Abstract
Brain-derived neurotrophic factor (BDNF) stimulates local dendritic mRNA translation and is involved in formation and consolidation of memory. 2H,3H,6aH-pyrrolidino[2″,1″-3′,2′]1,3-oxazino[6′,5′-5,4]-benzo[e]1,4-dioxan-10-one (CX614), one of the best-studied positive AMPA receptor modulators (also known as ampakines), increases BDNF mRNA and protein and facilitates long-term potentiation (LTP) induction. Several other ampakines also improve performance in various behavioral and learning tasks. Since local dendritic protein synthesis has been implicated in LTP stabilization and in memory consolidation, this study investigated whether CX614 could influence synaptic plasticity by upregulating dendritic protein translation. CX614 treatment of primary neuronal cultures and acute hippocampal slices rapidly activated the translation machinery and increased local dendritic protein synthesis. CX614-induced activation of translation was blocked by K252a [(9S,10R,12R)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid methyl ester], CNQX, APV, and TTX, and was inhibited in the presence of an extracellular BDNF scavenger, TrkB-Fc. The acute effect of CX614 on translation was mediated by increased BDNF release as demonstrated with a BDNF scavenging assay using TrkB-Fc during CX614 treatment of cultured primary neurons and was blocked by nifedipine, ryanodine, and lack of extracellular Ca2+ in acute hippocampal slices. Finally, CX614, like BDNF, rapidly increased dendritic translation of an exogenous translation reporter. Together, our results demonstrate that positive modulation of AMPA receptors rapidly stimulates dendritic translation, an effect mediated by BDNF secretion and TrkB receptor activation. They also suggest that increased BDNF secretion and stimulation of local protein synthesis contribute to the effects of ampakines on synaptic plasticity.
Introduction
Dendritic protein synthesis, an event essential for the formation and stabilization of synapses and neural circuits in brain, consists of three steps: initiation, elongation, and release; each of these steps involves multiple factors and is regulated by various proteins, including kinases. Memory stabilization/consolidation is protein synthesis dependent, as inhibition of mRNA translation to protein prevents long-term storage and consolidation of newly learned tasks and events (Wells and Fallon, 2000; Pfeiffer and Huber, 2006; Bramham, 2007). Regulation of dendritic protein synthesis has recently been shown to play a critical role in synaptic plasticity, in particular in long-term potentiation (LTP) and long-term depression (LTD) (Davis and Squire, 1984; Klintsova and Greenough, 1999; Huber et al., 2000; Sigrist et al., 2000; Wells and Fallon, 2000; Aakalu et al., 2001; Pfeiffer and Huber, 2006). Brain-derived neurotrophic factor (BDNF) has emerged as a key regulator of synaptic plasticity and learning (Minichiello et al., 2002; Narisawa-Saito et al., 2002; Jourdi et al., 2003; Rex et al., 2006, 2007; Bramham, 2007). BDNF activation of protein synthesis in neuronal cell bodies and dendrites is mediated by stimulation of the BDNF receptor, tyrosine receptor kinase B (TrkB), leading to increased translation via the mammalian target of rapamycin (mTOR) pathway and phosphorylation of several translation factors and kinases, such as initiation factor 4E binding protein 1 (4EBP1) and p70S6 kinase (p70S6K) (Takei et al., 2004).
Positive AMPA receptor modulators (PARMs, also known as ampakines) are allosteric ligands that increase glutamate-mediated responses of AMPA receptors by modifying the kinetics of the receptors (activation, deactivation, desensitization) (Arai and Kessler, 2007). 2H,3H,6aH-Pyrrolidino[2″,1″-3′,2′]1,3-oxazino[6′,5′-5,4]-benzo[e]1,4-dioxan-10-one (CX614) is one of the best characterized ampakines; it decreases desensitization and slows deactivation of AMPA receptors in excised patches, enhances the size (amplitude and duration) of field EPSPs in hippocampal slices, and increases autaptically evoked EPSCs in neuronal cultures (Arai et al., 2000). It also increases mRNA expression and protein levels of BDNF (Lauterborn et al., 2000, 2003). Previous studies suggested that the long-lasting effects of ampakines on learning and memory are mediated by increased BDNF expression (Granger et al., 1993; Ingvar et al., 1997; Lynch, 2004). Ampakines also facilitate LTP induction and improve memory retention and learning of several behavioral tasks. Because of all these properties, several types of positive AMPA receptor modulators are being examined as potential treatments for various neurodegenerative diseases, behavioral disorders, and cognitive deficits associated with aging (Lynch, 2004). Accordingly, the present study investigated the links between CX614, BDNF, and dendritic protein synthesis. Our results indicate that CX614 rapidly activates dendritic protein synthesis machinery and that these effects require BDNF release and activation of TrkB and mTOR.
Materials and Methods
Reagents and treatments.
Primary neuronal cultures and acute hippocampal slices were treated with the ampakine (CX614) (a generous gift from Cortex Pharmaceuticals; 50 μm for slices and 10 μm for primary neuronal cultures). BDNF was also used for 1 h (100 ng/ml in slices and 50 ng/ml in cultured neurons). Primary neuronal cultures and acute hippocampal slices were treated with the following blockers and inhibitors for 10 and 30 min, respectively, before being treated with CX614 or BDNF; these inhibitors and blockers were used at the following concentrations:(2R)-amino-5-phosphonovaleric acid (APV) (Tocris; 25 μm in cultured neurons), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (Tocris; 50 μm in slices; 25 μm in cultured neurons), (9S,10R,12R)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid methyl ester (K252a) (Tocris; 0.5 μm), rapamycin (EMD Biosciences; 1 μm), actinomycin D (EMD Biosciences; 10 μm), nifedipine (Sigma; 10 μm), ryanodine (EMD Biosciences; 100 μm), and EGTA (Sigma; 5 mm). TrkB-Fc, a cell membrane-impermeable extracellular scavenger of BDNF made from the extracellular domain of human TrkB fused to the C-terminal histidine-tagged Fc region of human IgG1 (R&D Systems or from Sigma) was used at 2 μg/ml in acute slices and in the BDNF precipitation-scavenging assay (see below) and at 0.5 μg/ml in cultured neurons. All reagents were applied directly from stock solutions into culture medium (for cultured neurons) or artificial CSF (aCSF) for acute hippocampal slices. CX614 (50 μm) and BDNF (100 ng/ml) were added directly into culture medium for the translation reporter experiment (see Fig. 6).
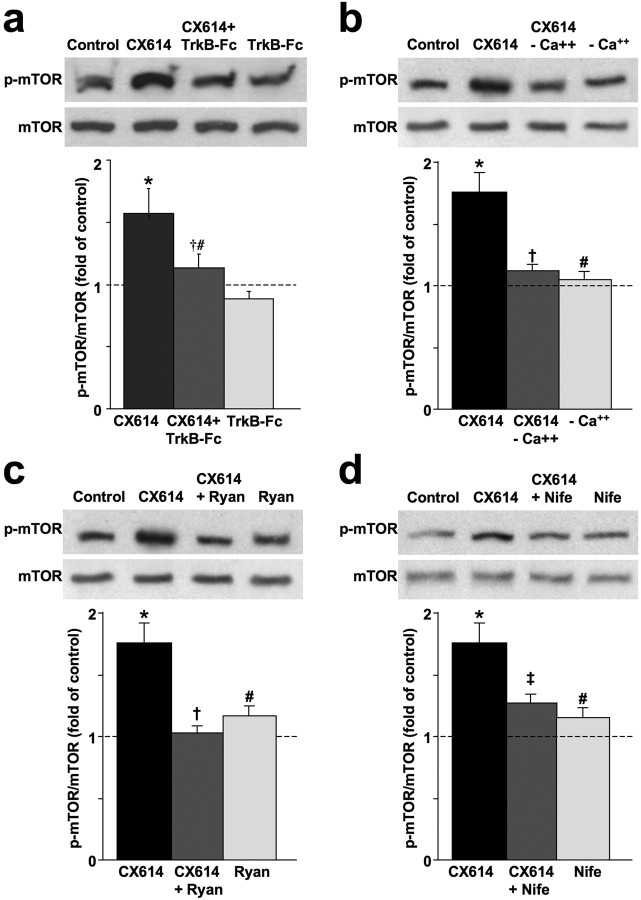
Figure 6.
