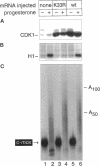
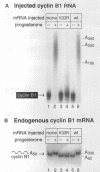
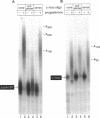
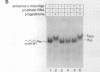
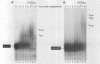
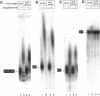
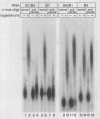
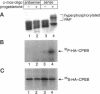
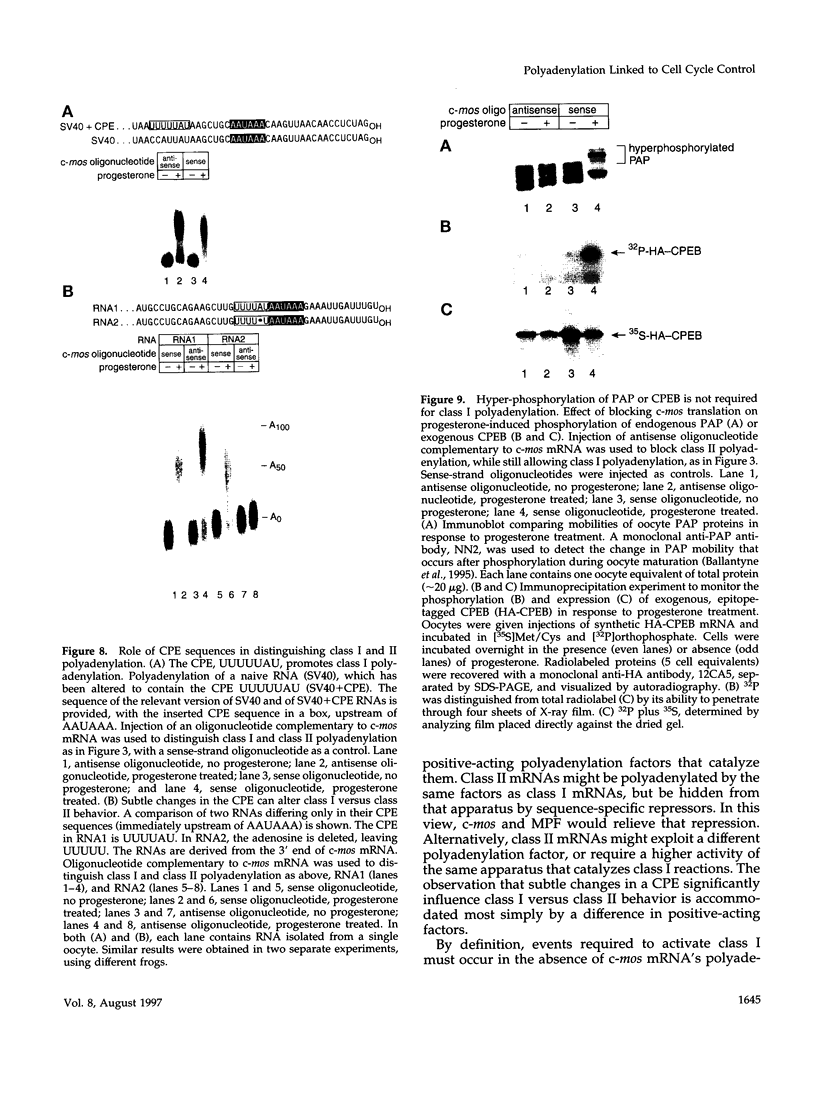
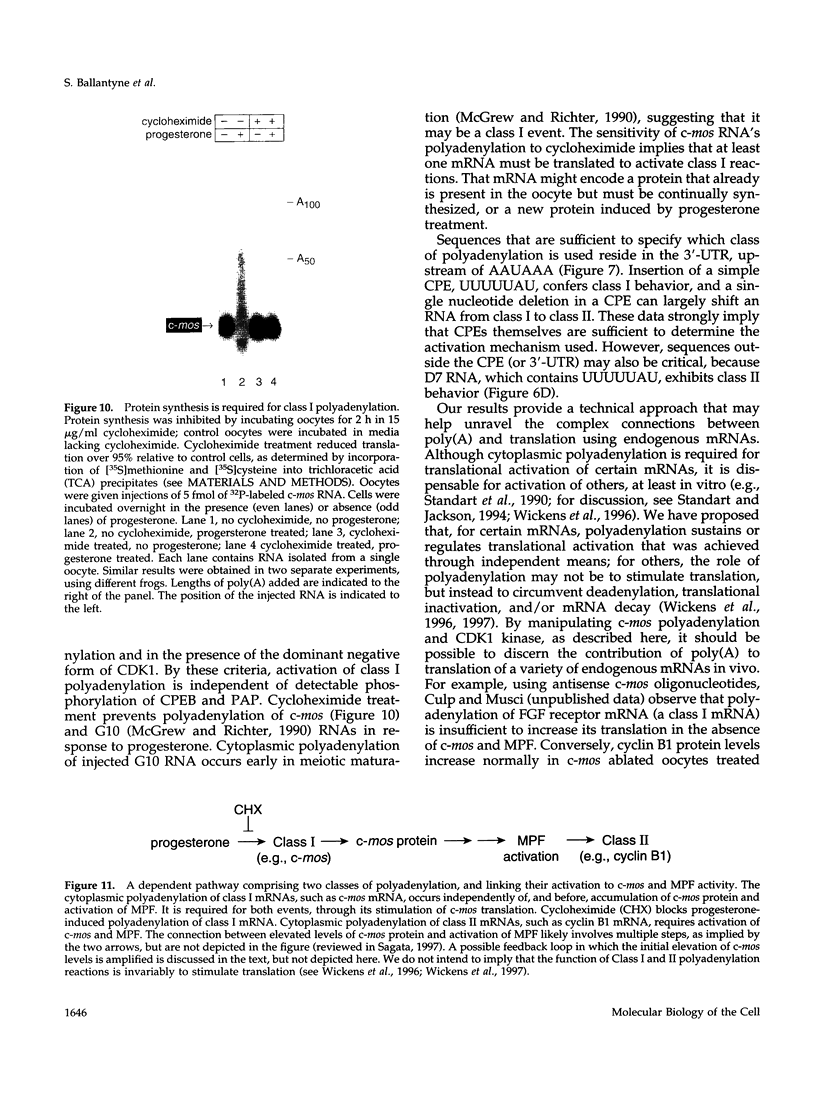
Abstract
During oocyte maturation and early development, mRNAs receive poly(A) in the cytoplasm at distinct times relative to one another and to the cell cycle. These cytoplasmic polyadenylation reactions do not occur during oogenesis, but begin during oocyte maturation and continue throughout early development. In this report, we focus on the link between cytoplasmic polyadenylation and control of the cell cycle during meiotic maturation. Activation of maturation promoting factor, a complex of CDK1 and cyclin, is required for maturation and dependent on c-mos protein kinase. We demonstrate here that two classes of polyadenylation exist during oocyte maturation, defined by their dependence of c-mos and CDK1 protein kinases. Polyadenylation of the first class of mRNAs (class I) is independent of c-mos and CDK1 kinase activities, whereas polyadenylation of the second class (class II) requires both of these activities. Class I polyadenylation, through its effects on c-mos mRNA, is required for class II polyadenylation. cis-acting elements responsible for this distinction reside in the 3'-untranslated region, upstream of the polyadenylation signal AAUAAA. Cytoplasmic polyadenylation elements (CPEs) are sufficient to specify class I polyadenylation, and subtle changes in the CPE can substantially, though not entirely, shift an RNA from class I to class II. Activation of class I polyadenylation events is independent of hyperphosphorylation of CPE-binding