and Raivich, 2005). Like Best1, the C-terminal cytoplasmic region of Best3 contains several phosphorylation sites for protein kinases which profoundly influence the functional expression of these proteins (Qu et al., 2007; Hartzell et al., 2008; Matchkov et al., 2008) and probably does occur after an injury.
For the first time, we provide evidence that Best1 is an injury-regulated gene in sensory neurons. In particular, by analyzing gene expression in DRG, we show that Best1 is preferentially expressed in medium and large sensory neurons. Previous work observed the same pattern of expression when recording CaCC in axotomized neurons (André et al., 2003). These findings strongly support the concept that CaCC expressed under these conditions is associated with Best1. Recently, global gene expression analysis, directed toward finding membrane proteins regulated by interleukin-4, identified TMEM16A as associated with the Ca2+-activated Cl− channel involved in secretion (Caputo et al., 2008). Numerous studies emphasize that CaCC may have a variety of different functions depending on colocalization with other channels and the type of physiological mechanism involved in raising [Ca2+]i (Scott et al., 1995). The discovery that different gene families code for these ionic channels implies a functional diversity. In line with this, the recently identified TMEM16 family seems a serious candidate for CaCC, and the strong protein expression of TMEM16A in small sensory neurons has led to the proposal that TMEM16A is a CaCC involved in nociception (Yang et al., 2008). Our data demonstrate that Tmem16a and Tmem16b genes are not regulated by nerve injury and do not support the CaCC expressed in injured sensory neurons. We also verified that, as for siRNA–Best1, siRNA–Tmem16a and siRNA–Tmem16b efficiently decreased their corresponding transcripts (data not shown). Hence, our study suggests that nerve injury promotes CaCC expression from a different set of genes to those expressed under physiological conditions or possibly from other types of neuropathies or trauma. However, the biological effects of bestrophins expressed in axotomized neurons remain to be solved. Indeed, we noticed that reduction or loss of Best1 or Best3 mRNA by silencing, or Best1 knock-out mice, does not promote cell death nor grossly modify the elongated mode of growth (data not shown). Thus, phenotypic changes are obviously subtle and need extensive work, such as analysis of neurite initiation and growth velocity, as well as studies of electrical activity and channel- or receptor-mediated calcium homeostasis to be fully understood.
This work provides the first demonstration that Best1 is a nerve injury-regulated gene whose function may be involved in the regeneration of sensory neurons. Its requirement for CaCC expression also defines chloride homeostasis as fundamental to cell repair and makes possible the evaluation of the function of these channels in this fundamental process.
Footnotes
For their support, we thank the Association Française contre les Myopathies, the Ministère de la Recherche (support to M.B.), the French Minister for Foreign Affairs (support to A.K.), National Institutes of Health (Grant R01 EY13160), and the Macular Vision Research Foundation (support to A.M.). We are very grateful to Dr. H. Boukhaddaoui and the regional imaging platform for their help with image acquisition and analysis.
References
- Al-Jumaily M, Kozlenkov A, Mechaly I, Fichard A, Matha V, Scamps F, Valmier J, Carroll P. Expression of three distinct families of calcium-activated chloride channel genes in the mouse dorsal root ganglion. Neurosci Bull. 2007;23:293–299. doi: 10.1007/s12264-007-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André S, Boukhaddaoui H, Campo B, Al-Jumaily M, Mayeux V, Greuet D, Valmier J, Scamps F. Axotomy-induced expression of calcium-activated chloride current in subpopulations of mouse dorsal root ganglion neurons. J Neurophysiol. 2003;90:3764–3773. doi: 10.1152/jn.00449.2003. [DOI] [PubMed] [Google Scholar]
- Bakall B, Marmorstein LY, Hoppe G, Peachey NS, Wadelius C, Marmorstein AD. Expression and localization of bestrophin during normal mouse development. Invest Ophthalmol Vis Sci. 2003;44:3622–3628. doi: 10.1167/iovs.03-0030. [DOI] [PubMed] [Google Scholar]