CX614-induced BDNF release activates/phosphorylates mTOR. a, Cultured cortical neurons (DIV14) were preincubated with TrkB-Fc (0.5 μg/ml, 1 h) before being treated with CX614 (10 μm, 1 h). At the end of treatment, cells were processed for immunoblotting with antibodies against actin and total and phosphorylated mTOR. Immunoblots were quantified and ratios of p-mTOR over total unphosphorylated mTOR were calculated; data were then expressed as fold of control and are means ± SEM from six independent experiments. *p < 0.001 compared with control; †p < 0.05 compared with CX614; #not significantly different from control (ANOVA followed by Tukey's posttest analysis). b–d, Acute hippocampal slices were treated with CX614 (50 μm) in the absence or presence of Ca2+ (b), the absence or presence of ryanodine (Ryan) (100 μm, c), or the absence or presence of nifedipine (Nife) (10 μm, d) for 1 h. At the end of treatment, tissues were processed for immunoblotting with antibodies against total and phosphorylated mTOR, and actin (as a loading control). Immunoblots were quantified and ratios of phosphorylated over total mTOR were calculated; data were then expressed as percentage of control and are means ± SEM from three or four independent experiments. *p < 0.01 compared with control; #not significantly different from control; †p < 0.01 compared with CX614-treated samples; ‡p < 0.05 compared with CX614 (ANOVA followed by Tukey's posttest analysis).
Acute hippocampal slice preparation.
Hippocampi were rapidly dissected from 2-month-old Sprague Dawley rats, submerged in chilled cutting medium containing (in mm) 220 sucrose, 20 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 2 ascorbic acid, and 2 MgSO4, bubbled with 95% O2–5% CO2, and cut into transverse slices (400 μm thick) using a McIlwain tissue chopper. Slices were then incubated in artificial CSF (aCSF) containing (in mm) 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 24 NaHCO3, 2 ascorbic acid, 10 glucose, 1.5 MgSO4, and 2.5 CaCl2, saturated with 95% O2–5% CO2, and incubated for a 1 h recovery period at 37°C. All reagents were added directly into aCSF, and slices were treated for the indicated lengths of time.
Primary cortical neuronal cultures.
The neocortices of embryonic day 19 (E19) Sprague Dawley rat embryos were dissected, digested with trypsin, and mechanically dissociated. Cell were resuspended in B27-Neurobasal medium (NBM) and plated onto poly-d-lysine-coated dishes at a density of 800–1000 cells/mm2 for Western blotting and onto poly-d-lysine-coated glass coverslips at a density of 100–200 cells/mm2 for immunostaining. The cells were maintained in B27-NBM supplemented with 0.5 mm glutamine and with penicillin and streptomycin. Day in vitro 14 (DIV14) cultures were used in all experiments.
BDNF release scavenging assay.
Twenty dishes of DIV14 primary cortical neurons were first washed with warm HBSS (3 × 10 min, 37°C), then incubated with TrkB-Fc (1 h, 2 μg/ml, total medium volume = 4 ml/culture dish) before being treated with vehicle or with CX614 (10 μm; 1 h). At the end of treatment, medium from three to four dishes was collected and concentrated (40×) using filter-centrifugation through a Vivaspin 20 column (Vivascience) at 4°C. Protein concentration was determined, and equal volume of lysis buffer [150 mm NaCl, 5 mm EDTA, 1% Triton X-100, 10 mm Tris-HCl, pH 7.4, 0.5 mm PMSF, 2 mg/ml leupeptin, and 1:1000 protease inhibitor mixture (Roche)] was then added. Thirty to forty micrograms of total proteins from concentrated medium were processed by SDS-PAGE for Western blotting as indicated below. Anti-BDNF antibodies (N-20, Santa Cruz Biotechnology) were used to label scavenged BDNF. Levels of TrkB-Fc from the concentrated medium were used as an indicator of equal protein loading from control- and CX614-treated samples as follows: TrkB-Fc was reacted with polyclonal anti-TrkB antibodies (Cell Signaling) followed by HRP-conjugated secondary antibodies or reacted directly with HRP-conjugated goat anti-human secondary antibody (Jackson ImmunoResearch Laboratories). Results were then calculated as levels of BDNF corrected for levels of TrkB-Fc. The experiment was repeated twice, generating six values for control and six values for CX614-treated cultures, which were used to generate means ± SEM.
Immunoblotting.
At the end of treatments, acute hippocampal slices and cultured neurons were collected in lysis buffer [150 mm NaCl, 5 mm EDTA, 1% Triton X-100, 10 mm Tris-HCl, pH 7.4, 0.5 mm PMSF, 2 mg/ml leupeptin, and 1:1000 protease inhibitor mixture (Roche)]. Five to forty micrograms of denatured total protein were separated by SDS-PAGE using 4–20% Precise Protein Gels (Pierce), and transferred onto polyvinylidene difluoride membranes. Membranes were then blocked with 5% nonfat milk or with 3% bovine serum albumin (BSA) dissolved in Tris-buffered saline (TBS) at room temperature for 1 h and reacted overnight with antibodies at 4°C. All primary antibodies were used at 1:1000 dilution except anti-β-actin, which was used at 1:10,000. We used the following primary antibodies: affinity-purified rabbit anti-Arc (Synaptic Systems), anti-phospho TrkB [Tyr516; also known as phospho-TrkA (Tyr490); Cell Signaling], anti-TrkB (Cell Signaling and Millipore), rabbit anti-GFAP (DAKO), mouse anti-β-actin (Millipore), anti-phospho mTOR (Ser2448) and anti-mTOR (both from Cell Signaling), anti-phospho p70S6K (Thr389) and anti-p70S6K (both from Cell Signaling), anti-phospho 4EBP1 (Thr37/46; Cell Signaling), and anti-4EBP1 (Cell Signaling and Santa Cruz Biotechnology). Following incubation with primary antibodies, membranes were washed in TBS containing 0.1% Tween 20 (TBST) and incubated with peroxidase-conjugated secondary antibodies (1:10,000; Vector Laboratories), for 1 h, developed with ECL plus solution (ECL kit, GE Healthcare) and exposed to negative film. Exposed films were developed, fixed, scanned, and analyzed quantitatively by densitometry with NIH ImageJ software. Data were calculated as fold of control and expressed as means ± SEM from four to six independent experiments. All results were normalized against the total levels of native nonphosphorylated proteins in each individual sample. In addition, all blots were stripped and reincubated with anti-β-actin as loading control, and all results were adjusted against actin signal.
Immunostaining.
Cultured primary cortical neurons and acute hippocampal slices were fixed in ice-cold 4% paraformaldehyde in 0.1 m phosphate-buffer for 1 h. Slices were postfixed overnight in the same solution and sectioned (25 μm) using a freezing microtome. Fixed cells and free-floating sections were washed in PBS (3 × 15 min) and permeabilized in 0.7% Triton X-100 in PBS for 1 h, washed with PBS (2 × 5 min), rinsed in 0.1 m glycine in PBS for 1 h, treated at room temperature with 1% sodium borohydride dissolved in water for 20 min, and incubated in preblock buffer (0.05% Triton X-100, 5% donkey serum in PBS) for 90 min and then reacted overnight (4°C) with primary antibodies diluted 1:200–500 in preblock buffer. The following antibodies were used: anti-phospho mTOR (Ser2448), anti-mTOR, anti-phospho 4EBP1 (Thr37/46), and anti-4EBP1 (all from Cell Signaling except anti-4EBP1, from Santa Cruz Biotechnology). After three washes (3 × 30 min) with the preblock buffer at room temperature, sections and cells were incubated with the following secondary antibodies: Alexa Fluor 488 (green)-conjugated anti-mouse IgG or Alexa Fluor 546 (red)-conjugated anti-rabbit IgG antibodies (Invitrogen) diluted (1:100) in the preblock buffer at room temperature for 1 h, rinsed with PBS (3 × 5 min) and mounted with Prolong Gold Antifade Reagents (Invitrogen). Immunostaining signals were not detected in the absence of primary antibodies, and results did not show any change in total labeling with antibodies against the nonphosphorylated proteins. Immunostaining was observed and captured (1392 × 1040 pixels/field) using an Axioskop fluorescent microscope (Zeiss) equipped with a digital camera (Olympus).
Local protein translation and image acquisition and analysis.
To quantify dendritic protein synthesis in living hippocampal neurons prepared from E18 BALB/c mouse embryos, we used a green fluorescent (GFP) reporter cDNA flanked by the 5′ and 3′ untranslated regions (UTRs) from the CaMKIIα subunit. This pcDNA3.1–5′ UTR-dGFP-3′ UTR plasmid (generous gift from Dr. Erin M. Schuman, Caltech, Pasadena, CA) was described previously (Aakalu et al., 2001). The UTRs of the CaMKIIα mRNA contain sequences sufficient for its dendritic localization (Mayford et al., 1996). This plasmid encodes a myristoylated GFP (myrGFP) protein, and myristoylation limits intracellular diffusion of the translated reporter protein, because the translated myrGFP is anchored to the cell membrane. Previous work with this construct established that myrGFP recovery in distal dendritic segments (>100 μm) was due to new protein synthesis rather than diffusion from other cell compartments. Twenty-four hours after transfection of this translation reporter plasmid, neurons cultured in a glass-bottom 35 mm dish were transferred to an imaging buffer (100 mm NaCl, 3 mm KCl, 10 mm HEPES, 2 mm CaCl2, 2 mm MgCl2, 10 mm glucose, and 2% B27 dissolved in 100 mm PBS) and washed three times with the imaging buffer to remove excess substrate. Healthy neurons that expressed myrGFP were chosen for experimentation. Selected dendrites (∼100 μm from the cell body) were photobleached by excitation at 488 nm (Argon 488 nm) for 10–60 s until the GFP signals were barely seen. Vehicle alone, BDNF, or CX614 was then added into the medium immediately after photobleaching. Fluorescent intensity in a distal segment of the photobleached dendrites was captured every 30 s immediately after photobleaching and drug application. The same acquisition parameters and settings were applied to vehicle-, BDNF-, and CX614-treated neurons.