protein or poly(A) polymerase, and requires cellular protein synthesis. The two classes of polyadenylation and of mRNA define a dependent pathway, in which polyadenylation of certain mRNAs requires the prior polyadenylation of another. We propose that this provides one method of regulating the temporal order of polyadenylation events, and links polyadenylation to the control of the meiotic cell cycle.
Full text
PDF




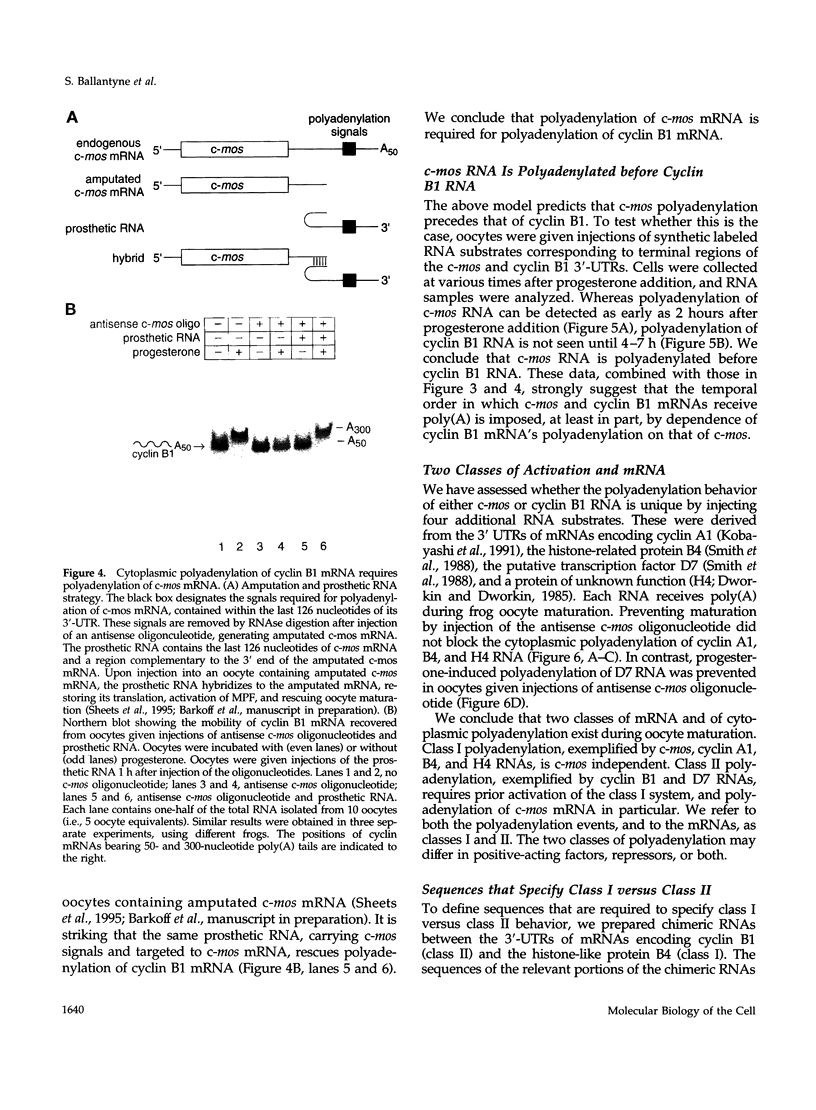

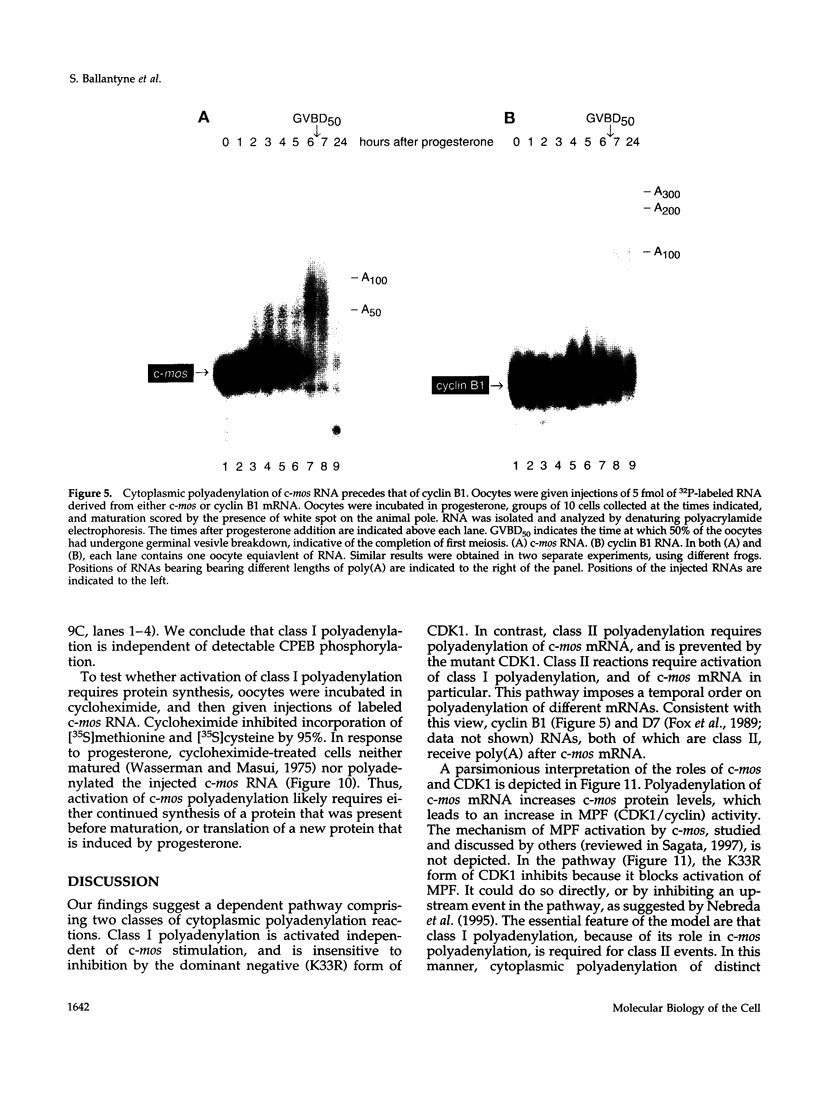
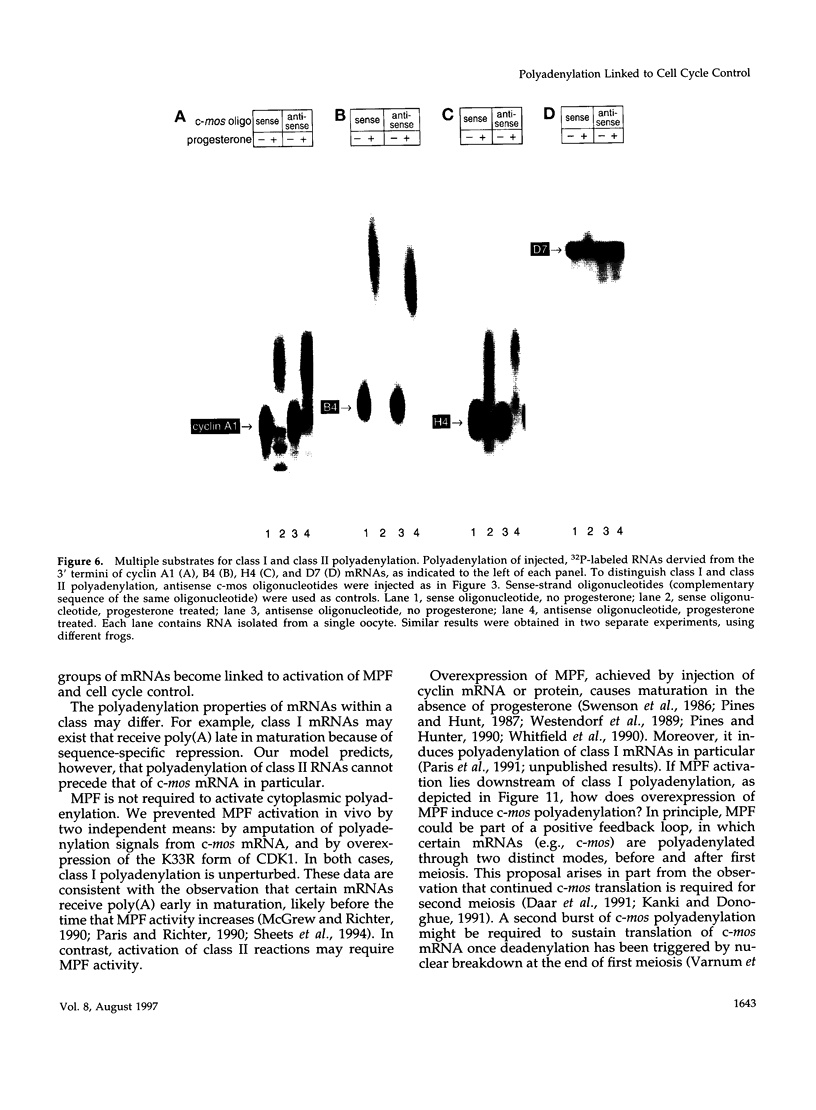
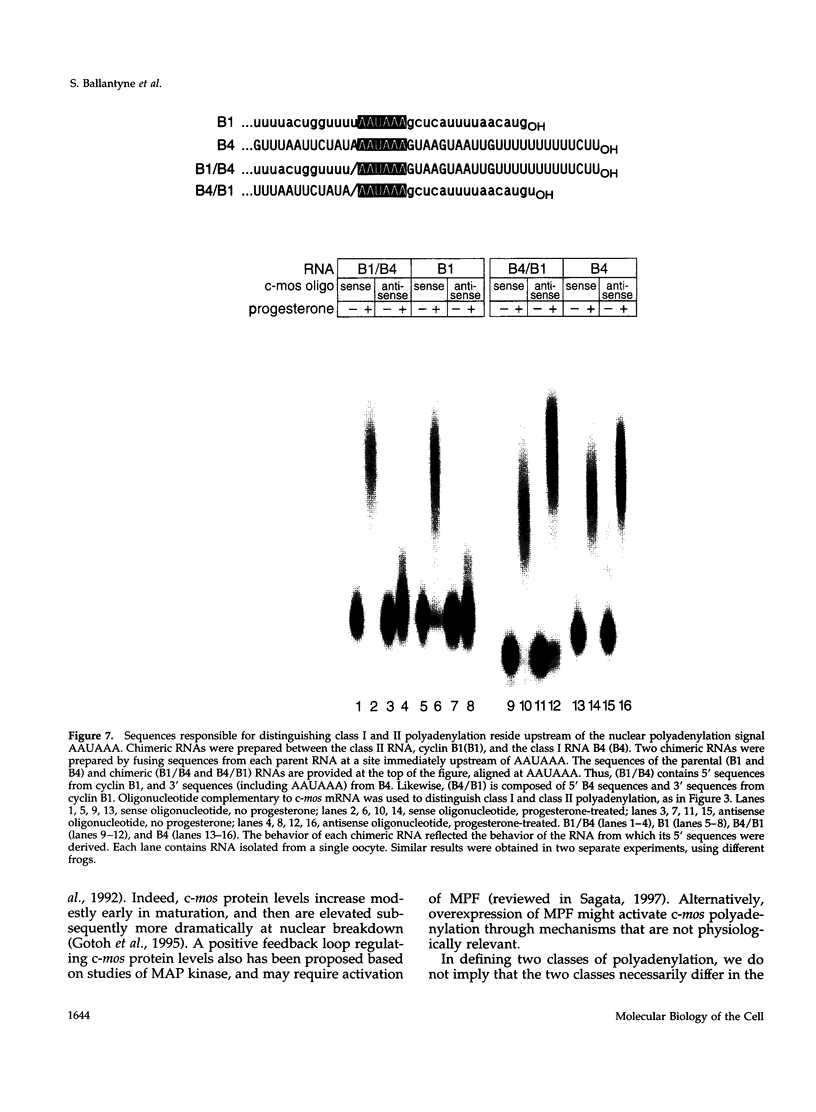


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audic Y., Omilli F., Osborne H. B. Postfertilization deadenylation of mRNAs in Xenopus laevis embryos is sufficient to cause their degradation at the blastula stage. Mol Cell Biol. 1997 Jan;17(1):209–218. doi: 10.1128/mcb.17.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne S., Bilger A., Astrom J., Virtanen A., Wickens M. Poly (A) polymerases in the nucleus and cytoplasm of frog oocytes: dynamic changes during oocyte maturation and early development. RNA. 1995 Mar;1(1):64–78. [PMC free article] [PubMed] [Google Scholar]
- Bilger A., Fox C. A., Wahle E., Wickens M. Nuclear polyadenylation factors recognize cytoplasmic polyadenylation elements. Genes Dev. 1994 May 1;8(9):1106–1116. doi: 10.1101/gad.8.9.1106. [DOI] [PubMed] [Google Scholar]
- Colgan D. F., Murthy K. G., Prives C., Manley J. L. Cell-cycle related regulation of poly(A) polymerase by phosphorylation. Nature. 1996 Nov 21;384(6606):282–285. doi: 10.1038/384282a0. [DOI] [PubMed] [Google Scholar]
- Curtis D., Lehmann R., Zamore P. D. Translational regulation in development. Cell. 1995 Apr 21;81(2):171–178. doi: 10.1016/0092-8674(95)90325-9. [DOI] [PubMed] [Google Scholar]