- Bakall B, McLaughlin P, Stanton JB, Zhang Y, Hartzell HC, Marmorstein LY, Marmorstein AD. Bestrophin-2 is involved in the generation of intraocular pressure. Invest Ophthalmol Vis Sci. 2008;49:1563–1570. doi: 10.1167/iovs.07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudes M, Pieraut S, Valmier J, Carroll P, Scamps F. Single-cell electroporation of adult sensory neurons for gene screening with RNA interference mechanism. J Neurosci Methods. 2008;170:204–211. doi: 10.1016/j.jneumeth.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D'Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie KP, Scott RH. Calcium-activated currents in cultured neurones from rat dorsal root ganglia. Br J Pharmacol. 1992;106:593–602. doi: 10.1111/j.1476-5381.1992.tb14381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez R, Boettger T, Stein V, Birkenhäger R, Otto E, Hildebrandt F, Jentsch TJ. Barttin is a Cl-channel beta-subunit crucial for renal Cl- reabsorption and inner ear K+ secretion. Nature. 2001;414:558–561. doi: 10.1038/35107099. [DOI] [PubMed] [Google Scholar]
- Esumi N, Kachi S, Hackler L, Jr, Masuda T, Yang Z, Campochiaro PA, Zack DJ. BEST1 expression in the retinal pigment epithelium is modulated by OTX family members. Hum Mol Genet. 2009;18:128–141. doi: 10.1093/hmg/ddn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings S, Reuter D, Kleene SJ. Neuronal Ca2+-activated Cl-channels–homing in on an elusive channel species. Prog Neurobiol. 2000;60:247–289. doi: 10.1016/s0301-0082(99)00027-1. [DOI] [PubMed] [Google Scholar]
- Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- Hartzell HC, Qu Z, Yu K, Xiao Q, Chien LT. Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev. 2008;88:639–672. doi: 10.1152/physrev.00022.2007. [DOI] [PubMed] [Google Scholar]
- Jacob JM, McQuarrie IG. Acceleration of axonal outgrowth in rat sciatic nerve at one week after axotomy. J Neurobiol. 1993;24:356–367. doi: 10.1002/neu.480240308. [DOI] [PubMed] [Google Scholar]
- Krämer F, Stöhr H, Weber BH. Cloning and characterization of the murine Vmd2 RFP-TM gene family. Cytogenet Genome Res. 2004;105:107–114. doi: 10.1159/000078016. [DOI] [PubMed] [Google Scholar]
- Lancaster E, Oh EJ, Gover T, Weinreich D. Calcium and calcium-activated currents in vagotomized rat primary vagal afferent neurons. J Physiol. 2002;540:543–556. doi: 10.1113/jphysiol.2001.013121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankford KL, Waxman SG, Kocsis JD. Mechanisms of enhancement of neurite regeneration in vitro following a conditioning sciatic nerve lesion. J Comp Neurol. 1998;391:11–29. doi: 10.1002/(sici)1096-9861(19980202)391:1<11::aid-cne2>3.0.co;2-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan IB. Signaling protein complexes associated with neuronal ion channels. Nat Neurosci. 2006;9:305–310. doi: 10.1038/nn1647. [DOI] [PubMed] [Google Scholar]
- Makwana M, Raivich G. Molecular mechanisms in successful peripheral regeneration. FEBS J. 2005;272:2628–2638. doi: 10.1111/j.1742-4658.2005.04699.x. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2000;97:12758–12763. doi: 10.1073/pnas.220402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein AD, Stanton JB, Yocom J, Bakall B, Schiavone MT, Wadelius C, Marmorstein LY, Peachey NS. A model of best vitelliform macular dystrophy in rats. Invest Ophthalmol Vis Sci. 2004;45:3733–3739. doi: 10.1167/iovs.04-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein LY, Wu J, McLaughlin P, Yocom J, Karl MO, Neussert R, Wimmers S, Stanton JB, Gregg RG, Strauss O, Peachey NS, Marmorstein AD. The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (best-1) J Gen Physiol. 2006;127:577–589. doi: 10.1085/jgp.200509473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt A, Stöhr H, Passmore LA, Krämer F, Rivera A, Weber BH. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best's disease) Hum Mol Genet. 1998;7:1517–1525. doi: 10.1093/hmg/7.9.1517. [DOI] [PubMed] [Google Scholar]
- Matchkov VV, Larsen P, Bouzinova EV, Rojek A, Boedtkjer DM, Golubinskaya V, Pedersen FS, Aalkjaer C, Nilsson H. Bestrophin-3 (vitelliform macular dystrophy 2-like 3 protein) is essential for the cGMP-dependent calcium-activated chloride conductance in vascular smooth muscle cells. Circ Res. 2008;103:864–872. doi: 10.1161/CIRCRESAHA.108.178517. [DOI] [PubMed] [Google Scholar]