Statistical analyses.
All statistics were performed using GraphPad Prism 4.03 software. One-way ANOVA was used to test whether the means of each experimental group were significantly different, and if the overall p value was <0.05, then multiple comparisons between the experimental groups were tested using Tukey post hoc analysis.
Results
Ampakine-induced rapid dendritic activation of translation factors and kinases
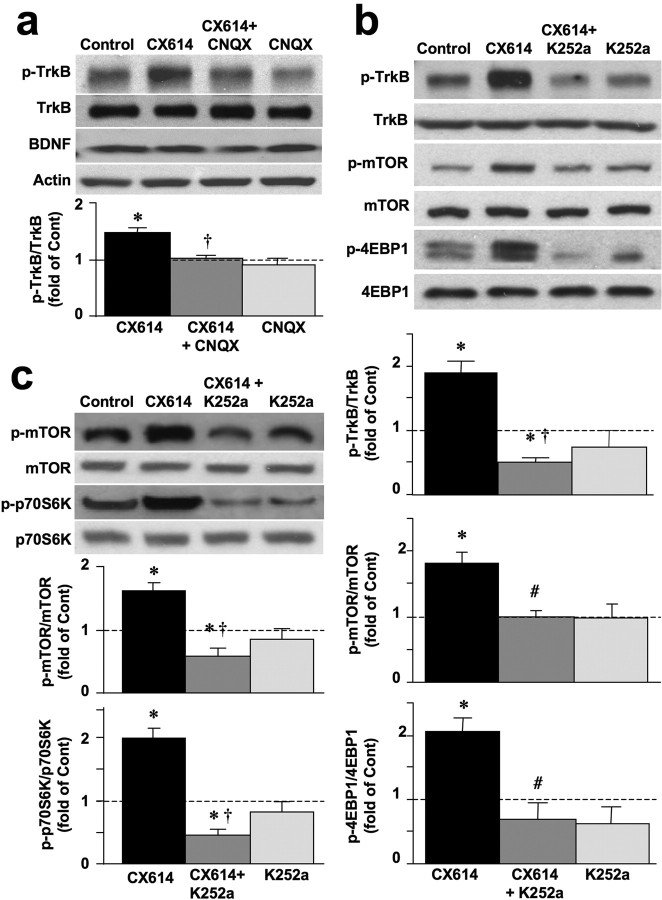
Eukaryotic initiation factor 4E (eIF4E) is normally bound to and inhibited by the nonphosphorylated form of eIF4E-binding protein (4EBP1). Phosphorylation of 4EBP1 by mTOR disrupts its association with eIF4E, thus allowing initiation of cap-dependent translation. Hippocampal slices, prepared from adult rats, were treated with CX614 (50 μm; 1 h), fixed, and immunostained with antibodies against phosphorylated and native forms of mTOR and 4EBP1. Labeling with antibodies against native forms of these two proteins was not altered by CX614 treatment. Immunostaining with phospho-mTOR and phospho-4EBP1 antibodies was significantly higher in pyramidal neurons in CA1 region of CX614-treated hippocampal slices than in vehicle-treated slices especially in cell bodies and apical dendrites (Fig. 1a). Similar results were observed in granule cells in the dentate gyrus. Effects of CX614 on protein synthesis regulators were further evaluated by immunoblotting of samples from acute hippocampal slices and from primary neuronal cultures. In acute hippocampal slices, CX614 treatment induced a marked increase in levels of phosphorylated/activated mTOR and 4EBP1, without affecting their total levels (Fig. 1b). CX614 stimulation of translation machinery required activation of AMPA receptors, as CX614-stimulated phosphorylation of mTOR and 4EBP1 was prevented by the AMPA receptor blocker, CNQX (50 μm) (Fig. 1b). In addition, CX614 treatment of primary neuronal cultures (10 μm, 1 h) significantly increased phosphorylation of 4EBP1 (data not shown) and of mTOR and p70S6K, a kinase implicated in phosphorylation of the S6 subunit of ribosomes and in activation of translation (Fig. 1c). In primary neuronal cultures, the effects of CX614 on activation of mTOR and p70S6K were blocked by CNQX (25 μm). Moreover, tetrodotoxin (TTX; 1–2 μm) and two NMDA receptor blockers, APV (25 μm) and MK801 (10 μm) prevented CX614-dependent activation of mTOR (supplemental Data 1, available at www.jneurosci.org as supplemental material). CX614 treatment of primary neuronal cultures also increased phosphorylation of ERK1/2 and AKT (supplemental Data 2, available at www.jneurosci.org as supplemental material).
Figure 1.
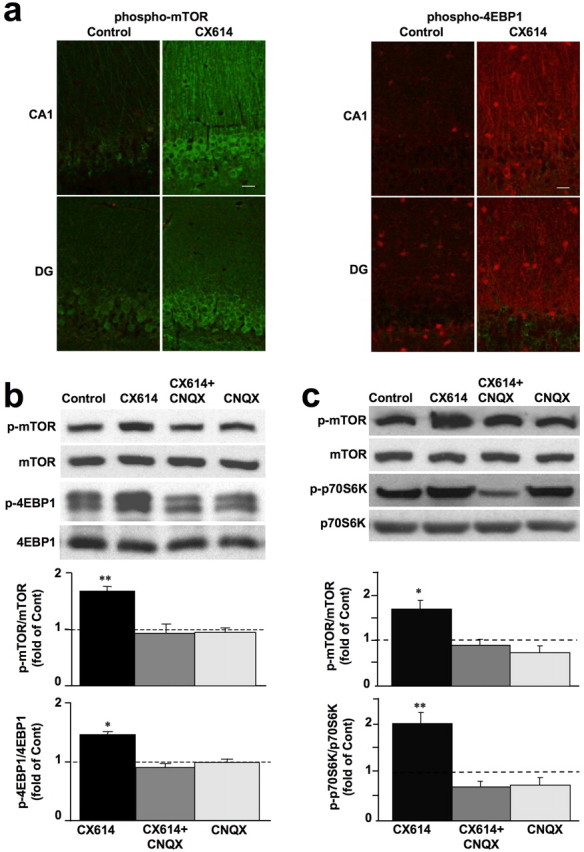
Effects of CX614 on mTOR and 4EBP1 phosphorylation in acute hippocampal slices and primary cortical neurons. a, Acute hippocampal slices from adult male rats incubated with vehicle (Control) or CX614 (50 μm) for 1 h were immediately processed for immunohistochemistry with antibodies against phospho-mTOR (left panels) or phospho-4EBP1 (right panels). Note the clear increase in immunoreactivity in stratum radiatum of CA1 and in stratum granulosum of the dentate gyrus (DG) in CX614-treated slices compared with control. Scale bar, 20 μm. b, Acute hippocampal slices from adult male rats were incubated with CX614 (50 μm) for 1 h in the presence or absence of CNQX (50 μm), and tissues were immediately processed for immunoblots with the following antibodies: phospho-mTOR, mTOR, phospho-4EBP1, and 4EBP1. c, Cultured cortical neurons were incubated with CX614 (10 μm) for 1 h in the presence of CNQX (25 μm) and were processed for immunoblotting with the following antibodies: phospho-mTOR, mTOR, phospho-p70S6K, and p70S6K. Immunoblots were quantified and ratios of phosphorylated over total antigens were calculated; data were then expressed as fold of control and are means ± SEM from six independent experiments. *p < 0.01, **p < 0.001 compared with control (ANOVA followed by Tukey's posttest analysis).
mTOR activation mediates CX614-induced rapid stimulation of translation machinery
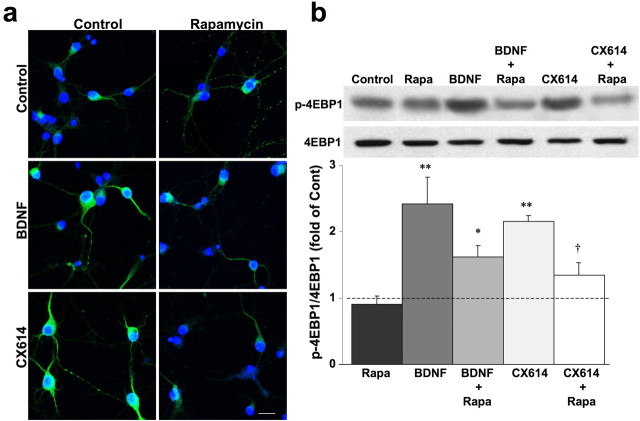
We then examined whether mTOR mediated the effects of CX614 on activation of the translation machinery using rapamycin, a specific mTOR inhibitor, and phosphorylation of 4EBP1 as an example of activation of downstream targets of mTOR. Cortical neurons were treated with CX614 and with BDNF (as a positive control) in the absence or presence of rapamycin. In both CX614- and BDNF-treated cultures, immunostaining with anti-phospho-4EBP1 was more intense in cell bodies and dendrites than in vehicle-treated control cultures (Fig. 2a); preincubation with rapamycin blocked both CX614- and BDNF-dependent increases in 4EBP1 phosphorylation (Fig. 2a). Similar results were obtained when culture homogenates were processed for immunoblotting with antibodies against phospho-4EBP1 (Fig. 2b) or phospho-p70S6K (data not shown; see also supplemental Data 2, available at www.jneurosci.org as supplemental material); rapamycin significantly reduced CX614-induced increase in levels of phosphorylated 4EBP1 and p70S6K. Similar results were obtained in acute hippocampal slices (data not shown). Incubation of cultures with AMPA alone (25 μm, 1 h) did not modify 4EBP1 phosphorylation but increased p70S6K phosphorylation (supplemental Data 2, available at www.jneurosci.org as supplemental material).