- Daar I., Paules R. S., Vande Woude G. F. A characterization of cytostatic factor activity from Xenopus eggs and c-mos-transformed cells. J Cell Biol. 1991 Jul;114(2):329–335. doi: 10.1083/jcb.114.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. B., Dworkin-Rastl E. Changes in RNA titers and polyadenylation during oogenesis and oocyte maturation in Xenopus laevis. Dev Biol. 1985 Dec;112(2):451–457. doi: 10.1016/0012-1606(85)90417-8. [DOI] [PubMed] [Google Scholar]
- Dworkin M. B., Shrutkowski A., Dworkin-Rastl E. Mobilization of specific maternal RNA species into polysomes after fertilization in Xenopus laevis. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7636–7640. doi: 10.1073/pnas.82.22.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. A., Sheets M. D., Wahle E., Wickens M. Polyadenylation of maternal mRNA during oocyte maturation: poly(A) addition in vitro requires a regulated RNA binding activity and a poly(A) polymerase. EMBO J. 1992 Dec;11(13):5021–5032. doi: 10.1002/j.1460-2075.1992.tb05609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. A., Sheets M. D., Wickens M. P. Poly(A) addition during maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev. 1989 Dec;3(12B):2151–2162. doi: 10.1101/gad.3.12b.2151. [DOI] [PubMed] [Google Scholar]
- Fox C. A., Wickens M. Poly(A) removal during oocyte maturation: a default reaction selectively prevented by specific sequences in the 3' UTR of certain maternal mRNAs. Genes Dev. 1990 Dec;4(12B):2287–2298. doi: 10.1101/gad.4.12b.2287. [DOI] [PubMed] [Google Scholar]
- Furuno N., Nishizawa M., Okazaki K., Tanaka H., Iwashita J., Nakajo N., Ogawa Y., Sagata N. Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J. 1994 May 15;13(10):2399–2410. doi: 10.1002/j.1460-2075.1994.tb06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F., Richter J. D. Cloning and characterization of a Xenopus poly(A) polymerase. Mol Cell Biol. 1995 Mar;15(3):1422–1430. doi: 10.1128/mcb.15.3.