- Méchaly I, Bourane S, Piquemal D, Al-Jumaily M, Ventéo S, Puech S, Scamps F, Valmier J, Carroll P. Gene profiling during development and after a peripheral nerve traumatism reveals genes specifically induced by injury in dorsal root ganglia. Mol Cell Neurosci. 2006;32:217–229. doi: 10.1016/j.mcn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Milenkovic VM, Rivera A, Horling F, Weber BH. Insertion and topology of normal and mutant bestrophin-1 in the endoplasmic reticulum membrane. J Biol Chem. 2007;282:1313–1321. doi: 10.1074/jbc.M607383200. [DOI] [PubMed] [Google Scholar]
- Petrukhin K, Koisti MJ, Bakall B, Li W, Xie G, Marknell T, Sandgren O, Forsman K, Holmgren G, Andreasson S, Vujic M, Bergen AA, McGarty-Dugan V, Figueroa D, Austin CP, Metzker ML, Caskey CT, Wadelius C. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998;19:241–247. doi: 10.1038/915. [DOI] [PubMed] [Google Scholar]
- Pieraut S, Laurent-Matha V, Sar C, Hubert T, Méchaly I, Hilaire C, Mersel M, Delpire E, Valmier J, Scamps F. NKCC1 phosphorylation stimulates neurite growth of injured adult sensory neurons. J Neurosci. 2007;27:6751–6759. doi: 10.1523/JNEUROSCI.1337-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifferi S, Pascarella G, Boccaccio A, Mazzatenta A, Gustincich S, Menini A, Zucchelli S. Bestrophin-2 is a candidate calcium-activated chloride channel involved in olfactory transduction. Proc Natl Acad Sci U S A. 2006;103:12929–12934. doi: 10.1073/pnas.0604505103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu ZQ, Yu K, Cui YY, Ying C, Hartzell C. Activation of bestrophin CL channels is regulated by C-terminal domains. J Biol Chem. 2007;282:17460–17467. doi: 10.1074/jbc.M701043200. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Bakall B, Kinnick T, Peachey N, Wimmers S, Wadelius C, Marmorstein A, Strauss O. Expression of bestrophin-1, the product of the VMD2 gene, modulates voltage-dependent Ca2+ channels in retinal pigment epithelial cells. FASEB J. 2006;20:178–180. doi: 10.1096/fj.05-4495fje. [DOI] [PubMed] [Google Scholar]
- Sánchez-Vives MV, Gallego R. Calcium-dependent chloride current induced by axotomy in rat sympathetic neurons. J Physiol. 1994;475:391–400. doi: 10.1113/jphysiol.1994.sp020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RH, Sutton KG, Griffin A, Stapleton SR, Currie KP. Aspects of calcium-activated chloride currents: a neuronal perspective. Pharmacol Ther. 1995;66:535–565. doi: 10.1016/0163-7258(95)00018-c. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton JB, Goldberg AF, Hoppe G, Marmorstein LY, Marmorstein AD. Hydrodynamic properties of porcine bestrophin-1 in Triton X-100. Biochim Biophys Acta. 2006;1758:241–247. doi: 10.1016/j.bbamem.2006.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Tsunenari T, Yau KW, Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci U S A. 2002;99:4008–4013. doi: 10.1073/pnas.052692999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A. A novel human Cl(-) channel family related to Drosophila flightless locus. J Biol Chem. 2004;279:22461–22468. doi: 10.1074/jbc.M313813200. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Zhang QL, Webster HD. Myelinated fiber regeneration after sciatic nerve crush: morphometric observations in young adult and aging mice and the effects of macrophage suppression and conditioning lesions. Exp Neurol. 1992;118:53–61. doi: 10.1016/0014-4886(92)90022-i. [DOI] [PubMed] [Google Scholar]
- Tsunenari T, Sun H, Williams J, Cahill H, Smallwood P, Yau KW, Nathans J. Structure-function analysis of the bestrophin family of anion channels. J Biol Chem. 2003;278:41114–41125. doi: 10.1074/jbc.M306150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- Yu K, Xiao Q, Cui G, Lee A, Hartzell HC. The best disease-linked Cl− channel hBest1 regulates CaV 1 (L-type) Ca2+ channels via src-homology-binding domains. J Neurosci. 2008;28:5660–5670. doi: 10.1523/JNEUROSCI.0065-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Davidson BR, Stamer WD, Barton JK, Marmorstein LY, Marmorstein AD. Enhanced inflow and outflow rates despite lower IOP in Bestrophin-2 deficient mice. Invest Ophthalmol Vis Sci. 2009;50:765–770. doi: 10.1167/iovs.08-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]