Figure 2.
mTOR mediates the effects of CX614 on 4EBP1 activation in cultured cortical neurons. Cultured cortical neurons were incubated with CX614 (10 μm) or BDNF (50 ng/ml) for 1 h in the absence or presence of rapamycin (1 μm). At the end of treatment, cultures were fixed and processed for immunostaining (a) with antibodies against phospho-4EBP1, or lysed and processed for immunoblotting (b) with antibodies against 4EPP1 and phospho-4EBP1. Immunoblots were quantified and ratios of phosphorylated over total 4EBP1 were calculated; data were then expressed as fold of control and are means ± SEM from six independent experiments. *p < 0.01, **p < 0.001 compared with control; †p < 0.01 compared with CX614 (ANOVA followed by Tukey's posttest analysis). Rapa, Rapamycin. Scale bar, 20 μm.
TrkB phosphorylation/activation mediates CX614-induced stimulation of protein translation machinery
BDNF affects translation by inducing activation/phosphorylation of its tyrosine kinase receptor, TrkB. Our results (Figs. 1, 2; supplemental Data 2, available at www.jneurosci.org as supplemental material) indicated similar effects of BDNF and CX614 on activation of several kinases and factors involved in translation and were in good agreement with previously reported effects of BDNF (Takei et al., 2004). Thus, our results implied convergence of CX614 and BDNF effects on the same signaling pathway. To examine this possibility, we first evaluated whether the effects of CX614 were mediated by activation of the TrkB receptor. Acute hippocampal slices and primary neuronal cultures were treated with CX614 (50 and 10 μm, respectively, for 1 h) and levels of phosphorylated TrkB (Fig. 3a) as well as those of downstream regulators of translation (Fig. 3b,c) were determined by Western blotting. CX614 treatment rapidly increased TrkB phosphorylation (Fig. 3a), and the effects of CX614 were specific for AMPA receptors, as they were blocked by cotreatment with CNQX. The specificity of TrkB activation/phosphorylation by CX614 was further evaluated by using an inhibitor of TrkB, K252a. K252 completely eliminated CX614-dependent activation of TrkB (Fig. 3b) and blocked CX614-dependent upregulation of phosphorylation of mTOR and 4EBP1 in acute slices (Fig. 3b) and of mTOR and p70S6K in primary neuronal cultures (Fig. 3c). As chronic CX614 treatment upregulates BDNF mRNA and protein levels (Lauterborn et al., 2000), we evaluated BDNF protein levels in the same samples (Fig. 3a) and found that they were not increased following 1 h CX614 treatment. This result instigated more detailed examination of the effects of CX614 treatment on BDNF release.
Figure 3.
BDNF mediates CX614-induced stimulation of protein translation in primary neuronal cultures and acute hippocampal slices. a, Acute hippocampal slices were treated with CX614 (50 μm) in the absence or presence of CNQX (50 μm) for 1 h. At the end of treatment, tissues were processed for immunoblotting with antibodies against TrkB, phospho-TrkB, BDNF, and actin. Immunoblots were quantified and ratios of phosphorylated over total TrkB were calculated; data were then expressed as fold of control (Cont) and are means ± SEM from six independent experiments. *p < 0.01 compared with control; †p < 0.01 compared with CX614 (ANOVA followed by Tukey's posttest analysis). b, Acute hippocampal slices were treated with CX614 (50 μm) in the absence or presence of K252a (0.5 μm) for 1 h. At the end of treatment, tissues were processed for immunoblotting with antibodies against total and phosphorylated TrkB, mTOR, and 4EBP1. Immunoblots were quantified and ratios of phosphorylated over total antigens were calculated; data were then expressed as fold of control and are means ± SEM from six independent experiments. *p < 0.05 compared with control; #p < 0.05 compared with CX614; †p < 0.01 compared with CX614 (ANOVA followed by Tukey's posttest analysis). c, Cultured cortical neurons were treated with CX614 (10 μm) in the absence or presence of K252a (0.5 μm) for 1 h. At the end of treatment, cells were processed for immunoblotting with antibodies against total and phosphorylated mTOR and p70S6K. Immunoblots were quantified and ratios of phosphorylated over total antigens were calculated; data were then expressed as fold of control and are means ± SEM from six independent experiments. *p < 0.01, †p < 0.001 compared with CX614 (ANOVA followed by Tukey's posttest analysis).
CX614 treatment increases BDNF release in cultured neurons
The possibility that CX614 stimulates BDNF release was assessed using TrkB-Fc, a cell membrane-impermeable scavenger of BDNF. TrkB-Fc application in culture medium eliminated CX614-induced TrkB phosphorylation (Fig. 4a), suggesting that BDNF release was involved. To further confirm this assumption, we used TrkB-Fc in primary cultured neurons to trap released BDNF in combination with Western blotting as indicated in Materials and Methods to quantify BDNF release (Fig. 4b). Cultured primary neurons were preincubated with TrkB-Fc (2 μg/ml, 1 h) and incubated in the absence or presence of CX614 (10 μm, 1 h). Culture medium was collected and concentrated. Proteins in concentrated samples were then processed for immunoblotting with antibodies against BDNF and unphosphorylated TrkB to assess levels of loaded TrkB-Fc. Normalized levels of BDNF were averaged for the various replicates and expressed as fold of control. Results of this experiment showed that CX614 treatment increased levels of TrkB-Fc-trapped BDNF by 4.07 ± 0.67-fold (means ± SEM of six experiments; p < 0.001, Student's t test).
Figure 4.
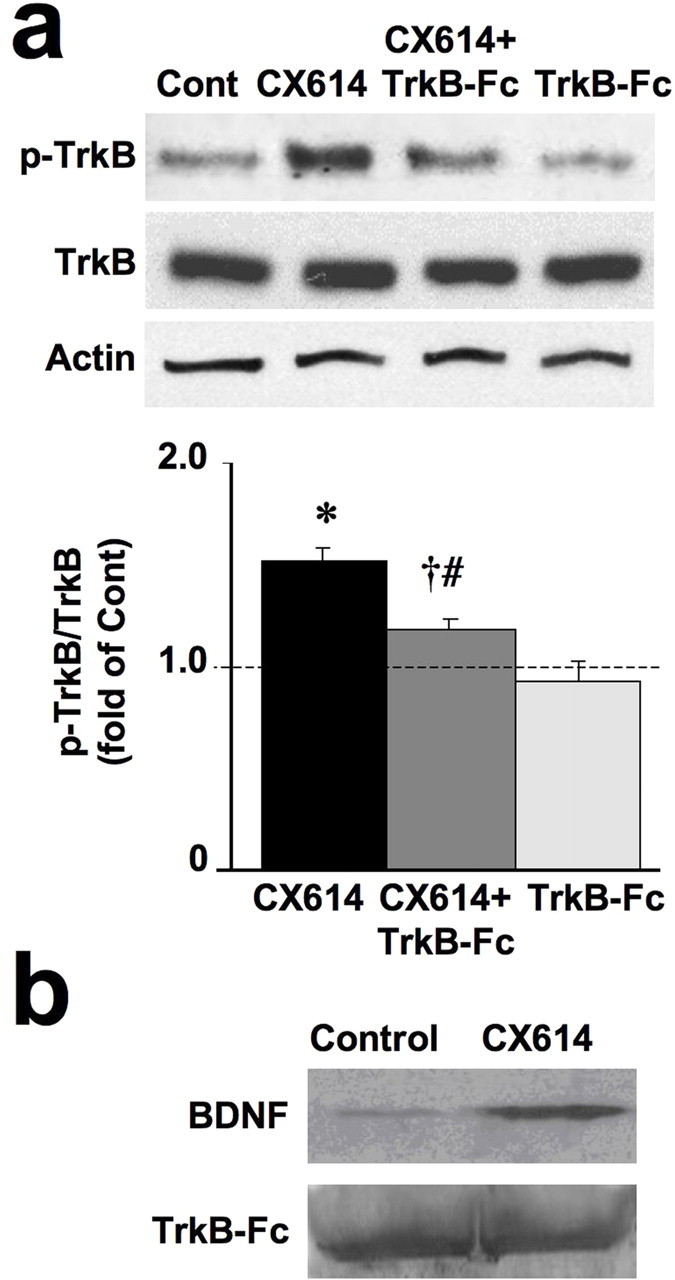
CX614 stimulates BDNF release in cultured neurons. a, Cultured cortical neurons (DIV14) were preincubated with TrkB-Fc (0.5 μg/ml, 1 h) and incubated in the absence or presence of CX614 (10 μm, 1 h). At the end of treatment, cells were processed for immunoblotting with antibodies against actin and total and phosphorylated TrkB. Immunoblots were quantified and ratios of p-TrkB over total unphosphorylated TrkB were calculated; data were then expressed as fold of control and are means ± SEM from six experiments. *p < 0.001 compared with control; †p < 0.05 compared with CX614; #not significantly different from control (ANOVA followed by Tukey's posttest analysis). Cont, Control. b, DIV14 cultured cortical neurons preincubated with TrkB-Fc (2 μg/ml) were treated with CX614 (10 μm, 1 h). Medium from three or four dishes was combined, and proteins were concentrated as indicated in Materials and Methods. Proteins in concentrated samples were then processed for immunoblotting with antibodies against BDNF and unphosphorylated TrkB to assess levels of loaded TrkB-Fc.