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F., Xu W., Cooper G. M., Richter J. D. Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J. 1994 Dec 1;13(23):5712–5720. doi: 10.1002/j.1460-2075.1994.tb06909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Masuyama N., Dell K., Shirakabe K., Nishida E. Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J Biol Chem. 1995 Oct 27;270(43):25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- Hake L. E., Richter J. D. CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell. 1994 Nov 18;79(4):617–627. doi: 10.1016/0092-8674(94)90547-9. [DOI] [PubMed] [Google Scholar]
- Huarte J., Stutz A., O'Connell M. L., Gubler P., Belin D., Darrow A. L., Strickland S., Vassalli J. D. Transient translational silencing by reversible mRNA deadenylation. Cell. 1992 Jun 12;69(6):1021–1030. doi: 10.1016/0092-8674(92)90620-r. [DOI] [PubMed] [Google Scholar]
- Hyman L. E., Wormington W. M. Translational inactivation of ribosomal protein mRNAs during Xenopus oocyte maturation. Genes Dev. 1988 May;2(5):598–605. doi: 10.1101/gad.2.5.598. [DOI] [PubMed] [Google Scholar]
- Kanki J. P., Donoghue D. J. Progression from meiosis I to meiosis II in Xenopus oocytes requires de novo translation of the mosxe protooncogene. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5794–5798. doi: 10.1073/pnas.88.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Minshull J., Ford C., Golsteyn R., Poon R., Hunt T. On the synthesis and destruction of A- and B-type cyclins during oogenesis and meiotic maturation in Xenopus laevis. J Cell Biol. 1991 Aug;114(4):755–765. doi: 10.1083/jcb.114.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Smibert C. A. Translational regulation of maternal mRNAs. Curr Opin Genet Dev. 1996 Aug;6(4):403–407. doi: 10.1016/s0959-437x(96)80060-8. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Dworkin-Rastl E., Dworkin M. B., Richter J. D. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989 Jun;3(6):803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Richter J. D. Translational control by cytoplasmic polyadenylation during Xenopus oocyte maturation: characterization of cis and trans elements and regulation by cyclin/MPF. EMBO J. 1990 Nov;9(11):3743–3751. doi: 10.1002/j.1460-2075.1990.tb07587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
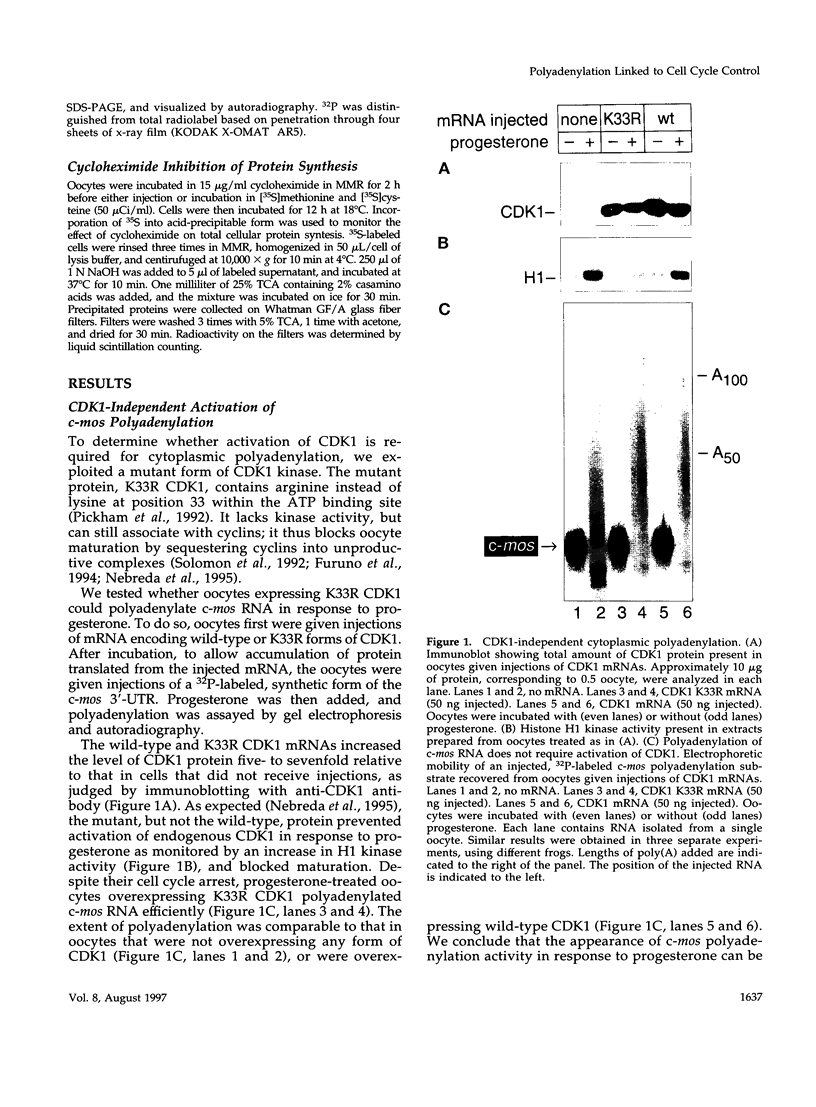
- Nebreda A. R., Gannon J. V., Hunt T. Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes. EMBO J. 1995 Nov 15;14(22):5597–5607. doi: 10.1002/j.1460-2075.1995.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995 Jun;17(6):471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- Paris J., Philippe M. Poly(A) metabolism and polysomal recruitment of maternal mRNAs during early Xenopus development. Dev Biol. 1990 Jul;140(1):221–224. doi: 10.1016/0012-1606(90)90070-y. [DOI] [PubMed] [Google Scholar]
- Paris J., Richter J. D. Maturation-specific polyadenylation and translational control: diversity of cytoplasmic polyadenylation elements, influence of poly(A) tail size, and formation of stable polyadenylation complexes. Mol Cell Biol. 1990 Nov;10(11):5634–5645. doi: 10.1128/mcb.10.11.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
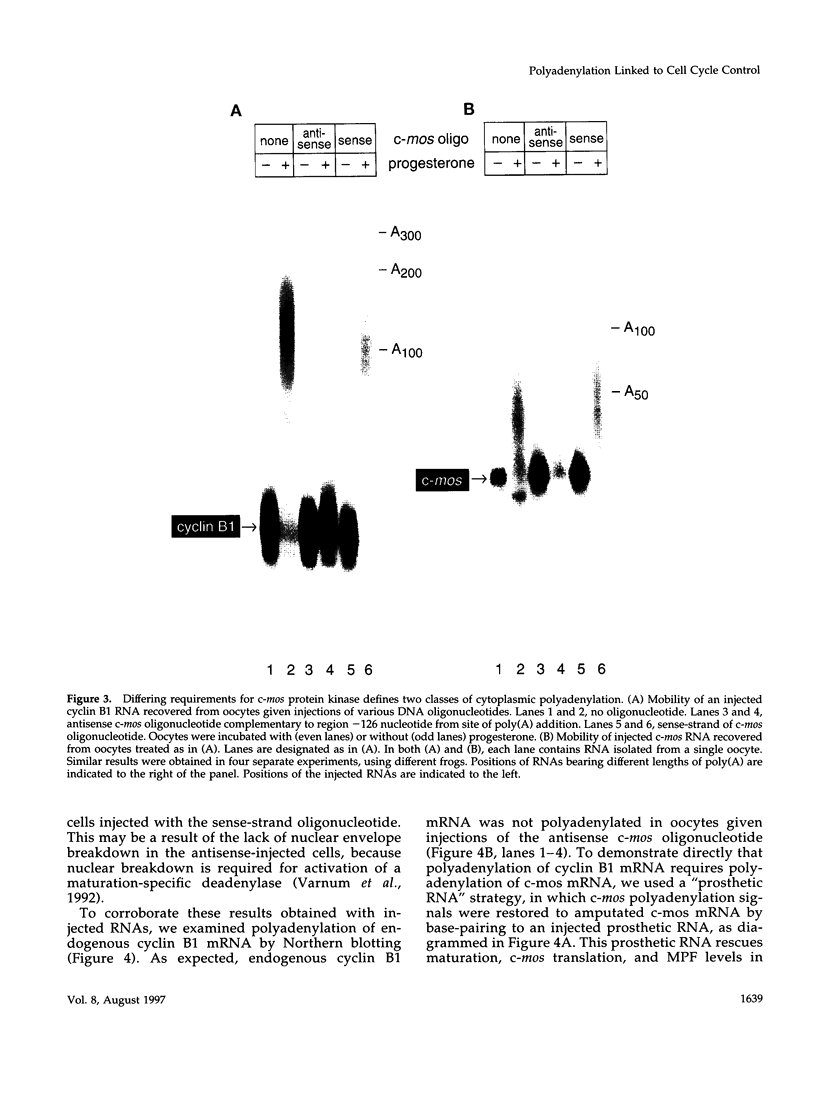
- Paris J., Swenson K., Piwnica-Worms H., Richter J. D. Maturation-specific polyadenylation: in vitro activation by p34cdc2 and phosphorylation of a 58-kD CPE-binding protein. Genes Dev. 1991 Sep;5(9):1697–1708. doi: 10.1101/gad.5.9.1697. [DOI] [PubMed] [Google Scholar]