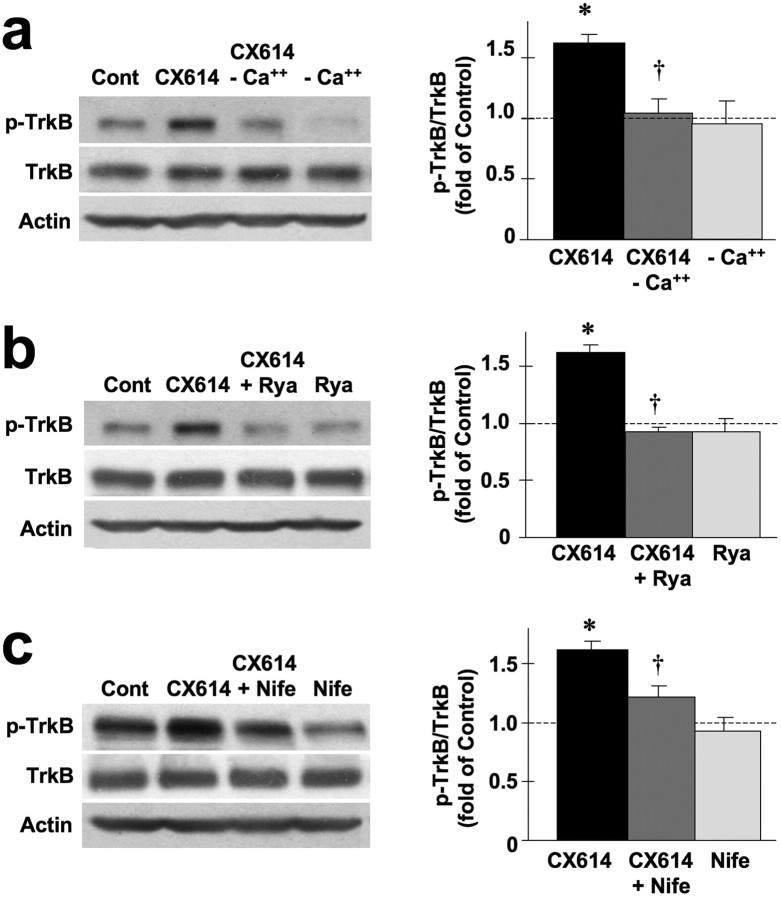
In hippocampal neurons, BDNF secretion requires activation of voltage-gated Ca2+ channels as well as Ca2+ release from internal stores (Kolarow et al., 2007). Reagents or conditions that alter Ca2+ levels were therefore also used to examine BDNF secretion in acute hippocampal slices (Fig. 5a–c). CX614-elicited TrkB phosphorylation in acute hippocampal slices was completely blocked in the absence of extracellular Ca2+ (Fig. 5a). Ryanodine, a blocker of Ca2+ release from intracellular stores also completely eliminated CX614-stimulated TrkB activation/phosphorylation (Fig. 5b). Finally, nifedipine, a blocker of voltage-gated Ca2+ channels, markedly reduced CX614-elicited TrkB phosphorylation (Fig. 5c).
Figure 5.
BDNF release mediates CX-614 activation of TrkB receptors in acute hippocampal slices. Acute hippocampal slices were treated with CX614 (50 μm) in the absence or presence of Ca2+ (a), the absence or presence of ryanodine (Rya) (100 μm, b), or the absence or presence of nifedipine (Nife) (10 μm, c) for 1 h. At the end of treatment, tissues were processed for immunoblotting with antibodies against total and phosphorylated TrkB, and actin [as a loading control (Cont)]. Immunoblots were quantified and ratios of phosphorylated over total TrkB were calculated; data were then expressed as percentage of control and are means ± SEM from six independent experiments. *p < 0.01 compared with control; †p < 0.01 compared with CX614 (ANOVA followed by Tukey's posttest analysis).
CX614-dependent activation of protein translation machinery is mediated by increased BDNF release
We also determined whether factors that alter BDNF release or interfere with released BDNF could affect downstream regulators of translation such as mTOR. CX614-dependent phosphorylation of mTOR was blocked following TrkB-Fc preincubation in cultured primary neurons (Fig. 6a). In addition, preincubation of primary neuronal cultures with TrkB-Fc completely prevented CX614-dependent phosphorylation of 4EBP1 (data not shown). CX614-stimulation of mTOR phosphorylation in acute hippocampal slices was also inhibited in the absence of extracellular Ca2+ and in the presence of ryanodine or nifedipine (Fig. 6b–d).
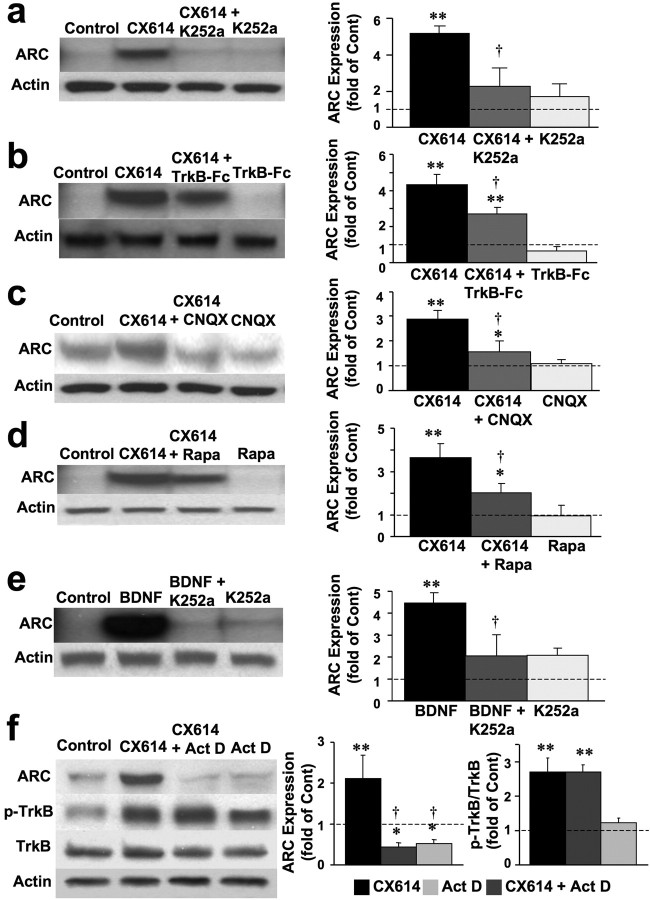
CX614 rapidly upregulates ARC and stimulates dendritic protein synthesis
We then studied the effects of CX614 on activity-regulated cytoskeleton-associated protein (ARC, also known as Arg3.1), an immediate early gene product that is markedly and rapidly upregulated by neuronal activity and plasticity-inducing stimuli; both ARC mRNA and protein are rapidly transported to dendrites and spines following LTP induction (Steward and Worley, 2002). ARC mRNA can also be locally translated in dendrites. The effects of CX614 on ARC expression were examined using primary neuronal cultures treated with CX614 alone or in combination with CNQX, rapamycin, K252a, TrkB-Fc, or actinomycin D, a transcription inhibitor. Primary cultures were also treated with BDNF to upregulate ARC protein levels as a positive control. CX614 and BDNF treatments elicited large increases in ARC levels, and these effects were completely blocked by CNQX, K252a, and actinomycin D, and significantly reduced by TrkB-Fc and rapamycin (Fig. 7). Note however that actinomycin D did not modify CX614-induced activation of TrkB (Fig. 7f).
Figure 7.
BDNF mediates CX614-induced stimulation of ARC synthesis in primary neuronal cultures. Cultured cortical neurons were incubated in the absence or presence of CX614 (10 μm) and K252a (0.5 μm, a), TrkB-Fc (0.5 μg/ml, b), CNQX (50 μm, c), or rapamycin (Rapa) (100 μm, d) for 1 h. At the end of treatment, cells were processed for immunoblotting with antibodies against ARC and actin (for loading control). Immunoblots were quantified and corrected ARC levels were calculated; data were then expressed as fold of control and are means ± SEM from six independent experiments. *p < 0.01, **p < 0.001 compared with control; †p < 0.01 compared with CX614 (ANOVA followed by Tukey's posttest analysis). Cultured cortical neurons were incubated in the absence or presence of BDNF (50 ng/ml) and K252a (0.5 μm) (e). At the end of treatment, cells were processed for immunoblotting with antibodies against ARC and actin (for loading control). Immunoblots were quantified and corrected ARC levels were calculated; data were then expressed as fold of control and are means ± SEM from six independent experiments. **p < 0.001 compared with control; †p < 0.01 compared with CX614 (ANOVA followed by Tukey's posttest analysis). f, Cultured cortical neurons incubated in the absence or presence of CX614 (10 μm) and actinomycin D (Act D) (10 μm) for 1 h. At the end of treatment, cells were processed for immunoblotting with antibodies against ARC, total and phosphorylated TrkB, and actin (for loading control; data not shown). Immunoblots were quantified and corrected ARC levels and ratios of phosphorylated over total TrkB were calculated; quantification of the blots from six independent experiments indicated that the effects of CX614 were highly significant, and the effects of CX614 on ARC but not on phospho-TrkB were significantly reduced by actinomycin D. Cont, Control.
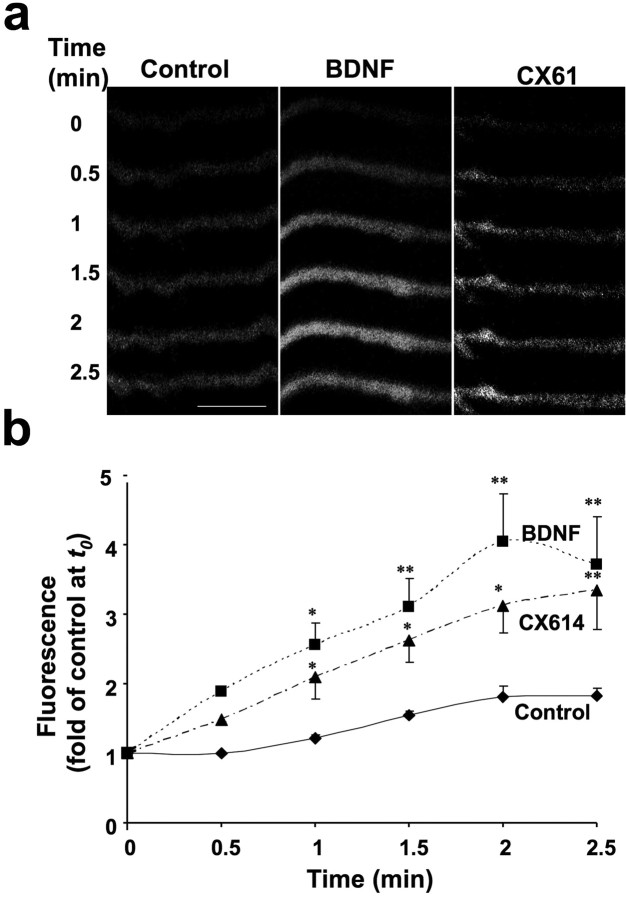
A new assay for evaluation of dendritic protein synthesis using a GFP protein-translation reporter under the control of the UTRs of CaMKIIα has been recently developed (Aakalu et al., 2001). Both BDNF and CX614 rapidly (within minutes) stimulated GFP synthesis in dendrites of cultured hippocampal neurons (Fig. 8a), and the rate of fluorescence recovery, i.e., the rate of protein synthesis, was comparable in CX614- and BDNF-treated cultures and faster than in vehicle-treated controls (Fig. 8b).
Figure 8.
Effects of BDNF and CX614 treatment on dendritic protein synthesis. a, Selected dendrites of cultured hippocampal neurons that were injected with the GFP reporter plasmid at 12 DIV were “photobleached” until fluorescence was almost invisible before being treated with vehicle (control), BDNF (100 ng/ml), or CX614 (50 μm). Time-lapse images were then taken at 30 s intervals. Scale bar, 5 μm. b, Fluorescence intensity was quantified and values were expressed as percentage of values measured at time 0 (t0). Results are means ± SEM of three experiments [results from each experiments were the averaged values determined from 3–5 dendrites (1–2 dendrites/neuron)] from three independent cultures. *p < 0.01, **p < 0.001 compared with control (ANOVA followed by Tukey's posttest analysis).
Discussion
Positive modulators of receptors have a number of advantages compared with receptor agonists to study the properties of receptors. In contrast to agonists, they do not elicit receptor desensitization, and they only enhance the responses of receptors activated by synaptically released neurotransmitters. CX614 is one of the best-studied positive AMPA receptor modulators and increases spontaneous activity in hippocampal slices for prolonged periods of time (Lauterborn et al., 2000, 2003), as well as the amplitude and frequency of miniature EPSPs (Arai et al., 2000). To understand the links between AMPA receptors, BDNF, and local protein synthesis, we used CX614, in cultured cortical neurons and in acute hippocampal slices. While the cultured neuron preparation provides a better model for detailed studies of mechanisms of action, the acute hippocampal slice preparation more closely resembles the in vivo conditions (Paulsen and Sejnowski, 2000). CX614 rapidly activated dendritic translational machinery and local protein synthesis in both preparations. These findings constitute the first indication that positive modulation of AMPA receptors leads to rapid activation/phosphorylation of mTOR and its downstream targets, such as 4EBP1 and p70S6K, which play important roles in local dendritic protein translation (Takei et al., 2004). TTX blocked CX614-dependent activation of translation, suggesting that, in the presence of CX614, basal levels of synaptic activity are sufficient to activate translation (supplemental Data 1, available at www.jneurosci.org as supplemental material). The effects of CX614 were significantly reduced or eliminated by rapamycin, further confirming the critical role of mTOR. Previous studies have indicated activation of translation by stimulation of metabotropic and NMDA glutamate receptors (Huber at al., 2000; Gong et al., 2006). In agreement with these findings, our results indicated that NMDA receptors are also involved, as the NMDA receptor blockers APV and MK801 prevented the effects of CX614 treatment of cultured neurons on the translation machinery (supplemental Data 1, available at www.jneurosci.org as supplemental material).
Another positive AMPA receptor modulator, aniracetam (1 μm), also stimulated local protein synthesis when applied in the presence of very high AMPA concentrations (500 μm) (Wu et al., 2004). In contrast to these results, our findings indicated activation of translation within minutes of CX614 application alone. Thus, activation of local dendritic translation can occur at basal or near-basal levels of glutamate release in the presence of CX614. In contrast, miniature AMPA receptor currents were proposed to exert a tonic suppression on local dendritic protein synthesis (Sutton et al., 2006). Our data imply the existence of previously unreported mechanisms that are rapidly induced/stimulated by ampakines and that activate local dendritic translation at near-basal levels of synaptic transmission.
CX614 also rapidly increased the dendritic synthesis of myristoylated GFP (myr-GFP) and of ARC, further confirming that positive modulation of AMPA receptors leads to increased dendritic protein synthesis. Since the cDNA encoding the myr-GFP reporter protein is inserted between the 5′ and 3′ UTRs of CaMKIIα, ampakine treatment is likely to also result in local dendritic translation of CaMKIIα. ARC synthesis was previously demonstrated to take place both in cell bodies and in dendrites (Bramham et al., 2008). The differential effects of actinomycin D and rapamycin on CX614-mediated increase in ARC levels suggest that both increased transcription and translation contribute to CX614-dependent upregulation of ARC. This likely involves increased ARC synthesis in cell bodies as well as within dendrites.