- Pickham K. M., Meyer A. N., Li J., Donoghue D. J. Requirement of mosXe protein kinase for meiotic maturation of Xenopus oocytes induced by a cdc2 mutant lacking regulatory phosphorylation sites. Mol Cell Biol. 1992 Jul;12(7):3192–3203. doi: 10.1128/mcb.12.7.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunt T. Molecular cloning and characterization of the mRNA for cyclin from sea urchin eggs. EMBO J. 1987 Oct;6(10):2987–2995. doi: 10.1002/j.1460-2075.1987.tb02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989 Nov 30;342(6249):512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Sagata N. What does Mos do in oocytes and somatic cells? Bioessays. 1997 Jan;19(1):13–21. doi: 10.1002/bies.950190105. [DOI] [PubMed] [Google Scholar]
- Sallés F. J., Darrow A. L., O'Connell M. L., Strickland S. Isolation of novel murine maternal mRNAs regulated by cytoplasmic polyadenylation. Genes Dev. 1992 Jul;6(7):1202–1212. doi: 10.1101/gad.6.7.1202. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets M. D., Fox C. A., Hunt T., Vande Woude G., Wickens M. The 3'-untranslated regions of c-mos and cyclin mRNAs stimulate translation by regulating cytoplasmic polyadenylation. Genes Dev. 1994 Apr 15;8(8):926–938. doi: 10.1101/gad.8.8.926. [DOI] [PubMed] [Google Scholar]
- Sheets M. D., Wu M., Wickens M. Polyadenylation of c-mos mRNA as a control point in Xenopus meiotic maturation. Nature. 1995 Apr 6;374(6522):511–516. doi: 10.1038/374511a0. [DOI] [PubMed] [Google Scholar]
- Smith R. C., Dworkin-Rastl E., Dworkin M. B. Expression of a histone H1-like protein is restricted to early Xenopus development. Genes Dev. 1988 Oct;2(10):1284–1295. doi: 10.1101/gad.2.10.1284. [DOI] [PubMed] [Google Scholar]
- Smith R. C., Dworkin M. B., Dworkin-Rastl E. Destruction of a translationally controlled mRNA in Xenopus oocytes delays progesterone-induced maturation. Genes Dev. 1988 Oct;2(10):1296–1306. doi: 10.1101/gad.2.10.1296. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Lee T., Kirschner M. W. Role of phosphorylation in p34cdc2 activation: identification of an activating kinase. Mol Biol Cell. 1992 Jan;3(1):13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standart N., Dale M., Stewart E., Hunt T. Maternal mRNA from clam oocytes can be specifically unmasked in vitro by antisense RNA complementary to the 3'-untranslated region. Genes Dev. 1990 Dec;4(12A):2157–2168. doi: 10.1101/gad.4.12a.2157. [DOI] [PubMed] [Google Scholar]
- Standart N., Jackson R. J. Regulation of translation by specific protein/mRNA interactions. Biochimie. 1994;76(9):867–879. doi: 10.1016/0300-9084(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B., Hake L. E., Richter J. D. CPEB controls the cytoplasmic polyadenylation of cyclin, Cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J. 1996 May 15;15(10):2582–2592. [PMC free article] [PubMed] [Google Scholar]
- Stebbins-Boaz B., Richter J. D. Multiple sequence elements and a maternal mRNA product control cdk2 RNA polyadenylation and translation during early Xenopus development. Mol Cell Biol. 1994 Sep;14(9):5870–5880. doi: 10.1128/mcb.14.9.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgess E. A., Ballantine J. E., Woodland H. R., Mohun P. R., Lane C. D., Dimitriadis G. J. Actin synthesis during the early development of Xenopus laevis. J Embryol Exp Morphol. 1980 Aug;58:303–320. [PubMed] [Google Scholar]
- Swenson K. I., Farrell K. M., Ruderman J. V. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986 Dec 26;47(6):861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Thuresson A. C., Aström J., Aström A., Grönvik K. O., Virtanen A. Multiple forms of poly(A) polymerases in human cells. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):979–983. doi: 10.1073/pnas.91.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum S. M., Hurney C. A., Wormington W. M. Maturation-specific deadenylation in Xenopus oocytes requires nuclear and cytoplasmic factors. Dev Biol. 1992 Oct;153(2):283–290. doi: 10.1016/0012-1606(92)90113-u. [DOI] [PubMed] [Google Scholar]