The effects of CX614 on protein synthesis machinery and dendritic protein synthesis were remarkably similar to the previously reported BDNF-induced stimulation of dendritic translation (Takei et al., 2004). Interestingly, acute application of CX614 also resulted in TrkB activation, indicating that both CX614 and BDNF converge on the mTOR pathway to stimulate translation. While such convergence could be due to the known effects of CX614 on upregulation of BDNF expression (Lauterborn et al., 2003), CX614-induced phosphorylation of TrkB was still observed in the presence of a transcription inhibitor, actinomycin D (Fig. 7f), clearly indicating that the effect of CX614 on TrkB phosphorylation was independent of its effect on BDNF mRNA. In addition, treatment of cultured primary neurons and acute hippocampal slices with CX614 even up to 1 h did not increase BDNF protein levels (Fig. 3a). Therefore, CX614-dependent increase in TrkB phosphorylation cannot involve increased BDNF expression, but rather increased release of BDNF and/or transactivation of the TrkB receptor. Together, the absence of BDNF protein upregulation and the significant reduction of TrkB phosphorylation with TrkB-Fc after 1 h CX614 treatment suggest that CX614 treatment of neurons or slices rapidly induces BDNF release.
TrkB-Fc, an extracellular scavenger of BDNF, has been extensively used in several studies to block the effects of extracellular (including endogenously secreted) BDNF in acute hippocampal slice preparations (Rex et al., 2006) or during electrophysiological recordings from the visual cortex (Liu et al., 2007; Kaneko et al., 2008). Using ELISA, Lockhart et al. (2007) reported that BDNF release remained below detection threshold after 5 h treatment with a different AMPA receptor modulator (S 18986). In contrast, our results indicated a robust CX614-dependent upregulation of BDNF release. Therefore, our results provide direct and conclusive evidence that acute CX614 treatment increases BDNF release and that extracellular BDNF stimulates TrkB phosphorylation and activates downstream regulators of dendritic protein translation.
Our results are thus in good agreement with those of Wu et al. (2004), who also observed rapid increase in BDNF release in cultured cerebellar granule cells following treatment with a high AMPA concentration in the presence of aniracetam. In addition, the use of pharmacological agents previously shown to block BDNF release provided further evidence that CX614-mediated stimulation of local protein synthesis was due to BDNF release and TrkB activation (Kolarow et al., 2007). In particular, Ca2+ release from internal stores is critical for BDNF release. While AMPA receptors in hippocampus are largely impermeable to Ca2+ ions (Lomeli et al., 1994; Jensen et al., 1998; Carlson et al., 2000; Iizuka et al., 2000; Krampfl et al., 2002; Kumar et al., 2002), increased Na+ influx through AMPA receptors can lead to increased Ca2+ release from intracellular stores (Hoyt et al., 1998; Zhang and Lipton, 1999). Indeed, blocking Ca2+ release from internal Ca2+ stores with ryanodine inhibited CX614-induced TrkB activation.
Ca2+ increase in the cytosol can also be due to activation of NMDA receptors, and/or of voltage-dependent Ca2+ channels (Shoshan-Barmatz et al., 1994). NMDA receptors appeared to be implicated in the effects of CX614 on mTOR phosphorylation, and it is possible that NMDA receptors could also be implicated in the effects of CX614 on BDNF release. Future studies should examine the interaction of NMDA receptor activation and positive modulation of AMPA receptors on BDNF release in acute hippocampal slices and in primary neuronal cultures. Voltage-dependent Ca2+ channels were also required for BDNF release as evidenced by TrkB phosphorylation (Fig. 5) and for activation of downstream regulators of translation, such as phosphorylation of mTOR (Fig. 6); absence and chelation of extracellular Ca2+ or presence of nifedipine completely prevented CX614-dependent TrkB phosphorylation, without affecting basal levels of TrkB phosphorylation. Therefore, our findings indicate that both intracellular and extracellular Ca2+ is involved in CX614-dependent phosphorylation of TrkB; these results are therefore in good agreement with those of Kolarow et al. (2007).
While other mechanisms could contribute to CX614 upregulation of translation such as TrkB transactivation, and activity-dependent membrane insertion of TrkB, our results with TrkB-Fc strongly argue against this. The similar profiles of reporter translation with CX614 and BDNF treatments imply that it is less likely that CX614 stimulates translation of BDNF mRNA rapidly enough (within minutes) for BDNF to be synthesized, packaged within vesicles, and released as mature BDNF. Although this cannot be ruled out completely, we propose that CX614's effects on local protein synthesis are due to the release of a pool of preexisting BDNF.
LTP and LTD have been implicated in synaptic plasticity and are widely considered as cellular models for certain forms of learning and memory. It is now well established that local protein synthesis is involved in synaptic plasticity, LTP, LTD, synaptic consolidation, and formation of spatial memory and learning (Alberini, 1999; Klintsova and Greenough, 1999; Wells and Fallon, 2000; Aakalu et al., 2001). In addition, it is well accepted that BDNF and activation of glutamate receptors play important roles in triggering local (dendritic) protein synthesis and in regulating several aspects of synaptic transmission and maturation (Akaneya et al., 1996, 1997; Narisawa-Saito et al., 1999a,b, 2002; Hayashi et al., 2000; Broutman and Baudry, 2001; Xiong et al., 2002; Yin et al., 2002; Jourdi et al., 2003; Lauterborn et al., 2003; Takei et al., 2004; Grooms et al., 2006; Rex et al., 2006; Bramham, 2007; Bramham et al., 2008; Newpher and Ehlers, 2008). Importantly, BDNF release has also been implicated in long-lasting synapse formation (also known as synaptic consolidation) (Taniguchi et al., 2006). Our findings that positive modulation of AMPA receptor function by ampakines results in BDNF release and activation of local protein synthesis machinery and local protein synthesis, in particular ARC and possibly CaMKIIα, shed new light on the cellular/molecular events implicated in synaptic plasticity. Furthermore, our results could account, at least in part, for the effects of positive AMPA receptor modulators on facilitation of LTP and improved learning and memory, and further justify the clinical development of these molecules for treatment of diseases associated with cognitive impairment.
Footnotes
This work was supported by Grant P01NS045260-01 from the National Institute of Neurological Disorders and Stroke (principal investigator: Dr. C. M. Gall).
The authors declare that they have no conflict of interest.
References
- Aakalu et al., 2001.Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Akaneya et al., 1996.Akaneya Y, Tsumoto T, Hatanaka H. Brain-derived neurotrophic factor blocks long-term depression in rat visual cortex. J Neurophysiol. 1996;76:4198–4201. doi: 10.1152/jn.1996.76.6.4198. [DOI] [PubMed] [Google Scholar]
- Akaneya et al., 1997.Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini, 1999.Alberini CM. Genes to remember. J Exp Biol. 1999;202:2887–2891. doi: 10.1242/jeb.202.21.2887. [DOI] [PubMed] [Google Scholar]
- Arai and Kessler, 2007.Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- Arai et al., 2000.Arai AC, Kessler M, Rogers G, Lynch G. Effects of the potent ampakine CX614 on hippocampal and recombinant AMPA receptors: interactions with cyclothiazide and GYKI 52466. Mol Pharmacol. 2000;58:802–813. doi: 10.1124/mol.58.4.802. [DOI] [PubMed] [Google Scholar]
- Bramham, 2007.Bramham CR. Control of synaptic consolidation in the dentate gyrus: mechanisms, functions, and therapeutic implications. Prog Brain Res. 2007;163:453–471. doi: 10.1016/S0079-6123(07)63025-8. [DOI] [PubMed] [Google Scholar]
- Bramham et al., 2008.Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene Arc/Arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broutman and Baudry, 2001.Broutman G, Baudry M. Involvement of the secretory pathway for AMPA receptors in NMDA-induced potentiation in hippocampus. J Neurosci. 2001;21:27–34. doi: 10.1523/JNEUROSCI.21-01-00027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson et al., 2000.Carlson NG, Howard J, Gahring LC, Rogers SW. RNA editing (Q/R site) and flop/flip splicing of AMPA receptor transcripts in young and old brains. Neurobiol Aging. 2000;21:599–606. doi: 10.1016/s0197-4580(00)00127-5. [DOI] [PubMed] [Google Scholar]
- Davis and Squire, 1984.Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Gong et al., 2006.Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- Granger et al., 1993.Granger R, Staubli U, Davis M, Perez Y, Nilsson L, Rogers GA, Lynch G. A drug that facilitates glutamatergic transmission reduces exploratory activity and improves performance in a learning-dependent task. Synapse. 1993;15:326–329. doi: 10.1002/syn.890150409. [DOI] [PubMed] [Google Scholar]
- Grooms et al., 2006.Grooms SY, Noh KM, Regis R, Bassell GJ, Bryan MK, Carroll RC, Zukin RS. Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons. J Neurosci. 2006;26:8339–8351. doi: 10.1523/JNEUROSCI.0472-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi et al., 2000.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hoyt et al., 1998.Hoyt KR, Stout AK, Cardman JM, Reynolds IJ. The role of intracellular Na+ and mitochondria in buffering of kainate-induced intracellular free Ca2+ changes in rat forebrain neurons. J Physiol. 1998;509:103–116. doi: 10.1111/j.1469-7793.1998.103bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber et al., 2000.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Iizuka et al., 2000.Iizuka M, Nishimura S, Wakamori M, Akiba I, Imoto K, Barsoumian EL. The lethal expression of the GluR2flip/GluR4flip AMPA receptor in HEK293 cells. Eur J Neurosci. 2000;12:3900–3908. doi: 10.1046/j.1460-9568.2000.00270.x. [DOI] [PubMed] [Google Scholar]
- Ingvar et al., 1997.Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, Schehr RS, Lynch G. Enhancement by an ampakine of memory encoding in humans. Exp Neurol. 1997;146:553–559. doi: 10.1006/exnr.1997.6581. [DOI] [PubMed] [Google Scholar]
- Jensen et al., 1998.Jensen JB, Schousboe A, Pickering DS. Development of calcium-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in cultured neocortical neurons visualized by cobalt staining. J Neurosci Res. 1998;54:273–281. doi: 10.1002/(SICI)1097-4547(19981015)54:2<273::AID-JNR15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Jourdi et al., 2003.Jourdi H, Iwakura Y, Narisawa-Saito M, Ibaraki K, Xiong H, Watanabe M, Hayashi Y, Takei N, Nawa H. Brain-derived neurotrophic factor signal enhances and maintains the expression of AMPA receptor-associated PDZ proteins in developing cortical neurons. Dev Biol. 2003;263:216–230. doi: 10.1016/j.ydbio.2003.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko et al., 2008.Kaneko M, Hanover JL, England PM, Stryker MP. TrkB kinase is required for recovery, but not loss, of cortical responses following monocular deprivation. Nat Neurosci. 2008;11:497–504. doi: 10.1038/nn2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova and Greenough, 1999.Klintsova AY, Greenough WT. Synaptic plasticity in cortical systems. Curr Opin Neurobiol. 1999;9:203–208. doi: 10.1016/s0959-4388(99)80028-2. [DOI] [PubMed] [Google Scholar]
- Kolarow et al., 2007.Kolarow R, Brigadski T, Lessmann V. Postsynaptic secretion of BDNF and NT-3 from hippocampal neurons depends on calcium calmodulin kinase II signaling and proceeds via delayed fusion pore opening. J Neurosci. 2007;27:10350–10364. doi: 10.1523/JNEUROSCI.0692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampfl et al., 2002.Krampfl K, Schlesinger F, Zörner A, Kappler M, Dengler R, Bufler J. Control of kinetic properties of GluR2 flop AMPA-type channels: impact of R/G nuclear editing. Eur J Neurosci. 2002;15:51–62. doi: 10.1046/j.0953-816x.2001.01841.x. [DOI] [PubMed] [Google Scholar]
- Kumar et al., 2002.Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22:3005–3015. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn et al., 2000.Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci. 2000;20:8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn et al., 2003.Lauterborn JC, Truong GS, Baudry M, Bi X, Lynch G, Gall CM. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther. 2003;307:297–305. doi: 10.1124/jpet.103.053694. [DOI] [PubMed] [Google Scholar]
- Liu et al., 2007.Liu Y, Zhang LI, Tao HW. Heterosynaptic scaling of developing GABAergic synapses: dependence on glutamatergic input and developmental stage. J Neurosci. 2007;27:5301–5312. doi: 10.1523/JNEUROSCI.0376-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart et al., 2007.Lockhart BP, Rodriguez M, Mourlevat S, Peron P, Catesson S, Villain N, Galizzi JP, Boutin JA, Lestage P. S18986: a positive modulator of AMPA-receptors enhances (S)-AMPA-mediated BDNF mRNA and protein expression in rat primary cortical neuronal cultures. Eur J Pharmacol. 2007;561:23–31. doi: 10.1016/j.ejphar.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Lomeli et al., 1994.Lomeli H, Mosbacher J, Melcher T, Höger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994;266:1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- Lynch, 2004.Lynch G. AMPA receptor modulators as cognitive enhancers. Curr Opin Pharmacol. 2004;4:4–11. doi: 10.1016/j.coph.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Mayford et al., 1996.Mayford M, Baranes D, Podsypanina K, Kandel ER. The 3′-untranslated region of CaMKII alpha is a cis-acting signal for the localization and translation of mRNA in dendrites. Proc Natl Acad Sci U S A. 1996;93:13250–13255. doi: 10.1073/pnas.93.23.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello et al., 2002.Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito et al., 1999a.Narisawa-Saito M, Carnahan J, Araki K, Yamaguchi T, Nawa H. Brain-derived neurotrophic factor regulates the expression of AMPA receptor proteins in neocortical neurons. Neuroscience. 1999a;88:1009–1014. doi: 10.1016/s0306-4522(98)00496-5. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito et al., 1999b.Narisawa-Saito M, Silva AJ, Yamaguchi T, Hayashi T, Yamamoto T, Nawa H. Growth factor-mediated Fyn signaling regulates alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor expression in rodent neocortical neurons. Proc Natl Acad Sci U S A. 1999b;96:2461–2466. doi: 10.1073/pnas.96.5.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa-Saito et al., 2002.Narisawa-Saito M, Iwakura Y, Kawamura M, Araki K, Kozaki S, Takei N, Nawa H. Brain-derived neurotrophic factor regulates surface expression of alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors by enhancing the N-ethylmaleimide-sensitive factor/GluR2 interaction in developing neocortical neurons. J Biol Chem. 2002;277:40901–40910. doi: 10.1074/jbc.M202158200. [DOI] [PubMed] [Google Scholar]
- Newpher and Ehlers, 2008.Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen and Sejnowski, 2000.Paulsen O, Sejnowski TJ. Natural patterns of activity and long-term synaptic plasticity. Curr Opin Neurobiol. 2000;10:172–179. doi: 10.1016/s0959-4388(00)00076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer and Huber, 2006.Pfeiffer BE, Huber KM. Current advances in local protein synthesis and synaptic plasticity. J Neurosci. 2006;26:7147–7150. doi: 10.1523/JNEUROSCI.1797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex et al., 2006.Rex CS, Lauterborn JC, Lin CY, Kramár EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex et al., 2007.Rex CS, Lin CY, Kramár EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz et al., 1994.Shoshan-Barmatz V, Weil S, Meyer H, Varsanyi M, Heilmeyer LM. Endogenous, Ca(2+)-dependent cysteine-protease cleaves specifically the ryanodine receptor/Ca2+ release channel in skeletal muscle. J Membr Biol. 1994;142:281–288. doi: 10.1007/BF00233435. [DOI] [PubMed] [Google Scholar]
- Sigrist et al., 2000.Sigrist SJ, Thiel PR, Reiff DF, Lachance PE, Lasko P, Schuster CM. Postsynaptic translation affects the efficacy and morphology of neuromuscular junctions. Nature. 2000;405:1062–1065. doi: 10.1038/35016598. [DOI] [PubMed] [Google Scholar]
- Steward and Worley, 2002.Steward O, Worley P. Local synthesis of proteins at synaptic sites on dendrites: role in synaptic plasticity and memory consolidation? Neurobiol Learn Mem. 2002;78:508–527. doi: 10.1006/nlme.2002.4102. [DOI] [PubMed] [Google Scholar]
- Sutton et al., 2006.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Takei et al., 2004.Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi et al., 2006.Taniguchi N, Shinoda Y, Takei N, Nawa H, Ogura A, Tominaga-Yoshino K. Possible involvement of BDNF release in long-lasting synapse formation induced by repetitive PKA activation. Neurosci Lett. 2006;406:38–42. doi: 10.1016/j.neulet.2006.06.071. [DOI] [PubMed] [Google Scholar]
- Wells and Fallon, 2000.Wells DG, Fallon JR. In search of molecular memory: experience-driven protein synthesis. Cell Mol Life Sci. 2000;57:1335–1339. doi: 10.1007/PL00000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al., 2004.Wu X, Zhu D, Jiang X, Okagaki P, Mearow K, Zhu G, McCall S, Banaudha K, Lipsky RH, Marini AM. AMPA protects cultured neurons against glutamate excitotoxicity through a phosphatidylinositol 3-kinase-dependent activation in extracellular signal-regulated kinase to upregulate BDNF gene expression. J Neurochem. 2004;90:807–818. doi: 10.1111/j.1471-4159.2004.02526.x. [DOI] [PubMed] [Google Scholar]
- Xiong et al., 2002.Xiong H, Futamura T, Jourdi H, Zhou H, Takei N, Diverse-Pierluissi M, Plevy S, Nawa H. Neurotrophins induce BDNF expression through the glutamate receptor pathway in neocortical neurons. Neuropharmacology. 2002;42:903–912. doi: 10.1016/s0028-3908(02)00043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin et al., 2002.Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci U S A. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang and Lipton, 1999.Zhang Y, Lipton P. Cytosolic Ca2+ changes during in vitro ischemia in rat hippocampal slices: major roles for glutamate and Na+-dependent Ca2+ release from mitochondria. J Neurosci. 1999;19:3307–3315. doi: 10.1523/JNEUROSCI.19-09-03307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]