- Varnum S. M., Wormington W. M. Deadenylation of maternal mRNAs during Xenopus oocyte maturation does not require specific cis-sequences: a default mechanism for translational control. Genes Dev. 1990 Dec;4(12B):2278–2286. doi: 10.1101/gad.4.12b.2278. [DOI] [PubMed] [Google Scholar]
- Wasserman W. J., Masui Y. Effects of cyclohexamide on a cytoplasmic factor initiating meiotic naturation in Xenopus oocytes. Exp Cell Res. 1975 Mar 15;91(2):381–388. doi: 10.1016/0014-4827(75)90118-4. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Walder J. A., Dagle J. M. Cyclin B mRNA depletion only transiently inhibits the Xenopus embryonic cell cycle. Development. 1991 Apr;111(4):1173–1178. doi: 10.1242/dev.111.4.1173. [DOI] [PubMed] [Google Scholar]
- Westendorf J. M., Swenson K. I., Ruderman J. V. The role of cyclin B in meiosis I. J Cell Biol. 1989 Apr;108(4):1431–1444. doi: 10.1083/jcb.108.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield W. G., Gonzalez C., Maldonado-Codina G., Glover D. M. The A- and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2-M transition. EMBO J. 1990 Aug;9(8):2563–2572. doi: 10.1002/j.1460-2075.1990.tb07437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M., Anderson P., Jackson R. J. Life and death in the cytoplasm: messages from the 3' end. Curr Opin Genet Dev. 1997 Apr;7(2):220–232. doi: 10.1016/s0959-437x(97)80132-3. [DOI] [PubMed] [Google Scholar]
- Wormington W. M. Developmental expression and 5S rRNA-binding activity of Xenopus laevis ribosomal protein L5. Mol Cell Biol. 1989 Dec;9(12):5281–5288. doi: 10.1128/mcb.9.12.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew N., Mellini M. L., Vande Woude G. F. Meiotic initiation by the mos protein in Xenopus. Nature. 1992 Feb 13;355(6361):649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]